Abstract
Rationale
Tumor necrosis factor alpha (TNF-alpha) has been implicated as a key cytokine in many inflammatory lung diseases. These effects are currently unclear, because a transgenic mouse overexpressing TNF-alpha in the lung has been shown in separate studies to produce elements of both emphysema and pulmonary fibrosis.Objectives
We sought to elucidate the phenotypic effects of TNF-alpha overexpression in a mouse model.Measurements
We established the phenotype by measuring lung impedance and thoracic gas volume, and using micro-computed tomography and histology.Main results
We found that airways resistance in this mouse was not different to control mice, but that lung tissue dampening, elastance, and hysteresivity were significantly elevated. Major heterogeneous abnormalities of the parenchyma were also apparent in histologic sections and in micro-computed tomography images of the lung. These changes included airspace enlargement, loss of small airspaces, increased collagen, and thickened pleural septa. We also found significant increases in lung and chest cavity volumes in the TNF-alpha-overexpressing mice.Conclusions
We conclude that TNF-alpha overexpression causes pathologic changes consistent with both emphysema and pulmonary fibrosis combined with a general lung inflammation, and consequently does not model any single human disease. Our study thus confirms the pleiotropic effects of TNF-alpha, which has been implicated in multiple inflammatory disorders, and underscores the necessity of using a wide range of investigative techniques to link gene expression and phenotype in animal models of disease.Free full text

Tumor Necrosis Factor–α Overexpression in Lung Disease
Abstract
Rationale: Tumor necrosis factor α (TNF-α) has been implicated as a key cytokine in many inflammatory lung diseases. These effects are currently unclear, because a transgenic mouse overexpressing TNF-α in the lung has been shown in separate studies to produce elements of both emphysema and pulmonary fibrosis. Objectives: We sought to elucidate the phenotypic effects of TNF-α overexpression in a mouse model. Measurements: We established the phenotype by measuring lung impedance and thoracic gas volume, and using micro–computed tomography and histology. Main Results: We found that airways resistance in this mouse was not different to control mice, but that lung tissue dampening, elastance, and hysteresivity were significantly elevated. Major heterogeneous abnormalities of the parenchyma were also apparent in histologic sections and in micro–computed tomography images of the lung. These changes included airspace enlargement, loss of small airspaces, increased collagen, and thickened pleural septa. We also found significant increases in lung and chest cavity volumes in the TNF-α–overexpressing mice. Conclusions: We conclude that TNF-α overexpression causes pathologic changes consistent with both emphysema and pulmonary fibrosis combined with a general lung inflammation, and consequently does not model any single human disease. Our study thus confirms the pleiotropic effects of TNF-α, which has been implicated in multiple inflammatory disorders, and underscores the necessity of using a wide range of investigative techniques to link gene expression and phenotype in animal models of disease.
Tumor necrosis factor α (TNF-α) is a potent inflammatory cytokine, which has been implicated in a variety of pulmonary diseases, including idiopathic pulmonary fibrosis (1, 2), asthma (3), chronic obstructive pulmonary disease (COPD) (4–6), pulmonary Langerhans' cell histiocytosis (7), emphysema (6, 8, 9), and pulmonary fibrosis (10–12). Most cells in the body express receptors for TNF-α, which can elicit a number of different responses depending on many factors, including the dose, route of administration, and duration of TNF-α (13). The release of TNF-α is triggered primarily by inflammatory stimuli. In the lungs, TNF-α production arises from numerous cell types, including epithelium, endothelium, activated macrophages and monocytes, and probably also smooth muscle.
From a phenotypic point of view, data from animal studies are contradictory and confusing. On one hand, a transgenic mouse overexpressing TNF-α has been reported to have fibrosis in the parenchyma, together with elevated lymphocytes, macrophages, and neutrophils within the interstitium, leading to the conclusion that it is a model of idiopathic pulmonary fibrosis (14). On the other hand, Fujita and coworkers (15) found that the inflammation in this mouse resolves by the age of 6 months, at which time the lung histology and physiologic function is more consistent with emphysema. Recent findings in a mouse with an inducible TNF-α gene construct also suggest that TNF-α expression in the lung may produce emphysema in conjunction with lymphoid follicles (9).
The phenotype of the transgenic TNF-α–overexpressing mouse is thus still unclear. At least part of the confusion can be attributed to the fact that the two key studies on this mouse strain (14, 15) each used only a limited set of investigative techniques that biased them toward their respective conclusions. However, the issue may also be clouded by the tacit expectation that TNF-α overexpression should result in any one disease. In fact, different inflammatory diseases share common pathways and mediators, so manipulating a single mediator may well lead to a pathology involving aspects of several diseases. In any case, the pathogenic ubiquity of TNF-α in lung inflammation makes elucidation of its phenotypic effects an important issue. Accordingly, the goal of the present study was to investigate the surfactant protein-C/TNF-α mouse from the perspectives of lung function, three-dimensional imaging, and histology to elucidate its phenotype in more detail.
METHODS
The techniques and methods outlined in this section are described in greater detail in the online supplement.
Animals
We studied female surfactant protein-C/TNF-α transgenic (TG+) mice (n = 10) and littermate control animals (n = 10) aged 7 to 11 months. The animals' weights were not significantly different between groups (23.25 ± 0.69 and 24.75 ± 1.14 g in TG+ and control animals, respectively). The Institutional Animal Care and Use Committee of the University of Vermont approved the experiments. We identified TG+ mice by polymerase chain reaction analysis of genomic DNA isolated from small tissue samples obtained by ear punch (14).
