Abstract
Free full text

Virulence determinants in Pseudomonas aeruginosa strains from urinary tract infections.
Abstract
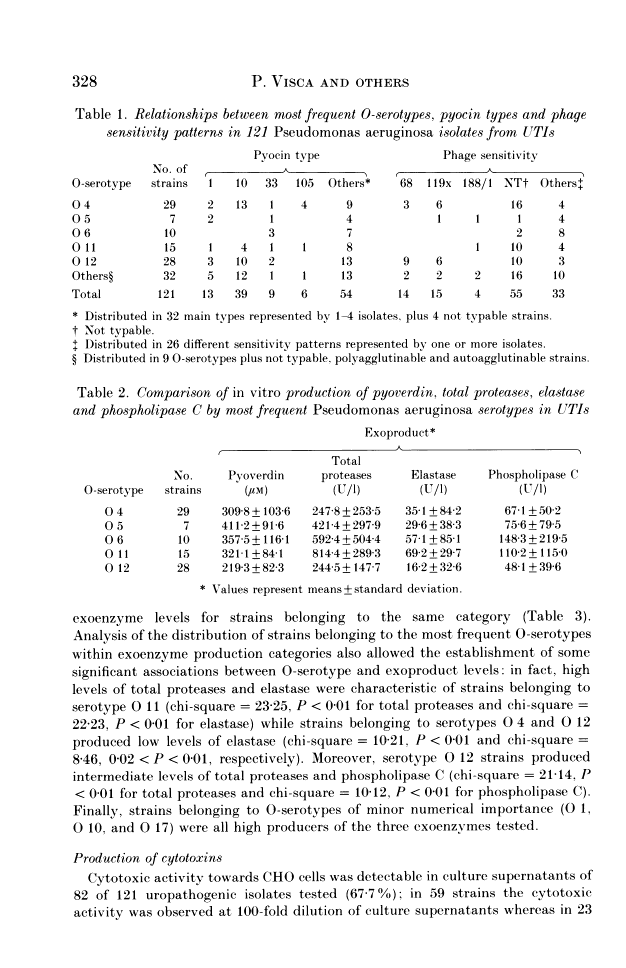
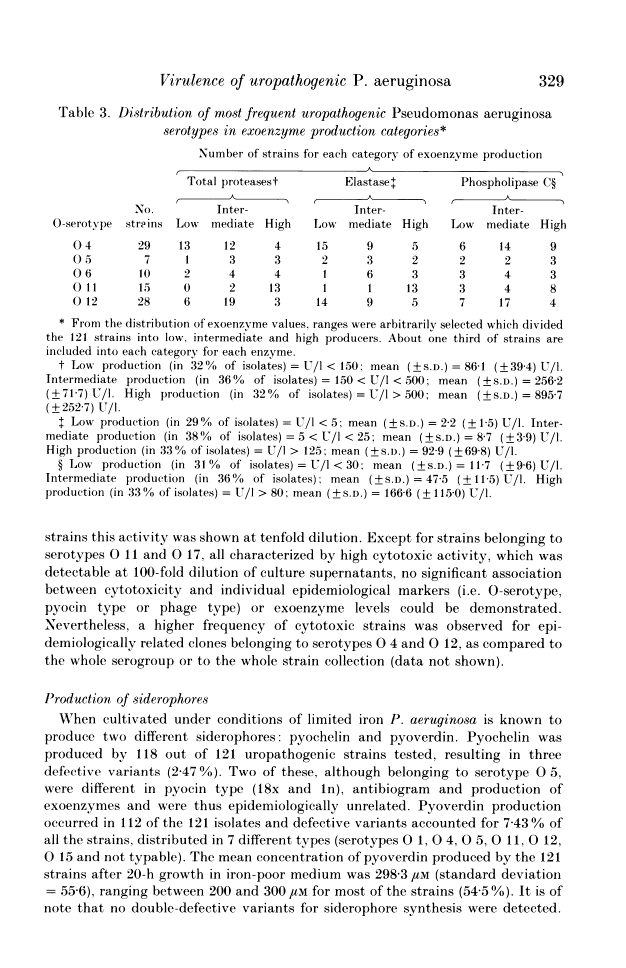
A total of 121 uropathogenic Pseudomonas aeruginosa strains were examined for production of several virulence-related factors. These strains were distributed in five predominant O-serotypes, i.e. O 4, O 12, O 11, O 6 and O 5, which accounted respectively for 23.9, 23.1, 12.3, 8.2 and 5.7% of isolates. Pyochelin and pyoverdin siderophores were produced by most of the isolates, defective variants occurring at very low frequency (2.4% for pyochelin and 7.4% for pyoverdin). Adherence to uroepithelial cells and production of cytotoxins was demonstrated in 52.8 and 67.7% of the strains, respectively, with higher frequencies for epidemiologically related strains belonging to serotypes O 4 and O 12. Titration of total proteases, elastase and phospholipase C revealed a high degree of heterogeneity among isolates. However, examination of individual O-serotypes by exoenzyme production showed that elevated levels of total proteases and elastase were characteristics of serotypes of minor numerical importance, i.e. O 1, O 10, O 11 and O 17, whilst low levels of elastase were produced by strains belonging to the predominant serotypes, namely O 4 and O 12. Moreover, epidemiologically related strains belonging to serotypes O 4 and O 12 appeared more homogeneous than the whole serogroup, when compared with other groups on the basis of exoenzyme levels.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey GP, Bolivar R, Fainstein V, Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. [Abstract] [Google Scholar]
- Brynger H, Brunner FP, Chantler C, Donckerwolcke RA, Jacobs C, Kramer P, Selwood NH, Wing AJ. Combined report on regular dialysis and transplantation in Europe. X, 1979. Proc Eur Dial Transplant Assoc. 1980;17:2–86. [Abstract] [Google Scholar]
- Kaye D. Urinary tract infections in the elderly. Bull N Y Acad Med. 1980 Mar;56(2):209–220. [Europe PMC free article] [Abstract] [Google Scholar]
- Nicolle LE, Muir P, Harding GK, Norris M. Localization of urinary tract infection in elderly, institutionalized women with asymptomatic bacteriuria. J Infect Dis. 1988 Jan;157(1):65–70. [Abstract] [Google Scholar]
- Woods DE, Straus DC, Johanson WG, Jr, Berry VK, Bass JA. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. [Europe PMC free article] [Abstract] [Google Scholar]
- Woods DE, Bass JA, Johanson WG, Jr, Straus DC. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramphal R, Guay C, Pier GB. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. [Europe PMC free article] [Abstract] [Google Scholar]
- Franklin AL, Todd T, Gurman G, Black D, Mankinen-Irvin PM, Irvin RT. Adherence of Pseudomonas aeruginosa to cilia of human tracheal epithelial cells. Infect Immun. 1987 Jun;55(6):1523–1525. [Europe PMC free article] [Abstract] [Google Scholar]
