Abstract
Free full text

Evidence that Bone Marrow Cells Do Not Contribute to the Alveolar Epithelium
Abstract
An ongoing controversy is the role of marrow cells in populating the alveolar epithelium. In this study, we employed flow cytometry and histologic techniques to evaluate this process. Donor bone marrow was harvested from transgenic mice expressing the LacZ or eGFP gene ubiquitously, or under the control of the human surfactant protein (SP)-C promoter, and transplanted into lethally irradiated, neonatal mice. In recipients transplanted with marrow that express eGFP or lacZ ubiquitously, light microscopy revealed cells whose morphology and location were compatible with a type II cell phenotype. Consistent with this, fluorescent microscopy suggested colocalization of eGFP and pro–SP-C proteins in single cells. In mice transplanted with SP-C–eGFP marrow, engraftment was not detectable by histology or flow cytometry. We therefore used deconvolution microscopy to reanalyze histologic sections that were thought to show marrow-derived type II cells. We found that all putative marrow-derived pneumocytes resulted from the overlapping fluorescent signals of an endogenous pro–SP-C+ type II cell and a donor-derived eGFP+ cell. Taken together, our observations underscore the technical difficulties associated with evaluating engraftment in lung, and argue against a contributory role for marrow cells in populating the alveolar epithelium.
Based on the findings of several different laboratories, it has been postulated that cells mobilized from the bone marrow serve as precursors of differentiated cells specific to nonhematopoietic organs, including the heart, brain, liver, and lung (1–5). Despite the considerable biological and therapeutic implications of these findings, it remains controversial whether solid-organ engraftment by bone marrow cells actually occurs. One issue is the fact that several laboratories have been unable to detect engraftment after conducting similar experiments (6–8). Though differences in experimental set-up and technique may explain this discrepancy (9), an alternate explanation is that the accepted methods of detecting engraftment have not been sufficiently rigorous. For instance, conventional microscopy may not be able to resolve overlapping or juxtaposed cells, leading them to be erroneously identified as a single cell with co-localized markers. These issues are particularly relevant for the alveolar regions of the lung because of the close apposition of capillaries, epithelium, and air interface.
In this study, we sought to systematically evaluate marrow cells that by conventional microscopy appeared to engraft as type II cells. Chimeric animals were generated by transplanting irradiated neonatal mice with unfractionated bone marrow cells that express the transgenes LacZ (Escherichia coli β-galactosidase) or eGFP (enhanced green fluorescent protein). In mice transplanted with marrow cells expressing eGFP or lacZ ubiquitously, the presence of engrafted alveolar epithelial cells was initially suggested by light microscopy, by co-localization of eGFP and pro-surfactant protein C (pro–SP-C) during fluorescent microscopy, and by flow cytometry of fixed and stained cells. On the other hand, immunostaining of cytospin preparations revealed no type II cells that express eGFP. Furthermore, we did not detect type II cell engraftment in mice transplanted with marrow from donors that express eGFP under the influence of the SP-C promoter. After reexamination of “engrafted” cells by deconvolution microscopy, we found that the apparent coexpression of markers instead arose from the tight juxtaposition of an endogenous type II cell and a marrow-derived, transgene-positive cell. These findings indicate that analysis of lung engraftment by conventional methods gives rise to false positives, and argue against a role for mobilized marrow cells in populating the alveolar epithelium.
MATERIALS AND METHODS
Mice
Wild-type B6129SF1 mice were transplanted with bone marrow from B6129SRosa 26 mice that ubiquitously express the LacZ gene. The LacZ gene encodes for the E. coli β-galactosidase, whose activity can be detected by its cleavage of the chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal) to release an insoluble bright blue precipitate. Wild-type C57BL/6J mice underwent transplantation with bone marrow from C57BL/6-Tg(ACTbEGFP)1Osb/J mice that ubiquitously express eGFP (actin-eGFP) (10), or from mice that express eGFP under the direction of a type II pneumocyte–specific promoter (SP-C–eGFP) (11). eGFP is a recombinant protein with 35-fold more intense fluorescence than the original Aequorea Victoria green fluorescent protein. Negative control mice were transplanted with strain-matched, wild-type bone marrow. The SP-C–eGFP transgenic mouse was kindly provided by Dr. Michael O'Reilly (University of Rochester, Rochester, NY). All other mice were purchased from Jackson Laboratories (Bar Harbor, ME).