Animal Preparation
Anesthetized mice were installed in a custom-designed whole-body plethysmograph and connected to a small animal ventilator (flexiVent; SCIREQ, Inc., Montreal, PQ, Canada), as previously described (16).
Experimental Protocol
After 30 seconds of regular ventilation, two baseline measurements of respiratory input impedance (Zrs) were obtained (see below). Next, a standard lung volume history was established by delivering a pressure–volume (PV) loop (see below) followed by four deep sighs to a pressure limit of 25 cm H2O. The Zrs was then measured every 30 seconds for 5 minutes followed by another four deep sighs and one more PV loop. The last PV loop was used in the data analysis. After this, the thoracic gas volume (VTG) was measured (see below). This sequence of events ensured that a standard volume history preceded each measurement, and was repeated at each of four different positive end-expiratory pressure (PEEP) levels (0, 1, 3, and 6 cm H2O) applied in random order. At the end of the experiment, the mice were killed, and the trachea was tied off after exhalation against a PEEP of 3 cm H2O. We then removed the cannula and waited for the mouse to stiffen to prevent motion artifacts during subsequent imaging in a micro–computed tomography (micro-CT) scanner (see below). When the scan was completed, the trachea was recannulated and the lungs fixed for histologic analysis (see below).
Determination of Input Impedance
We interpreted the measurement of Zrs in terms of the constant-phase model (17), where RN is the frequency-independent Newtonian resistance reflecting that of the conducting airways and any Newtonian component of the tissue, Iaw is airway gas inertance, G characterizes tissue resistance, and H characterizes tissue stiffness. Hysteresivity (η = G/H) was also calculated; η tends to increase when regional heterogeneity in the lung develops (17, 18).
Analysis of PV Loops
Quasi-static PV loops were obtained by stepwise inflation and deflation of the lungs. The shape factor (k) of the descending limb of the PV loop was calculated by fitting the data to the Salazar-Knowles equation (19). The value of the parameter k is believed to change characteristically with both fibrosis and emphysema (20, 21).
Measurement of VTG
VTG was measured plethysmographically (VTG,Pleth) by external chest compression as described previously (16, 22).
Micro-CT
Lung volume (VL,CT) and thoracic volume (VTh,CT) were calculated by integrating the total volume occupied by pixels within the lung and total thoracic regions, respectively, of the micro-CT images. Isosurface renderings of the images were constructed to allow visualization of the air–tissue interface within the lung. Maximum intensity projections of intensity-inverted images were constructed to visualize the airways. A number of the mice were not successfully scanned because of motion artifacts. Consequently, the micro-CT lung and chest volumes reported are based on a subset of the animals studied (n = 7 for TG+ and n = 6 for control mice).
Histologic Analysis
Histologic slides of the excised lungs were stained with hematoxylin and eosin and Sirius red and fast green FCF (visualizes collagen under polarized light). The Sirius red and fast green FCF slides were scored using index as either 1 (least amount of collagen staining), 2, or 3 (most intense/organized staining). We also analyzed the structure of the airspaces within each slide by digitizing the image and converting it to black and white and then identifying the length of every pixel line segment lying within an airspace. The number of line segments of each length was then multiplied by that length, and the results expressed as a histogram representing the fraction of the airspace area in the image composed of line segments of each length. The centroid of the histogram (Lc) was calculated as a reflection of mean airspace dimension (see online supplement).
Statistics
Data are presented as means ± SEM. Zrs, VTG, and k data were compared using one-way analysis of variance. Body weights and LC were compared using two-sample independent t tests. Histologic scores were compared using Wilcoxon rank sum test. p Values less than 0.05 were taken as significant.
RESULTS
Figure 1 shows parameters of Zrs (obtained from the constant-phase model) versus time at each of the four PEEP levels in control and TG+ mice. The RN decreased with increasing PEEP in both TG+ and control animals. There was no significant difference between the two groups of mice at any PEEP level. G was significantly higher in the TG+ group compared with control mice at all PEEP levels (p < 0.001). Furthermore, G in the control mice decreased monotonically with PEEP, whereas it started to increase again at a PEEP of 6 cm H2O in the TG+ animals. H showed yet another pattern, increasing with time but decreasing with PEEP in both groups. H was significantly higher in control mice at PEEP levels of 0, 1, and 3 cm H2O, but at 6 cm H2O, H was significantly higher in the TG+ mice (p < 0.01). Finally, η increased with PEEP in a similar pattern for both groups of mice, but was significantly higher in the TG+ than in control mice (p < 0.001).
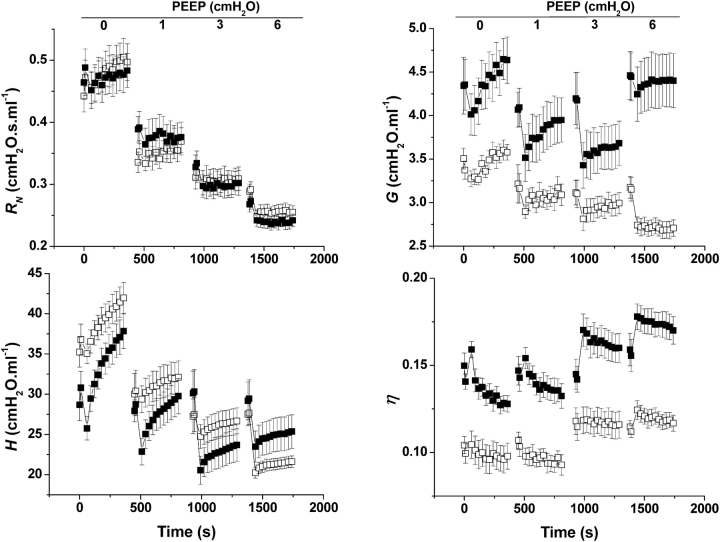
Mean values of the parameters of the constant-phase model of respiratory input impedance (Zrs) in TG+ (n = 10; solid symbols) and control (n = 10; open symbols) mice versus time. RN, H, G, and η reflect airway resistance, tissue stiffness, tissue damping, and hysteresivity, respectively. Error bars indicate SEM. Zrs was measured at a positive end-expiratory pressure (PEEP) of 0, 1, 3, and 6 cm H2O in randomized order. After the first two Zrs at each PEEP, a pressure–volume (PV) loop and deep inhalation were administered to generate a standard volume history.
