Abstract
Free full text

cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase.
Abstract
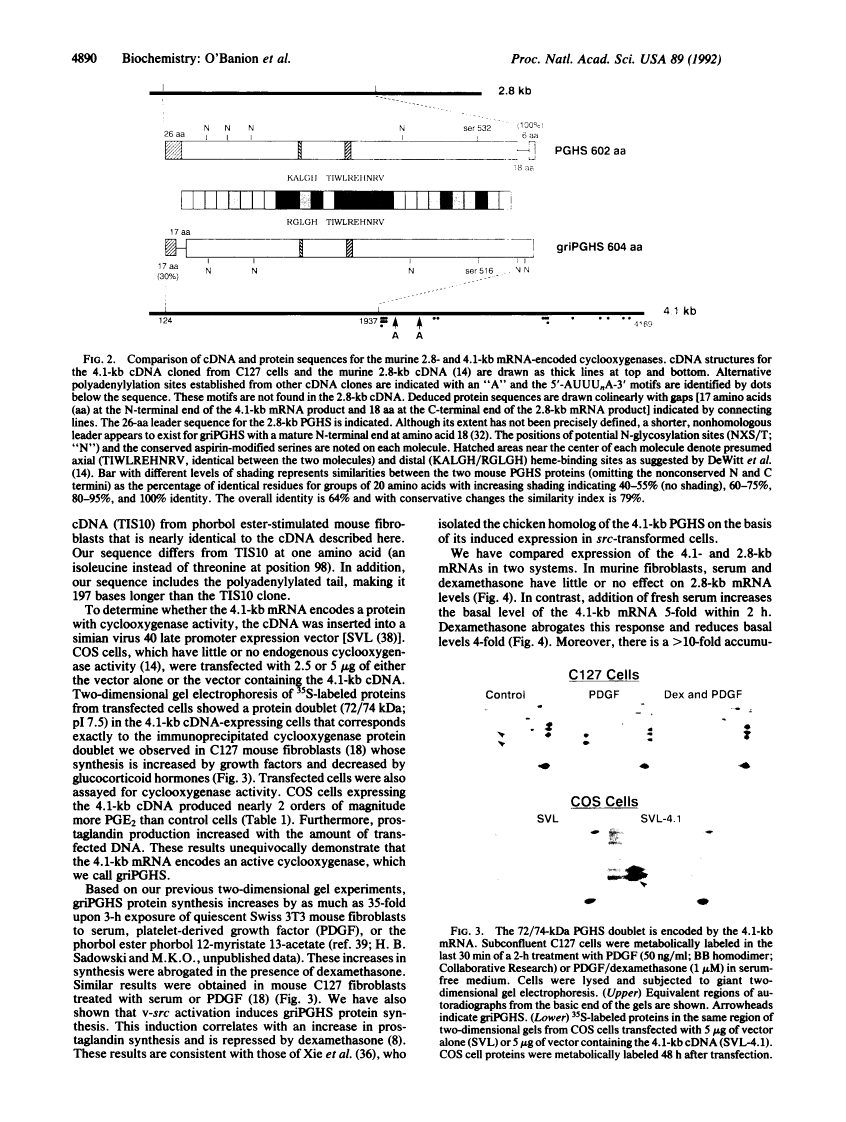
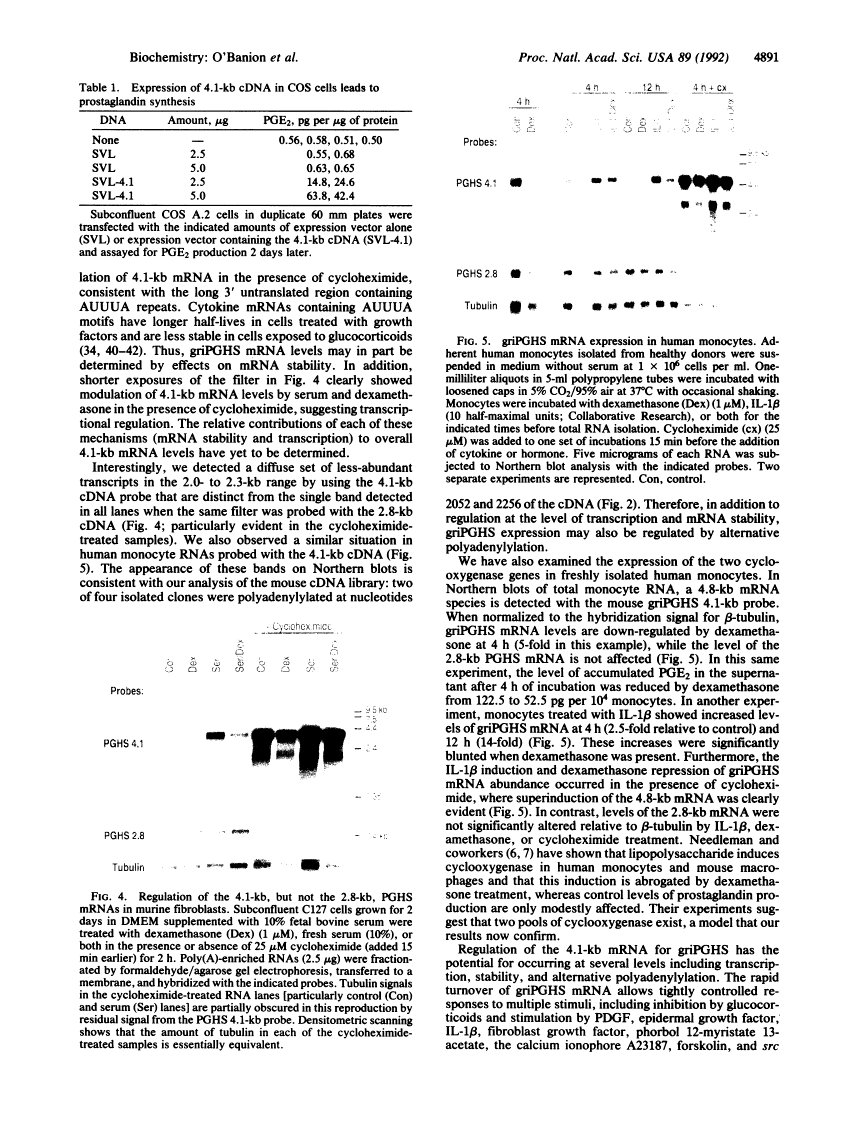
The antiinflammatory glucocorticoids are potent inhibitors of cyclooxygenase, a key regulator of prostaglandin synthesis; yet, the mechanism(s) by which this occurs is not fully understood. We have cloned a 4.1-kilobase (kb) cDNA, distinct from the previously cloned cyclooxygenase (2.8 kb), that confers cyclooxygenase activity to transfected cells. The mRNA for this newly discovered cyclooxygenase is unique for its long 3' untranslated region containing many AUUUA repeats. Levels of the 4.1-kb cyclooxygenase mRNA are rapidly increased by serum or interleukin 1 beta in mouse fibroblasts and human monocytes, respectively, and decreased by glucocorticoids, whereas levels of the 2.8-kb cyclooxygenase mRNA do not change. Similar effects are seen in the presence of cycloheximide where the 4.1-kb, but not the 2.8-kb, mRNA is greatly superinduced. Thus, there are both constitutive (2.8 kb) and regulated (4.1 kb) cyclooxygenase species, the latter most likely being a major mediator of inflammation.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
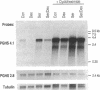
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Raz A, Wyche A, Siegel N, Needleman P. Regulation of fibroblast cyclooxygenase synthesis by interleukin-1. J Biol Chem. 1988 Feb 25;263(6):3022–3028. [Abstract] [Google Scholar]
- Raz A, Wyche A, Needleman P. Temporal and pharmacological division of fibroblast cyclooxygenase expression into transcriptional and translational phases. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1657–1661. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu KK, Sanduja R, Tsai AL, Ferhanoglu B, Loose-Mitchell DS. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2384–2387. [Europe PMC free article] [Abstract] [Google Scholar]
- Frasier-Scott K, Hatzakis H, Seong D, Jones CM, Wu KK. Influence of natural and recombinant interleukin 2 on endothelial cell arachidonate metabolism. Induction of de novo synthesis of prostaglandin H synthase. J Clin Invest. 1988 Dec;82(6):1877–1883. [Europe PMC free article] [Abstract] [Google Scholar]
- Maier JA, Hla T, Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [Abstract] [Google Scholar]
- Masferrer JL, Zweifel BS, Seibert K, Needleman P. Selective regulation of cellular cyclooxygenase by dexamethasone and endotoxin in mice. J Clin Invest. 1990 Oct;86(4):1375–1379. [Europe PMC free article] [Abstract] [Google Scholar]
- Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [Abstract] [Google Scholar]
- Han JW, Sadowski H, Young DA, Macara IG. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1990 May;87(9):3373–3377. [Europe PMC free article] [Abstract] [Google Scholar]
- Koehler L, Hass R, DeWitt DL, Resch K, Goppelt-Struebe M. Glucocorticoid-induced reduction of prostanoid synthesis in TPA-differentiated U937 cells is mainly due to a reduced cyclooxygenase activity. Biochem Pharmacol. 1990 Sep 15;40(6):1307–1316. [Abstract] [Google Scholar]
- Raz A, Wyche A, Fu J, Seibert K, Needleman P. Regulation of prostanoids synthesis in human fibroblasts and human blood monocytes by interleukin-1, endotoxin, and glucocorticoids. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:22–27. [Abstract] [Google Scholar]
- Hemler M, Lands WE. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem. 1976 Sep 25;251(18):5575–5579. [Abstract] [Google Scholar]
- DeWitt DL, Smith WL. Primary structure of prostaglandin G/H synthase from sheep vesicular gland determined from the complementary DNA sequence. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1412–1416. [Europe PMC free article] [Abstract] [Google Scholar]
- Merlie JP, Fagan D, Mudd J, Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [Abstract] [Google Scholar]
- DeWitt DL, el-Harith EA, Kraemer SA, Andrews MJ, Yao EF, Armstrong RL, Smith WL. The aspirin and heme-binding sites of ovine and murine prostaglandin endoperoxide synthases. J Biol Chem. 1990 Mar 25;265(9):5192–5198. [Abstract] [Google Scholar]
- Hla T, Farrell M, Kumar A, Bailey JM. Isolation of the cDNA for human prostaglandin H synthase. Prostaglandins. 1986 Dec;32(6):829–845. [Abstract] [Google Scholar]
- Bailey JM, Makheja AN, Pash J, Verma M. Corticosteroids suppress cyclooxygenase messenger RNA levels and prostanoid synthesis in cultured vascular cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1159–1163. [Abstract] [Google Scholar]
- Funk CD, FitzGerald GA. Eicosanoid forming enzyme mRNA in human tissues. Analysis by quantitative polymerase chain reaction. J Biol Chem. 1991 Jul 5;266(19):12508–12513. [Abstract] [Google Scholar]
- O'Banion MK, Sadowski HB, Winn V, Young DA. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J Biol Chem. 1991 Dec 5;266(34):23261–23267. [Abstract] [Google Scholar]
- Chen TR. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. [Abstract] [Google Scholar]
- Law MF, Lowy DR, Dvoretzky I, Howley PM. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. [Europe PMC free article] [Abstract] [Google Scholar]