Irradiation and Bone Marrow Transplantation
Donor bone marrow was flushed from the femurs, tibias, and humeri of 6- to 8-wk-old mice with cold sterile PBS. The cell suspension was repetitively aspirated until no visible fragments remained. Cells were pelleted at 300 × g for 10 min at 4°C before counting.
Recipient mice at 3 d of age underwent whole body split-dose irradiation (5 Gy × 2 doses) from a cesium radiator, followed by intraperitoneal injection of whole bone marrow cells (2 × 107 cells in 0.1 ml PBS). This dose of radiation results in myeloablation of the bone marrow, with no survival at 10–14 d in the absence of marrow transplantation (n = 3). In bone marrow recipients, reconstitution of the bone marrow resulted in levels of eGFP expression in CD45+ bone marrow cells similar to eGFP marrow donors (data not shown). After irradiation and bone marrow transplantation, mice were kept with their mothers under standard housing conditions, and fed acidic water and soft gel food. All animal studies adhered to protocols approved by the National Institute of Health and the Boston University Institutional Animal Care and Use Committee.
Immunohistochemistry of Recipient Lungs
At intervals of 2 wk, 1 mo, or 3 mo after transplantation, engraftment was evaluated. For histologic detection of marrow-derived cells in LacZ-transplanted mice, lungs were processed with the β-galactosidase substrate X-gal. Mice were killed and lungs were inflation-fixed with 1.5 ml of 0.25% glutaraldehyde/2 mM magnesium chloride for 15 min. Lungs were reinflated and deflated three consecutive times with PBS. Lungs were then inflated with Xgal solution (0.1% X-gal, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM magnesium chloride in PBS, pH 7.4), removed, and immersed in Xgal solution overnight at room temperature. The following day, lobes were dissected out, rinsed in PBS, formalin-fixed, and paraffin-embedded.
To detect marrow-derived cells in mice transplanted with eGFP bone marrow, we performed immunohistochemical (IHC) staining for eGFP. Lung sections were deparaffinized with xylene and sequentially hydrated with graded alcohols. Sections were quenched with 3% hydrogen peroxide/methanol to reduce endogenous peroxidase activity and blocked with 1% goat serum to reduce nonspecific antibody binding. Rabbit anti-GFP antibody (Chemicon, Temecula, CA) was applied. Secondary detection was performed with a biotinylated goat anti-rabbit antibody followed by incubation with the avidin–biotin complex (ABC kit; Vector Labs, Burlingame, CA) and the chromogenic substrate 3, 3′-diaminobenzidine. Slides were nuclear counterstained with methyl green.
To determine whether marrow-derived cells had acquired the molecular phenotype of type II pneumocytes, we performed dual immunofluorescent (IF) staining for eGFP and pro–SP-C. After deparaffination and graded hydration, slides were quenched with 1% sodium borohydride and blocked with 1% goat serum. Rabbit anti–pro–SP-C antibody (Chemicon) was added, followed with goat anti-rabbit antibody conjugated to Alexa Fluoro 568 (Molecular Probes, Eugene, OR). Slides were then blocked with 1% rabbit serum and stained with Alexa Fluoro 488–conjugated rabbit anti-GFP antibody (Molecular Probes). Nuclear counterstaining was performed with 4′6-Diamindino-2-phenylindole dihydrochloride (DAPI; Vector Labs).
Microscopy
Light microscopy.
Slides were viewed using a Zeiss N HBO100 microscope (Thornwood, NY) equipped with a mercury vapor-short-arc lamp, with double bandpass filters for fluorescence (DAPI: excitation 390/20 nm and emission 460/50 nm; Alexa Fluoro 488 or eGFP: excitation 475/40 nm and emission 530/50 nm; Alexa Fluoro 568: excitation 560/40 nm and emission 630/75 nm). Images were captured with an AxioCam MR CCD digital camera and further processed using Axiovision 3.1 software (Zeiss). Light microscopy images were photographed in color, while fluorescent images were photographed in black and white under each filter, then electronically merged and pseudo-colored.
Deconvolution microscopy.