Figure 2A shows the PV loops obtained in the two groups of mice at the four PEEP levels studied. The PV loops are plotted relative to VTG,Pleth at each PEEP level. VTG,Pleth was significantly different between control and TG+ mice at all PEEP levels. At PEEP levels of 0 and 1 cm H2O, the PV loops of the TG+ mice were more vertical than in control animals, indicating a more compliant lung. However, as PEEP increased to 6 cm H2O, the PV loop from the TG+ group moved to the right of the control loop, indicating a less compliant lung. Figure 2B shows the shape factor k determined from the descending limbs of the PV loops. k was significantly different between TG+ and control animals at all PEEP levels, being smaller in the TG+ animals at PEEPs of 0 and 1 cm H2O and larger at PEEPs of 3 and 6 cm H2O. These differences mirror those of the relative positions of the PV loops in Figure 2A.
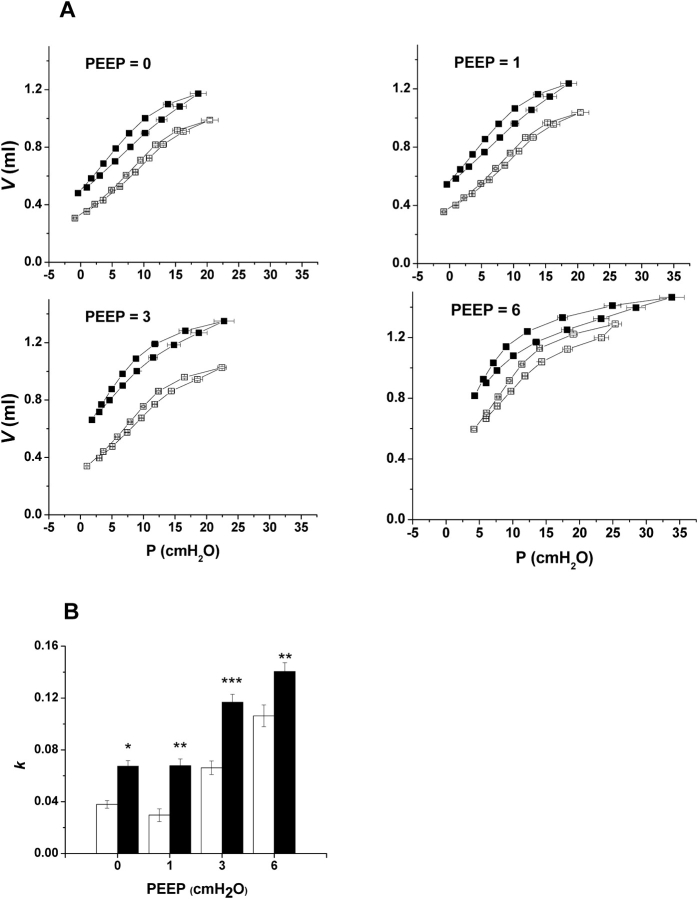
(A) Quasi-static PV loops from TG+ (n = 10; solid symbols) and control (n = 10; open symbols) at four different PEEP levels (in cm H2O). Loops proceed in a counterclockwise direction beginning with inspiration. Error bars indicate SEM. (B) Shape factor k (means ± SEM) calculated from PV loops in A using the Salazar-Knowles equation. Open bars are control animals (n = 10) and solid bars are TG+ mice (n = 10). Significant differences between groups are shown as follows: *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 3 shows representative micro-CT images obtained from a control mouse (Figures 3A and 3C) and a TG+ mouse (Figures 3B and 3D). The pleural fissures are clearly visible in the latter, and suggestive of peripheral fibrosis, which was confirmed by the pathology. The lungs of the TG+ mouse also show areas of enlarged airspaces indicative of emphysema, as well as areas with multiple irregular cystic spaces and parenchymal nodular changes. In addition, when the lungs of the TG+ mice were removed from the chest cavity, they exhibited a yellowish surface with a reticular pattern. They also remained expanded and stiff, in contrast to the control lungs, which collapsed. VL,CT was larger in the TG+ compared with the control mice (0.677 ± 0.062 vs. 0.489 ± 0.043 ml, p < 0.01). VTh,CT was also significantly larger in the TG+ compared with the control mouse (2.87 ± 0.17 vs. 1.99 ± 0.07 ml, p < 0.001).