- Doig P, Smith NR, Todd T, Irvin RT. Characterization of the binding of Pseudomonas aeruginosa alginate to human epithelial cells. Infect Immun. 1987 Jun;55(6):1517–1522. [Europe PMC free article] [Abstract] [Google Scholar]
- Anastassiou ED, Mintzas AC, Kounavis C, Dimitracopoulos G. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1987 Apr;25(4):656–659. [Europe PMC free article] [Abstract] [Google Scholar]
- Liu PV. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. [Abstract] [Google Scholar]
- Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohman DE, Burns RP, Iglewski BH. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980 Oct;142(4):547–555. [Abstract] [Google Scholar]
- Cross AS, Sadoff JC, Iglewski BH, Sokol PA. Evidence for the role of toxin A in the pathogenesis of infection with Pseudomonas aeruginosa in humans. J Infect Dis. 1980 Oct;142(4):538–546. [Abstract] [Google Scholar]
- Iglewski BH, Sadoff J, Bjorn MJ, Maxwell ES. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. [Europe PMC free article] [Abstract] [Google Scholar]
- Sokol PA, Iglewski BH, Hager TA, Sadoff JC, Cross AS, McManus A, Farber BF, Iglewski WJ. Production of exoenzyme S by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1981 Oct;34(1):147–153. [Europe PMC free article] [Abstract] [Google Scholar]
- Woods DE, Sokol PA. Use of transposon mutants to assess the role of exoenzyme S in chronic pulmonary disease due to Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985 Apr;4(2):163–169. [Abstract] [Google Scholar]
- Blackwood LL, Stone RM, Iglewski BH, Pennington JE. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983 Jan;39(1):198–201. [Europe PMC free article] [Abstract] [Google Scholar]
- Pavlovskis OR, Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. [Europe PMC free article] [Abstract] [Google Scholar]
- Woods DE, Cryz SJ, Friedman RL, Iglewski BH. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. [Europe PMC free article] [Abstract] [Google Scholar]
- Ohman DE, Cryz SJ, Iglewski BH. Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980 Jun;142(3):836–842. [Europe PMC free article] [Abstract] [Google Scholar]
- Jagger KS, Bahner DR, Warren RL. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibrosis. J Clin Microbiol. 1983 Jan;17(1):55–59. [Europe PMC free article] [Abstract] [Google Scholar]
- Berka RM, Vasil ML. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol. 1982 Oct;152(1):239–245. [Europe PMC free article] [Abstract] [Google Scholar]
- Berka RM, Gray GL, Vasil ML. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox CD, Rinehart KL, Jr, Moore ML, Cook JC., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox CD. Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun. 1982 Apr;36(1):17–23. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox CD, Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. [Europe PMC free article] [Abstract] [Google Scholar]