After initial viewing of lung sections under wide-field fluorescent microscopy, cells that appeared to coexpress eGFP and pro–SP-C were situated with a Lovins Micro-Slide Field Finder (Gurley Precision Instruments, Troy, NY) and reanalyzed with deconvolution microscopy. Slides were visualized on an upright Olympus IX70 microscope (Melville, NY) with a mercury arc lamp as the illumination source, and double bandpass filters for fluorescence (DAPI: excitation 360/40 nm and emission 457/50 nm; Alexa Fluoro 488 or eGFP: excitation 490/20 nm and emission 528/38 nm; Alexa Fluoro 568: excitation 555/28 nm and emission 617/73 nm). Sequential images of each cell of interest were captured by a Coolsnap_HQ/ICX 285 CCD camera (Photometrics, Tucson, AZ) at intervals of 0.1–0.2 μm through the z-axis using the Deltavision SoftWoRx Resolve 3D Software version 3.3.1 (Applied Precision, Issaquah, WA). Stacked images were deconvolved using the conservative approach with 15 iterations and a smoothing function of 0.5, with an image size of 512 × 512 pixels and a Bin of 1 × 1. A three-dimensional reconstruction of the deconvolved images was rendered using the Volume Viewer option, and was rotated 360 degrees around four separate axes. Cells were designated as co-expressing eGFP and pro–SP-C fluorescence if the two signals remained merged on rotational viewing (60 views total). Images were also analyzed with distance measurement tools.
Preparation of Lung Suspensions for Flow Cytometric Analysis
Lung tissue from the transplanted mice was digested using a modification of the methods described by Corti and coworkers (12). Mice were anesthetized with isofluorane and the left renal vessels were severed to reduce blood volume. To further minimize erythrocytes, the right ventricle was perfused with 10 ml of cold sterile PBS. The trachea was cannulated with a 22-gauge catheter, through which 3 ml of dispase at 37°C (BD Discovery Labware, Bedford, MA) then 0.5 ml of 1% low-melt agarose at 45°C (Sigma, St. Louis, MO) was infused. The lungs were iced in situ for 2 min, then removed into 1 ml of dispase at room temperature for 45 min. The proximal airways were removed and the remaining lung tissue was transferred to a 60-mm culture dish with 7 ml of 0.01% DNase (Sigma) in DMEM and further dissociated with the curved tips of fine forceps. The cell suspension was filtered sequentially through 100-μm and 40-μm Falcon cell strainers (Becton Dickinson, Franklin Lakes, NJ) and 25-μm nylon mesh (Sefar America, Kansas City, MO). The modified Papanicolaou (PAP) stain was performed to confirm type II cell content (13).
Antibody Staining for Flow Cytometry Analysis
Prior to pro–SP-C antibody staining, lung cells were fixed with 2% paraformaldehyde. Cells were then incubated with rabbit anti–pro–SP-C antibody (Chemicon) or rabbit immunoglobulin, followed by phycoerythrin (PE)-conjugated goat anti-rabbit IgG F (ab′)2 (Caltag, Burlingame, CA). Antibody dilution and post-staining washes were done using a permeabilization buffer (PBS plus 0.1% saponin).
In mice transplanted with SP-C–eGFP bone marrow, no antibody staining was performed because donor-derived type II cells were expected to be directly identified by eGFP fluorescence. After preparation of the initial lung suspension, unfixed cells were kept on ice until analysis. Propidium iodide (PI) (2 μg/ml; Sigma) was added at the onset of flow cytometry to exclude dead cells.
Flow cytometric analysis was performed on a Mo-Flo instrument (Cytomation, Fort Collins, CO). A 488-nm air-cooled argon-ion laser was used for excitation of eGFP, PI, and PE, and a 647-nm argon-krypton diode laser for excitation of APC. Data was analyzed with Summit software (Cytomation). For some experiments, we used a FACScan (Becton Dickinson) instrument and Flow-Jo software (Flow-Jo, Ashland, OR).
Cytocentrifuge Analysis
After proteolytic digestion, aliquots from the initial lung cell suspension and lung cells sorted for eGFP fluorescence were prepared for cytocentrifuge analysis using a Thermo-Shandon cytocentrifuge (Thermo-Shandon, Pittsburgh, PA) at 750 rpm for 4 min, followed by 4% paraformaldehyde fixation. Immunofluorescent staining was performed for eGFP and pro–SP-C. Cell content for eGFP was analyzed as follows: first, negative control slides prepared from recipients of wild-type bone marrow were viewed on the computer screen as digitized images, and the exposure time of the camera was adjusted as needed. Then, the same exposure time was used to generate live online images from cytospun slides of mice transplanted with eGFP bone marrow. Using only the digitized images on screen as our guide, we scanned through multiple fields of view at ×40 magnification, during which three independent observers counted the number of positive staining cells. Counts were averaged and divided by the total number of viewed nuclei. At least 300 nuclei were counted for each slide. Pro–SP-C content was assessed similarly, using isotype slides incubated with rabbit immunoglobulin to set exposure time.