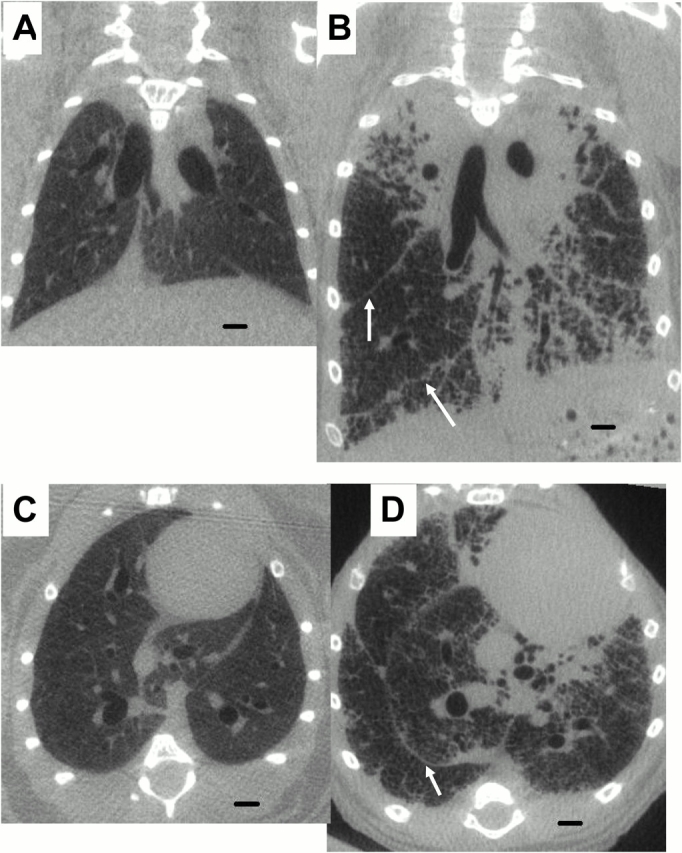
Representative micro–computed tomography (micro-CT) images of control (A and C) and TG+ mice (B and D). A and B are coronal sections and C and D are transverse sections. The mice were scanned at 80 kV(peak), 450 mA, 720 views for 80 minutes. The scans have a resolution of 47 μm/voxel side. Arrows point at interlobular fissures. Scale bar equals 1 mm.
Figure 4 and Figure E2 show representative isosurface renderings of the lung from a control mouse and a TG+ mouse. These images represent the interface between air and tissue in the lung. In the control lung (Figure 4A and Figure E2A) the interface is smooth, and indentations from the ribs are clearly visible. By contrast, the air–tissue interface of the TG+ lung (Figure 4B and Figure E2B) is rough with a nodular appearance. This is from patchy consolidation of the parenchyma, and reveals some intralobar airways that are not visible in the control lung. The maximum intensity projection images of the same lungs are also shown in Figure 4, and demonstrate that the diameters of the larger airways are similar in control (Figure 4C) and TG+ (Figure 4D) mice. Video sequences showing the isosurface renderings from all angles can be viewed on the online supplement (Figure E2).
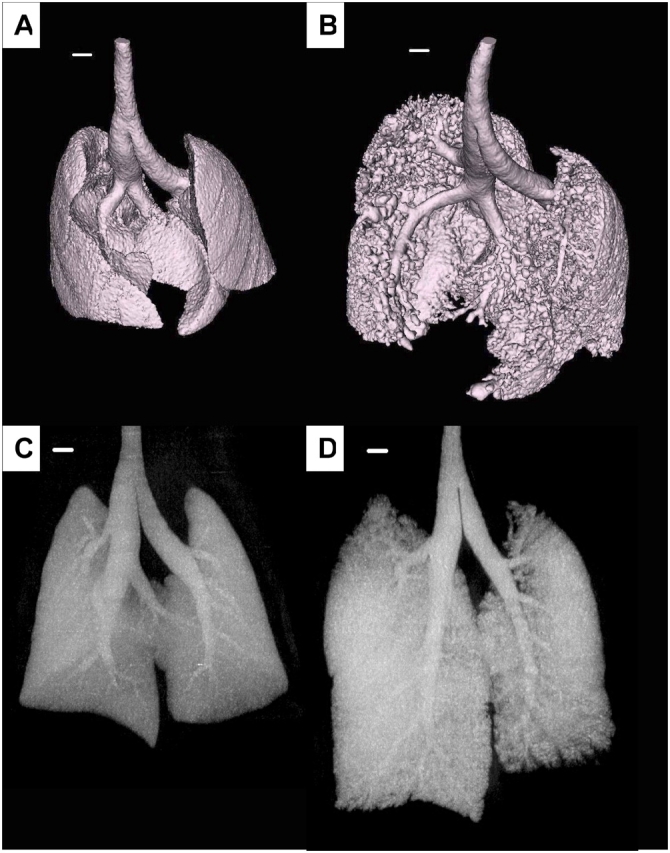
Surface renderings of the air–tissue interface from micro-CT scans of control (A) and TG+ (B) mice. Also shown are maximum intensity projection images of the same control (C) and TG+ (D) lungs from which the airway tree structure can be seen. Scale bar equals 1 mm.
Figure 5 shows representative histologic images (hematoxylin–eosin stain) obtained from a control mouse (Figure 5A) and a TG+ mouse (Figure 5B). The TG+ animal shows infiltration of lymphocytes and macrophages into the parenchyma, which was most prominent in the subpleural regions. The alveolar structure in the TG+ mouse is also distorted because of septal thickening caused by increased numbers of fibroblasts and collagen deposition, with some areas of consolidation. Many of the macrophages were laden with hemosiderine, indicating phagocytosis of red blood cells. Other macrophages were vacuolarized and had multiple nuclei. The pathology of the TG+ animals was patchy, however, with occasional alveolar regions that looked normal, without cell infiltrate or thickened membranes, next to highly affected areas.