- Ankenbauer R, Sriyosachati S, Cox CD. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. [Europe PMC free article] [Abstract] [Google Scholar]
- Visca P, Chiarini F, Vetriani C, Mansi A, Serino L, Orsi N. Epidemiological typing of uropathogenic Pseudomonas aeruginosa strains from hospitalized patients. J Hosp Infect. 1991 Nov;19(3):153–165. [Abstract] [Google Scholar]
- Pitt TL, Livermore DM, Pitcher D, Vatopoulos AC, Legakis NJ. Multiresistant serotype O 12 Pseudomonas aeruginosa: evidence for a common strain in Europe. Epidemiol Infect. 1989 Dec;103(3):565–576. [Europe PMC free article] [Abstract] [Google Scholar]
- Gilmore DS, Bruce SK, Jimenez EM, Schick DG, Morrow JW, Montgomerie JZ. Pseudomonas aeruginosa colonization in patients with spinal cord injuries. J Clin Microbiol. 1982 Nov;16(5):856–860. [Europe PMC free article] [Abstract] [Google Scholar]
- Fyfe JA, Harris G, Govan JR. Revised pyocin typing method for Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jul;20(1):47–50. [Europe PMC free article] [Abstract] [Google Scholar]
- Farmer JJ., 3rd Mnemonic for reporting bacteriocin and bacteriophage types. Lancet. 1970 Jul 11;1(7663):96–96. [Abstract] [Google Scholar]
- TOMARELLI RM, CHARNEY J, HARDING ML. The use of azoalbumin as a substrate in the colorimetric determination or peptic and tryptic activity. J Lab Clin Med. 1949 Mar;34(3):428–433. [Abstract] [Google Scholar]
- Bjorn MJ, Sokol PA, Iglewski BH. Influence of iron on yields of extracellular products in Pseudomonas aeruginosa cultures. J Bacteriol. 1979 Apr;138(1):193–200. [Europe PMC free article] [Abstract] [Google Scholar]
- Woods DE, Schaffer MS, Rabin HR, Campbell GD, Sokol PA. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J Clin Microbiol. 1986 Aug;24(2):260–264. [Europe PMC free article] [Abstract] [Google Scholar]
- Iglewski BH, Sadoff JC. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. [Abstract] [Google Scholar]
- Woods DE, Que JU. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987 Mar;55(3):579–586. [Europe PMC free article] [Abstract] [Google Scholar]
- Cox CD, Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. [Europe PMC free article] [Abstract] [Google Scholar]
- WAHBA AH. HOSPITAL INFECTION WITH PSEUDOMONAS PYOCYANEA: AN INVESTIGATION BY A COMBINED PYOCINE AND SEROLOGICAL TYPING METHOD. Br Med J. 1965 Jan 9;1(5427):86–89. [Europe PMC free article] [Abstract] [Google Scholar]
- Moody MR, Young VM, Kenton DM, Vermeulen GD. Pseudomonas aeruginosa in a center for cancer research. I. Distribution of intraspecies types from human and environmental sources. J Infect Dis. 1972 Feb;125(2):95–101. [Abstract] [Google Scholar]
- Brokopp CD, Gomez-Lus R, Farmer JJ., 3rd Serological typing of Pseudomonas aeruginosa: use of commercial antisera and live antigens. J Clin Microbiol. 1977 Jun;5(6):640–649. [Europe PMC free article] [Abstract] [Google Scholar]
- Pitt TL. A comparison of flagellar typing and phage typing as means of subdividing the O groups of Pseudomonas aeruginosa. J Med Microbiol. 1981 Aug;14(3):261–270. [Abstract] [Google Scholar]