RESULTS
Marrow Engraftment Assessment by Light Microscopy and Widefield Fluorescent Microscopy
To begin to determine whether bone marrow–derived cells are mobilized to engraft as type II pneumocytes, we first viewed histologic lung sections from recipient mice under light microscopy at 1 mo after transplantation. In lung sections from four mice transplanted with LacZ bone marrow, donor-derived inflammatory cells were frequent and occurred both within the alveolar space, and around the airway (data not shown). In addition, we identified occasional blue X-gal–stained cells in the corner of alveoli (Figure 1A). These cells exhibited cuboidal and granular morphology compatible with the type II pneumocyte phenotype. No X-gal–positive cells were observed in mice that received wild-type bone marrow transplantation (data not shown).
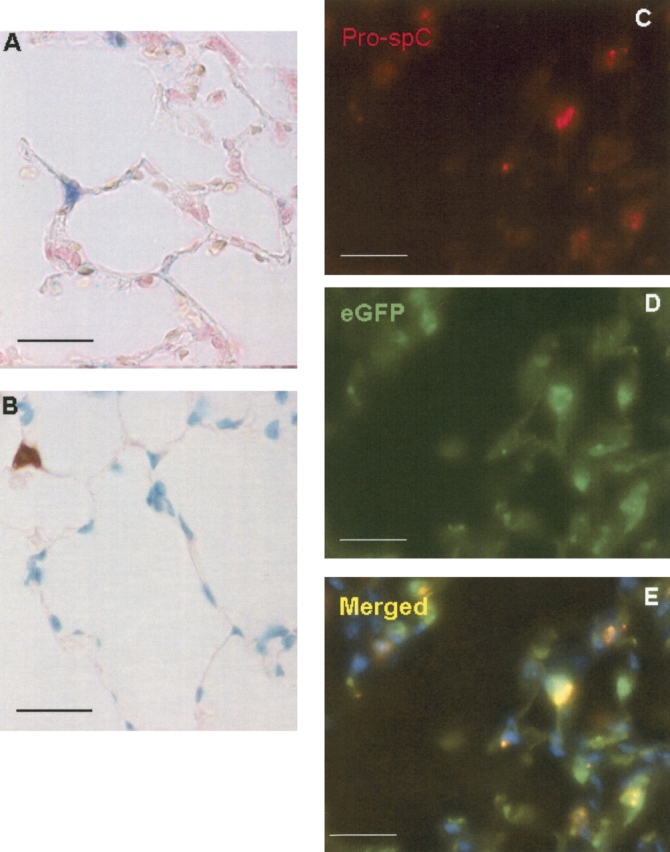
Light microscopy analysis of alveolar engraftment after transplantation with ubiquitous lacZ or eGFP bone marrow. (A) X-gal staining was performed on lungs from mice 1 mo after transplantion with LacZ bone marrow, to label marrow-derived cells blue. In this representative section, an X-gal+ cell is located in the corner of the alveolus. Its compact, granular morphology is suggestive of the type II epithelial cell. (B) In mice transplanted with actin-eGFP bone marrow, IHC was performed to localize eGFP. In this section taken from a mouse 1 mo after transplantation, an eGFP+ cell is shown whose location and shape is compatible with the type II cell. (C–E) Dual IF staining against pro–SP-C (red) and eGFP (green) was performed in mice transplanted with actin-eGFP bone marrow. Nuclei were counterstained with DAPI. In the composite image, co-localization of the green eGFP+ and red pro–SP-C fluorescent signals results in a yellow color. All sections: 5 μm, ×1,000 magnification. Size bar = 20 μm.
To confirm these findings, we analyzed histologic sections from four mice transplanted with eGFP-labeled bone marrow. Immunoperoxidase antibody staining against eGFP suggested that engraftment as type II cells also occurred in these mice (Figure 1B). Again, marrow-derived cells with a cuboidal, granular morphology at the corner of alveoli were detected. These cells contained numerous granules suggestive of lamellar bodies, and were identified in all four recipient mice.
Next, immunofluorescent staining was performed to establish co-localization of donor marrow (eGFP) and type II pneumocyte markers (Figures 1C–1E). To identify type II pneumocytes, we used the polyclonal antibody to pro–SP-C, which localizes exclusively to these cells in the murine lung by Embryonic Day 17 (14). The fluorescent signal of pro–SP-C is predominantly granular, as it is confined to multivesicular bodies adjacent to lamellar bodies (15). Using this technique, cells that seemed to stain for both eGFP and pro–SP-C were identified, further suggesting that bone marrow–derived cells had engrafted as type II pneumocytes. No eGFP-positive cells were detected in the lungs of mice transplanted with wild-type bone marrow (data not shown).