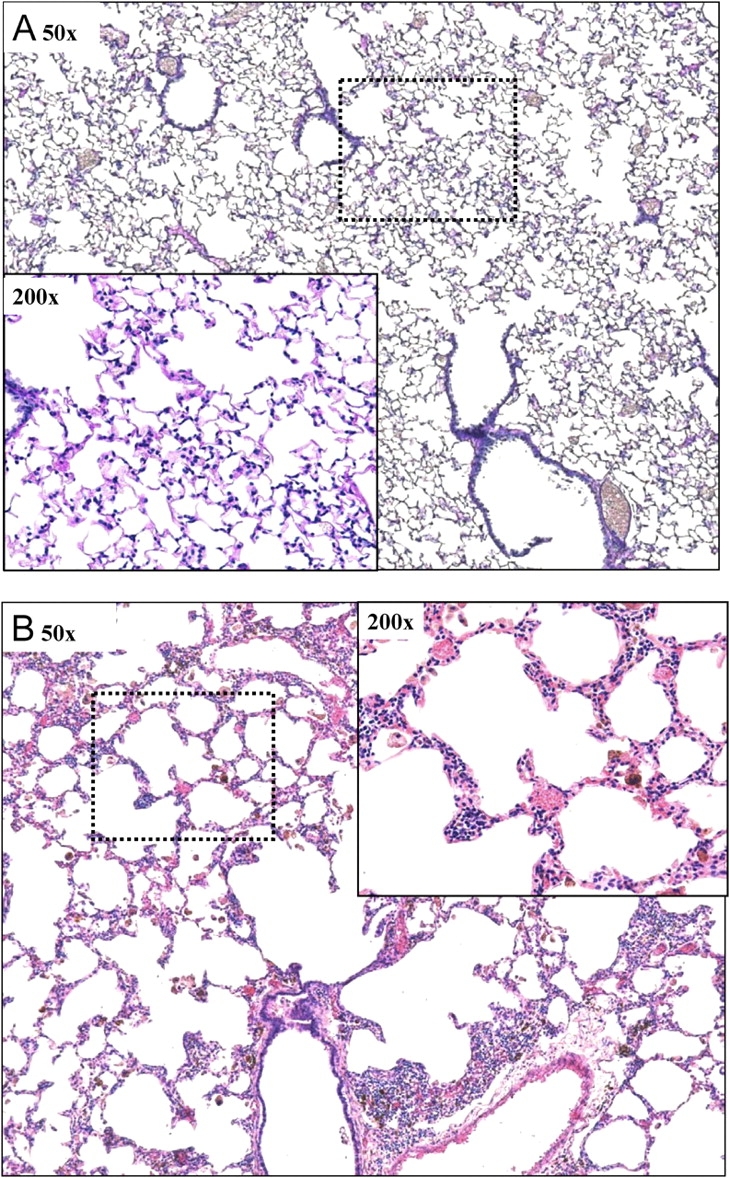
Representative images of lung tissue from control (A) and TG+ (B) mice stained with hematoxylin and eosin. The overview image was obtained at a magnification of 50×, whereas the breakout image was obtained at 200×. The dashed rectangular box indicates where the 200× image was obtained.
Figure 6 shows histologic images from Sirius red and fast green FCF stained slides viewed with polarized light from a control mouse (Figure 6A) and a TG+ mouse (Figure 6B). These images again show distortion of lung structure and thickened septa because of collagen deposition in the TG+ animals. We also observed clear signs of fibrosis along the larger airways as evidenced by Sirius red staining of collagen. The collagen scores in TG+ mice were significantly higher than the scores in the control animals (2.75 ± 0.089 vs. 1.35 ± 0.094, p < 0.001).
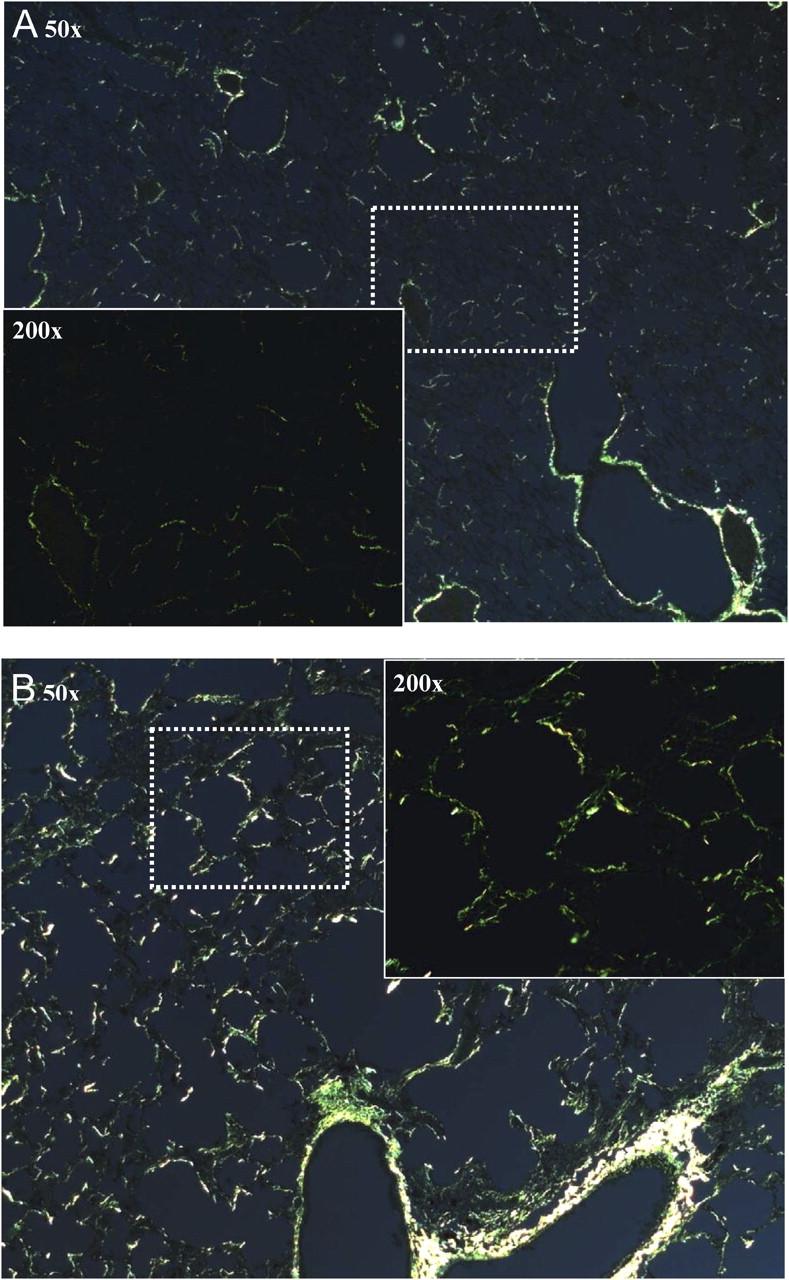
Representative images of lung tissue from control (A) and TG+ (B) mice stained with Sirius red and fast green FCF. The overview image was obtained at a magnification of 50×, whereas the breakout image was obtained at 200×. The dashed rectangular box indicates where the 200× image was obtained.