- Roberts NJ, Jr, Steigbigel RT. Effect of in vitro virus infection on response of human monocytes and lymphocytes to mitogen stimulation. J Immunol. 1978 Sep;121(3):1052–1058. [Abstract] [Google Scholar]
- Young DA, Voris BP, Maytin EV, Colbert RA. Very-high-resolution two-dimensional electrophoretic separation of proteins on giant gels. Methods Enzymol. 1983;91:190–214. [Abstract] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. [Abstract] [Google Scholar]
- Aviv H, Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. [Europe PMC free article] [Abstract] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. [Abstract] [Google Scholar]
- Del Sal G, Manfioletti G, Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [Abstract] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. [Europe PMC free article] [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- O'Banion MK, Young DA. Bovine papillomavirus type 1 alters the processing of host glucose- and calcium-modulated endoplasmic reticulum proteins. J Virol. 1991 Jul;65(7):3481–3488. [Europe PMC free article] [Abstract] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. [Abstract] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. [Abstract] [Google Scholar]
- Smith WL, DeWitt DL, Kraemer SA, Andrews MJ, Hla T, Maciag T, Shimokawa T. Structure-function relationships in sheep, mouse, and human prostaglandin endoperoxide G/H synthases. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:14–21. [Abstract] [Google Scholar]
- Shaw G, Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. [Abstract] [Google Scholar]
- Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. [Europe PMC free article] [Abstract] [Google Scholar]
- Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. [Europe PMC free article] [Abstract] [Google Scholar]
- Kujubu DA, Fletcher BS, Varnum BC, Lim RW, Herschman HR. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [Abstract] [Google Scholar]
- Breathnach R, Harris BA. Plasmids for the cloning and expression of full-length double-stranded cDNAs under control of the SV40 early or late gene promoter. Nucleic Acids Res. 1983 Oct 25;11(20):7119–7136. [Europe PMC free article] [Abstract] [Google Scholar]
- Levenson R, Iwata K, Klagsbrun M, Young DA. Growth factor- and dexamethasone-induced proteins in Swiss 3T3 cells. Relationship to DNA synthesis. J Biol Chem. 1985 Jul 5;260(13):8056–8063. [Abstract] [Google Scholar]
- Peppel K, Vinci JM, Baglioni C. The AU-rich sequences in the 3' untranslated region mediate the increased turnover of interferon mRNA induced by glucocorticoids. J Exp Med. 1991 Feb 1;173(2):349–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Boggaram V, Smith ME, Mendelson CR. Posttranscriptional regulation of surfactant protein-A messenger RNA in human fetal lung in vitro by glucocorticoids. Mol Endocrinol. 1991 Mar;5(3):414–423. [Abstract] [Google Scholar]