- Legakis NJ, Aliferopoulou M, Papavassiliou J, Papapetropoulou M. Serotypes of Pseudomonas aeruginosa in clinical specimens in relation to antibiotic susceptibility. J Clin Microbiol. 1982 Sep;16(3):458–463. [Europe PMC free article] [Abstract] [Google Scholar]
- Farmer JJ, 3rd, Weinstein RA, Zierdt CH, Brokopp CD. Hospital outbreaks caused by Pseudomonas aeruginosa: importance of serogroup O11. J Clin Microbiol. 1982 Aug;16(2):266–270. [Europe PMC free article] [Abstract] [Google Scholar]
- Del Piano M, La Palombara P, Picci A, Nicosia R. Serological and pyocin typing and antibiotic sensitivity of Pseudomonas aeruginosa strains. Microbiologica. 1986 Apr;9(2):253–258. [Abstract] [Google Scholar]
- Dette GA, Knothe H, Schäfer V, Pirlet-Berninger U. Serotyping, sites of isolation and resistance of Pseudomonas aeruginosa. Microbiologica. 1988 Apr;11(2):129–135. [Abstract] [Google Scholar]
- Giammanco A, Di Stefano R, Arista S, Sinatra A, Chiarini A. Infections caused by Pseudomonas aeruginosa: relatively frequent isolation of serogroup 12 from clinical specimens. Eur J Epidemiol. 1985 Jun;1(2):104–109. [Abstract] [Google Scholar]
- Roberts AP, Phillips R. Bacteria causing symptomatic urinary tract infection or asymptomatic bacteriuria. J Clin Pathol. 1979 May;32(5):492–496. [Europe PMC free article] [Abstract] [Google Scholar]
- Fegan M, Francis P, Hayward AC, Davis GH, Fuerst JA. Phenotypic conversion of Pseudomonas aeruginosa in cystic fibrosis. J Clin Microbiol. 1990 Jun;28(6):1143–1146. [Europe PMC free article] [Abstract] [Google Scholar]
- Janda JM, Bottone EJ. Pseudomonas aeruginosa enzyme profiling: predictor of potential invasiveness and use as an epidemiological tool. J Clin Microbiol. 1981 Jul;14(1):55–60. [Europe PMC free article] [Abstract] [Google Scholar]
- Morihara K, Tsuzuki H. Production of protease and elastase by Pseudomonas aeruginosa strains isolated from patients. Infect Immun. 1977 Mar;15(3):679–685. [Europe PMC free article] [Abstract] [Google Scholar]
- Shand GH, Anwar H, Kadurugamuwa J, Brown MR, Silverman SH, Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. [Europe PMC free article] [Abstract] [Google Scholar]
- Ninane G, Harper PB. The in vitro activity of ceftazidime against a multi-resistant serotype 12 Pseudomonas aeruginosa. Infection. 1983;11 (Suppl 1):S16–S19. [Abstract] [Google Scholar]
- Legakis NJ, Koukoubanis N, Malliara K, Michalitsianos D, Papavassiliou J. Importance of carbenicillin and gentamicin cross-resistant serotype 0:12 Pseudomonas aeruginosa in six Athens hospitals. Eur J Clin Microbiol. 1987 Jun;6(3):300–303. [Abstract] [Google Scholar]
- Allemeersch D, Beumer J, Devleeschouwer M, De Maeyer S, Dony J, Godard C, Osterrieth P, Pithsy A, Van der Auwera P, Van Poppel H, et al. Marked increase of Pseudomonas aeruginosa serotype 012 in Belgium since 1982. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):265–269. [Abstract] [Google Scholar]