Marrow Engraftment Assessment by Flow Cytometry and Staining of Cytospin Preparations
Flow cytometry was performed to confirm the presence of marrow-derived type II pneumocytes. By pro–SP-C immunostaining, type II cell content of digested lung preparations ranged from 10–30%, with similar percentages obtained by PAP staining. In the actin-eGFP mouse, eGFP-fluorescent cells comprised 50–55% of all lung cells, and 70% of type II cells.
Twelve mice that received actin-eGFP bone marrow were analyzed at intervals of 1 mo and 3 mo after transplantation. In the lungs of these transplanted mice, eGFP+ cells accounted for 2–25% of the digested lung population, and in some animals, a small number of cells appeared to co-express eGFP and pro–SP-C (Figure 2). The percentage of presumptive donor-derived type II cells ranged from 0–1% of the whole lung population, and comprised 0–8% (1.81 ± 3.05%; n = 12) of all type II pneumocytes. Apparent pneumocyte engraftment did not differ significantly between 1 mo and 3 mo after transplantation.
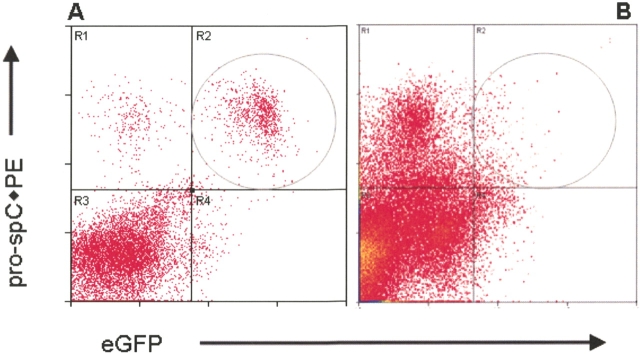
Flow cytometry analysis of alveolar engraftment after transplantation with ubiquitous eGFP bone marrow. From 1 mo to 3 mo after transplantation, lungs from recipient mice were enzymatically dispersed, fixed with paraformaldehyde, then sequentially incubated with a pro–SP-C polyclonal antibody and a PE-conjugated secondary antibody. In these density dot plots, the circled region denotes eGFP+/pro–SP-C+ cells. In the actin-eGFP mouse (A), 70% of the pro–SP-C+ cells expressed eGFP. In a representative mouse analyzed 1 mo after transplantation (B), 1% of the pro–SP-C cells were eGFP+, suggesting that they derived from the bone marrow.
To evaluate this further, we examined eGFP+ and pro–SP-C+ cells in directly cytospun preparations from the actin-eGFP mouse (Figure 3A) and transplanted mice (Figure 3B). Of all pro–SP-C+ cells, the vast majority contained abundant cytoplasm and exhibited a granular pro–SP-C staining pattern consistent with the type II cell phenotype. eGFP+, pro–SP-C+ cells with this staining pattern were frequently detected in the actin-eGFP mouse (Figure 3A). A subgroup of pro–SP-C+ cells, however, was smaller with scant cytoplasm—an appearance that was not consistent with a type II pneumocyte. These round, smaller cells were infrequent, forming < 0.5% of the pro–SP-C+ cells in all mice analyzed by this method. In mice transplanted with eGFP bone marrow, however, these small cells accounted for all of the double-positive cells (Figure 3B). In contrast to our previous findings, these data argue against marrow engraftment.