Figure 7 shows the length-weighted histograms of line segment lengths from the airspaces in lung slices from control and TG+ mice. The two histograms differ mostly for lengths of less than 200 μm, indicating that the TG+ mice had fewer small airspaces in their lungs than control animals, but similar numbers of airspaces of larger dimension. As a consequence, LC was significantly greater in the TG+ mice (200.6 ± 12.3 μm) than in the control group (145.1 ± 8.27 μm).
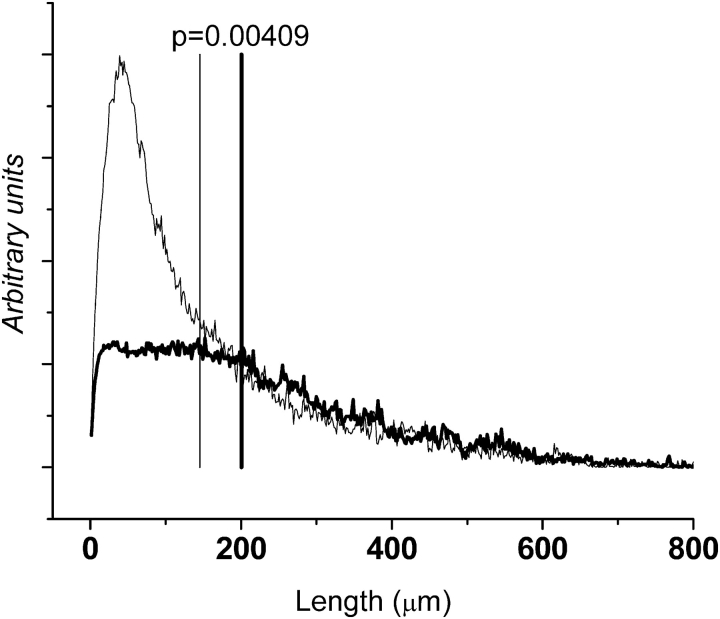
Frequency distributions of line segment lengths across airspaces from histologic images, weighted by segment length (arbitrary units). Mean distributions for control mice (thin line) and TG+ mice (thick line) are shown with vertical lines showing mean centroid of the histogram (LC) values. The shift of LC to the right indicates that TG+ mice have fewer small airspaces than do control mice.
DISCUSSION
We have performed a detailed assessment of the pathology and pathophysiology resulting from TNF-α overexpression in mouse lungs, and have demonstrated dramatic changes in both lung structure and function. This confirms and extends the results of two previous reports (14, 15). We have further shown that these changes are confined to the lung periphery, because RN was not affected by TNF-α overexpression and the maximum intensity projection images show similar dimensions for the larger airways in both control and TG+ mice (Figures 4C and 4D). By contrast, the parameters G, H, and their ratio η, which pertain to the lung periphery, were significantly affected in the TG+ animals, and G and H also exhibited qualitatively different dependencies on PEEP compared with control mice (Figure 1). The TG+ mice have more compliant lungs than control mice at lower PEEP but exhibit stiffer lungs at the highest PEEP of 6 cm H2O. Also, the PV loops from the two groups of mice exhibited different dependencies on PEEP (Figures 2A and 2B). Finally, the TG+ animals had lung volumes that were significantly larger, at any given PEEP, than the control animals (Figure 2A).
One possible explanation for an increase in η is increased regional heterogeneity throughout the lungs (17). Indeed, the micro-CT images (Figures 3 and and4,4, and Figure E2) and the lung micrographs (Figure 5) both show a patchy morphology in the TG+ animals, consistent with increased heterogeneity of lung tissue structure. However, heterogeneity is expected to decrease with increasing PEEP as the airways become tethered open by the forces of airway-parenchymal interdependence, yet we found that η was uniformly elevated in the TG+ mice at all PEEP levels (Figure 1). This finding suggests that heterogeneities are likely less important and that the uniform increase in G is caused by a change in the intrinsic rheologic properties of the lung tissue. Changes in intrinsic tissue properties are also supported by the isosurface rendering of the parenchyma from TG+, which show a substantial fraction of the lung to be consolidated with the remaining aerated regions exhibiting airspace enlargement (Figure 4 and Figure E2). Loss of alveolar septa was also visible in the micrographs (Figure 5). Interestingly, not all tissue pathology operates in this way. For example, Ito and coworkers (23) studied elastase-induced emphysema in the mouse and found that, even though η was increased at high PEEP levels, both H and G were reduced. These differences may reflect the different pathologic processes involved in chronic inflammation and proteolysis.