- Tzouvelekis LS, Tumah H, Malliara K, Legakis NJ. Relationship of antibiotic resistance phenotype to the R-pyocin susceptibility pattern in clinical isolates of Pseudomonas aeruginosa. J Chemother. 1989 Aug;1(4):226–230. [Abstract] [Google Scholar]
- Vieu JF, Allos G, Hassan-Massoud B, Santos-Ferreira MO, Tselentis G. Existe-t-il une épidémiologie géographique des sérogroupes O de Pseudomonas aeruginosa? Bull Soc Pathol Exot Filiales. 1984 May-Jun;77(3):288–294. [Abstract] [Google Scholar]
Associated Data
Articles from Epidemiology and Infection are provided here courtesy of Cambridge University Press
Full text links
Read article at publisher's site: https://doi.org/10.1017/s0950268800049797
Read article for free, from open access legal sources, via Unpaywall:
https://www.cambridge.org/core/services/aop-cambridge-core/content/view/B5B8DE7A86C06CBF8A826F81635CA827/S0950268800049797a.pdf/div-class-title-virulence-determinants-in-span-class-italic-pseudomonas-aeruginosa-span-strains-from-urinary-tract-infections-div.pdf
Citations & impact
Impact metrics
Article citations
Design, Synthesis, and Quorum Quenching Potential of Novel Catechol-Zingerone Conjugate to Find an Elixir to Tackle Pseudomonas aeruginosa Through the Trojan Horse Strategy.
Front Chem, 10:902719, 15 Jun 2022
Cited by: 0 articles | PMID: 35783213 | PMCID: PMC9240400
An Organ System-Based Synopsis of Pseudomonas aeruginosa Virulence.
Virulence, 12(1):1469-1507, 01 Dec 2021
Cited by: 22 articles | PMID: 34180343 | PMCID: PMC8237970
Review Free full text in Europe PMC
Enterobacteria secrete an inhibitor of Pseudomonas virulence during clinical bacteriuria.
J Clin Invest, 127(11):4018-4030, 25 Sep 2017
Cited by: 21 articles | PMID: 28945201 | PMCID: PMC5663353
Urinary tract infections: epidemiology, mechanisms of infection and treatment options.
Nat Rev Microbiol, 13(5):269-284, 08 Apr 2015
Cited by: 1388 articles | PMID: 25853778 | PMCID: PMC4457377
Review Free full text in Europe PMC
Characterization of phenotype variations of luminescent and non-luminescent variants of Vibrio harveyi wild type and quorum sensing mutants.
J Fish Dis, 39(3):317-327, 10 Apr 2015
Cited by: 2 articles | PMID: 25865123
Go to all (20) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phenotypic characters of urinary isolates of Pseudomonas aeruginosa & their association with mouse renal colonization.
Indian J Med Res, 123(1):67-72, 01 Jan 2006
Cited by: 15 articles | PMID: 16567871
Iron dictates the virulence of Pseudomonas aeruginosa in urinary tract infections.
J Biomed Sci, 15(6):731-741, 09 Aug 2008
Cited by: 23 articles | PMID: 18688758
Genotypic and phenotypic characterization of Pseudomonas aeruginosa isolates from urinary tract infections.
Int J Med Microbiol, 301(4):282-292, 30 Dec 2010
Cited by: 30 articles | PMID: 21193347
[Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation].
Ann Biol Clin (Paris), 69(4):393-403, 01 Jul 2011
Cited by: 42 articles | PMID: 21896403
Review