- Iwai Y, Bickel M, Pluznik DH, Cohen RB. Identification of sequences within the murine granulocyte-macrophage colony-stimulating factor mRNA 3'-untranslated region that mediate mRNA stabilization induced by mitogen treatment of EL-4 thymoma cells. J Biol Chem. 1991 Sep 25;266(27):17959–17965. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.11.4888
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/pnas/89/11/4888.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.89.11.4888
Article citations
Sirtuin 6 ameliorates arthritis through modulating cyclic AMP-responsive element binding protein/CCN1/cyclooxygenase 2 pathway in osteoblasts.
J Bone Miner Metab, 41(6):772-784, 29 Oct 2023
Cited by: 1 article | PMID: 37898986
Regulation of Cyclooxygenase-2 Expression in Human T Cells by Glucocorticoid Receptor-Mediated Transrepression of Nuclear Factor of Activated T Cells.
Int J Mol Sci, 23(21):13275, 31 Oct 2022
Cited by: 2 articles | PMID: 36362060 | PMCID: PMC9653600
COX-2-PGE2-EPs in gynecological cancers.
Arch Gynecol Obstet, 301(6):1365-1375, 03 May 2020
Cited by: 39 articles | PMID: 32363546 | PMCID: PMC7246249
Review Free full text in Europe PMC
Postnatal changes in constitutive cyclooxygenase‑2 expression in the mice hippocampus and its function in synaptic plasticity.
Mol Med Rep, 19(3):1996-2004, 15 Jan 2019
Cited by: 7 articles | PMID: 30664214 | PMCID: PMC6390017
Eicosanoids in platelets and the effect of their modulation by aspirin in the cardiovascular system (and beyond).
Br J Pharmacol, 176(8):988-999, 19 Apr 2018
Cited by: 33 articles | PMID: 29512148 | PMCID: PMC6451075
Review Free full text in Europe PMC
Go to all (407) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein.
J Biol Chem, 266(34):23261-23267, 01 Dec 1991
Cited by: 283 articles | PMID: 1744122
Induction of cyclooxygenase-2 is responsible for interleukin-1 beta-dependent prostaglandin E2 synthesis by human lung fibroblasts.
Am J Respir Cell Mol Biol, 12(3):358-365, 01 Mar 1995
Cited by: 54 articles | PMID: 7873203
Anti-inflammatory glucocorticoid action: inhibition of griPGHS, a new cyclooxygenase.
J Lipid Mediat, 6(1-3):101-111, 01 Mar 1993
Cited by: 8 articles | PMID: 8357976
Human cyclooxygenase-2 cDNA.
Proc Natl Acad Sci U S A, 89(16):7384-7388, 01 Aug 1992
Cited by: 769 articles | PMID: 1380156 | PMCID: PMC49714
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: CA47650
NIDDK NIH HHS (1)
Grant ID: DK1677