The question we now face is how the physiology of the TG+ mouse relates to human lung disease, the understanding of which was the motivation for producing this transgenic strain in the first place. A previous study of this animal (14) concluded that it is a model of emphysema, and our own observations support this in many respects. For example, the rightward movement of the PV loop with increasing PEEP in the TG+ mouse (Figures 2A and 2B) indicates a lung that is more compliant than normal at low volumes but becomes stiffer than normal as total lung capacity is approached, similar to observations in patients with COPD (24). Humans with emphysema have overexpanded lungs, precisely as we found in the TG+ mouse (Figures 2A and and3),3), and have enlarged alveoli as is evident in the TG+ mice (Figure 5). Emphysema may also lead to what is known as a barrel chest, and indeed we found that the TG+ mice had a VTh,CT 1.5 times larger than control animals, despite a somewhat smaller body weight (Figure 3). These results thus seem to indicate that the TG+ mouse is a model of COPD, perhaps not surprisingly given that TNF-α is believed to contribute to the apoptotic lesions that eventually lead to emphysema in COPD (6), possibly mediated via apoptotic macrophages (25). Cigarette smoke has also been shown to cause release of TNF-α in the lungs of both humans and mice (8, 9).
However, the lung pathology of the TG+ mouse is not entirely consistent with the conventional view of emphysema. Micro-CT images of the lungs of this animal (Figure 3) do not show the classical pattern of large air-filled spaces and bullae, but rather exhibit patches of airspace dilatation coupled with consolidation and fibrosis. Histology corroborated this impression (Figure 5), showing large areas of consolidated tissue consisting primarily of lymphocytes, consistent with the lymphocytic pneumonitis previously reported in this transgenic mouse (14). The septa in the TG+ lungs were also thicker than those in the control lungs (Figure 5) and contained more collagen (Figure 6). Also, the increase of LC in the TG+ mice was mostly caused by a reduction in the number of small airspaces rather than an expansion of large ones (Figure 7), suggesting more of a consolidative process rather than a destructive one. These features are consistent with a diagnosis of pulmonary fibrosis, which is consistent with the original report of this transgenic mouse (14).
The link to fibrosis is to be expected, because it has been shown in humans that the polymorphic TNF-α2 microsatellite is associated with a risk of chemotherapy-induced pulmonary fibrosis (10), and that there is a significant association between idiopathic pulmonary fibrosis and the TNF-α (−308 A) allele (12). Also, TNF-α antibodies protect against bleomycin-induced lung fibrosis in mice (11), although, curiously, there is evidence that lung-specific TNF-α overexpression may protect against bleomycin-induced fibrosis, at least in young mice (26). Taken together, these data implicate TNF-α as playing a potential role in the development of pulmonary fibrosis.
Our findings thus appear to represent a conflict between emphysema and fibrosis in the lung of the TG+ mouse, which raises the question as to whether this animal is representative of any particular human disease. On the other hand, although emphysema is perhaps the major hallmark of human COPD, airway wall fibrosis is also considered a feature of this disease (27). Indeed, increased collagen has been found in lung specimens from patients with COPD (28, 29), particularly in the smaller airways (30–32). Several studies have also found that emphysema may be accompanied by fibrotic changes in the lungs of both humans and animal models (33–37). Treating the lungs of the hamster with elastase, for example, has been shown to lead to both emphysema and expression of collagen mRNA (38). In any case, the development of pulmonary pathologies consistent with two disease states, emphysema and pulmonary fibrosis, in the TG+ mouse is not surprising given the pleiotropic nature of TNF-α signaling.
TNF-α signals via two receptors, TNF-RI and TNF-RII, through which distinct intracellular cascades can be elicited (39). TNF-RI activation promotes intracellular signaling involving c-Jun N-terminal kinase (JNK) and nuclear factor (NF)-κB (40). NF-κB activation often induces the expression of intracellular proteins that inhibit apoptosis (41), whereas activation of JNK can promote apoptosis in a mitochondria-dependent or mitochondria-independent manner (42). Signaling through TNF-RII promotes intracellular signaling without directly inducing apoptosis, but may promote TNF-RI–dependent cell death. Even within an individual cell, which signaling pathway is activated will likely be influenced by the local microenvironment, such as the presence of reactive oxygen species (43). Although TNF-RI and TNF-RII are broadly expressed, it is now appreciated that the majority of inflammatory signaling is elicited through TNF-RI, which may impact the pathologies elicited in this mouse as it induces the release of additional bioactive molecules that elicit autocrine, paracrine, and even endocrine effects. These molecules may also play important roles in the development and perpetuation of the pulmonary pathologies seen in the TG+ mouse, while eliciting some of the systemic effects that are hallmarks of both emphysema and pulmonary fibrosis. We must bear in mind, however, that we have examined the effects of TNF-α in a mature animal that expresses TNF-α constitutively throughout its entire life, in contrast to the human situation in which TNF-α overexpression typically begins at a more mature stage of development (e.g., in smoke-induced COPD or in idiopathic pulmonary fibrosis). This may be another reason why the pathology of the TG+ mouse does not correspond to any single human disease entity.
Our findings and the conclusions that arise from them are, of course, critically dependent on the investigative techniques we used. The use of forced oscillations to measure lung mechanical function is a well-established technique that has been applied to a variety of animal disease models (44–46), and the interpretation of Zrs in terms of the constant-phase model has been shown previously to allow a useful partitioning of lung mechanics into central and peripheral components (45, 47). Our recently developed plethysmographic technique for measuring VTG,Pleth in paralyzed mice has been previously validated against measurements made in spontaneously breathing mice over the PEEP range of 2 to 4 cm H2O, and also against measurements of VTG made using Archimedes' principle at 3 cm H2O (16). By comparing VTG,Pleth against measurements of lung volume made from micro-CT scans in the present study, we have further validated the plethysmographic technique.
Micro-CT itself is a relatively new technique, in terms of its application to mice, and provides a unique means for accurately studying anatomy in situ. As well as providing a means for integrating the volume of air in the lungs to calculate VTG, micro-CT also yields direct visual evidence of structural alterations in parenchyma (we used a resolution of 47 μm/voxel side) and can resolve the larger airways (Figures 3 and and4).4). A fundamental requirement for CT imaging of any kind, of course, is that the subject remain still throughout the scan. Although modern CT scanners for humans have reduced this period to a matter of seconds, the technologic development of animal micro-CT scanners is considerably less advanced, so our mice had to be motionless for more than an hour. We attempted to achieve this by waiting for the animal to stiffen before being placed in the scanner and by allowing the animal to settle in the scanner for approximately 15 minutes before scanning. Even so, in some cases, there was sufficient movement during the scan period to make the resulting images unusable, so we were not able to obtain high-quality images on all of the mice we studied.
In conclusion, we have used a comprehensive array of advanced physiologic and imaging methodologies to characterize the pathology of the TG+ mouse. Our results show that constitutive overexpression of TNF-α in the lungs of this animal produces a phenotype sharing key features of both emphysema and pulmonary fibrosis, which explains the apparent dichotomous conclusions of previous studies (14, 15). Our results also show that introduction of a single proinflammatory cytokine can lead to a phenotype that crosses a number of classical disease categories. This has important implications for the study of pathophysiology in general, much of which is motivated by the desire to find the cause of a particular disease. Many diseases, particularly those described as being “complex,” have been resistant to this type of elucidation. Perhaps the results of the present study provide some insight into why this is the case: if a single cause can produce features of multiple diseases, then a single disease may require multiple simultaneous causes. In any case, it is clear that comprehensive studies of animal models, using a wide variety of investigative techniques, are required to gain an understanding of the relationship between genetic expression and phenotype.
Acknowledgments
The authors acknowledge the professional assistance of Ms. Mieke Bevelander (genotyping) and Mr. Ryan Norton and Ms. Lisa Rinaldi (histology scoring). The SP-C/TNF-α breeding pairs were a gift from Dr. Robert Mason at the National Jewish Center for Respiratory Medicine and Pulmonology in Denver.
Notes
Supported by National Institutes of Health grants R01 HL67273 and NCRR-COBRE P20 RR15557.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: L.K.A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; J.T.-F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; T.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.E.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.G.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; J.H.T.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
Articles from American Journal of Respiratory and Critical Care Medicine are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1164/rccm.200410-1349oc
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2718479?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Adam19 Deficiency Impacts Pulmonary Function: Human GWAS Follow-up in a Mouse Knockout Model.
Lung, 202(5):659-672, 17 Aug 2024
Cited by: 0 articles | PMID: 39153120 | PMCID: PMC11427501
Safety evaluation of 5-hydroxytryptophan and S-(2-aminoethyl)isothiouronium bromide hydrobromide on rodent lungs.
Indian J Pharmacol, 56(1):28-36, 01 Jan 2024
Cited by: 0 articles | PMID: 38454586 | PMCID: PMC11001180
Cytotoxic CD4+ tissue-resident memory T cells are associated with asthma severity.
Med, 4(12):875-897.e8, 20 Oct 2023
Cited by: 3 articles | PMID: 37865091
Short-range interactions between fibrocytes and CD8+ T cells in COPD bronchial inflammatory response.
Elife, 12:RP85875, 26 Jul 2023
Cited by: 3 articles | PMID: 37494277 | PMCID: PMC10371228
Involvement of langerin in the protective function of a keratan sulfate-based disaccharide in an emphysema mouse model.
J Biol Chem, 299(8):105052, 15 Jul 2023
Cited by: 1 article | PMID: 37454739 | PMCID: PMC10448169
Go to all (153) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Overexpression of tumor necrosis factor-α in the lungs alters immune response, matrix remodeling, and repair and maintenance pathways.
Am J Pathol, 180(4):1413-1430, 07 Feb 2012
Cited by: 33 articles | PMID: 22322299
Lymphoid tissue and emphysema in the lungs of transgenic mice inducibly expressing tumor necrosis factor-alpha.
Am J Respir Cell Mol Biol, 30(4):438-448, 11 Sep 2003
Cited by: 48 articles | PMID: 12972399
Critical role of tumor necrosis factor receptor 1 in the pathogenesis of pulmonary emphysema in mice.
Int J Chron Obstruct Pulmon Dis, 11:1705-1712, 28 Jul 2016
Cited by: 11 articles | PMID: 27555760 | PMCID: PMC4968668
Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung.
Am J Respir Cell Mol Biol, 32(4):311-318, 24 Jan 2005
Cited by: 274 articles | PMID: 15668323
Funding
Funders who supported this work.
NCRR NIH HHS (1)
Grant ID: P20 RR15557
NHLBI NIH HHS (1)
Grant ID: R01 HL67273