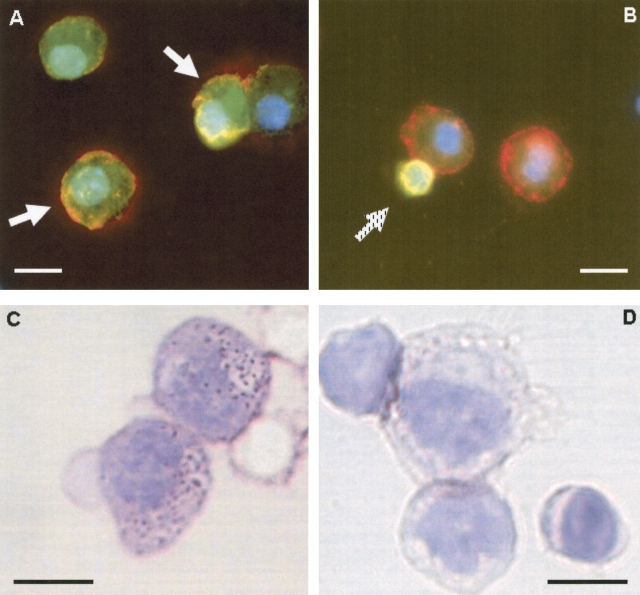
Lack of alveolar engraftment by cytocentrifuge analysis. After proteolytic lung digestion, cytocentrifuge slides were prepared and immunostained for pro–SP-C (red) and eGFP (green) in (A) actin-eGFP mouse and (B) recipient mouse transplanted with actin-eGFP marrow. In the actin-eGFP mouse, two eGFP+, pro–SP-C+ cells are identified by white arrows. Note size of cells and the granular staining of pro–SP-C. By contrast, all eGFP+, pro–SP-C+ lung cells in transplanted mice did not have the appearance of a type II cell (hatched arrow in B). These cells were smaller, lacked granular staining, and possessed scant cytoplasm. Two neighboring eGFP-negative cells exhibit size and granular pro–SP-C staining compatible with type II cells. Next, we collected eGFP+ lung cells by flow cytometry and performed PAP staining. While type II cells were readily identified by this method among eGFP+ cells from the actin-eGFP mouse (C), no type II cells were found among eGFP+, marrow-derived lung cells from a mouse transplanted with actin-eGFP marrow (D). Size bar = 10 μm.
To further resolve the identity of these small round cells, we performed PAP staining on all eGFP+ lung cells derived from transplanted mice. PAP staining of eGFP+ lung cells from a transplanted mouse did not reveal any cells consistent with a type II cell phenotype (Figure 3D). Characteristic type II cell staining was only observed in eGFP-negative cells. Importantly, PAP staining of eGFP+ lung cells from an actin-eGFP donor mouse imparted a characteristic type II cell appearance, with dark violet staining of lamellar bodies (Figure 3C).
Marrow Engraftment Assessment after Transplantation with SP-C–eGFP Bone Marrow
To help resolve these conflicting findings, we employed a transplantation model in which type II epithelial cells could be recognized without use of antibody binding and cell fixation. We performed transplantation studies using donor mice that express eGFP under the direction of the human SP-C promoter (SP-C–eGFP), thereby limiting eGFP-labeling to type II cells (11). With this donor mouse, type II cells have been readily identified by eGFP fluorescence on flow cytometry (11). Six mice transplanted with SP-C-eGFP bone marrow were analyzed by flow cytometry at 2 wk, 1 mo, and 3 mo after transplantation. No live eGFP+ cells were detected in any transplanted mice, though a small number of dead cells exhibited nonspecific green fluorescence (Figure 4). One million live cells from each transplanted mouse were analyzed.
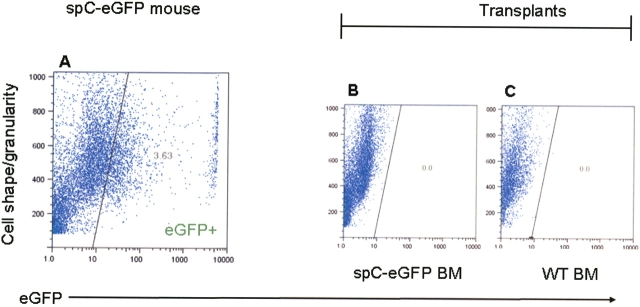
Lack of alveolar engraftment by flow cytometry after transplantation with SP-C–eGFP bone marrow. Bone marrow was procured from donor mice expressing eGFP under the control of the human SP-C promoter. From 2 wk to 3 mo after transplantation, lungs were enzymatically dispersed and treated with PI to exclude dead cells (90% live). During flow cytometry, density dot plot analysis of the PI-negative fraction demonstrated the presence of eGFP-expressing cells in a nontransplanted, SP-C–eGFP mouse (A), but no eGFP+ cells in mice transplanted with SP-C–eGFP bone marrow (B; n = 6) or negative-control mice transplanted with wild-type bone marrow (C; n = 4). Representative images here were taken from mice 1 mo after transplantation.
In addition, lungs from eight mice transplanted with SP-C–eGFP marrow underwent histologic analysis at intervals of 2 wk, 1 mo, and 3 mo, with detection of eGFP performed by viewing frozen, unfixed sections, and by performing IHC and IF staining on formalin-fixed, paraffin-embedded sections (Figure 5). Over 30 lung sections from each transplanted mouse were examined. For all transplanted mice, not a single eGFP+ cell was observed.
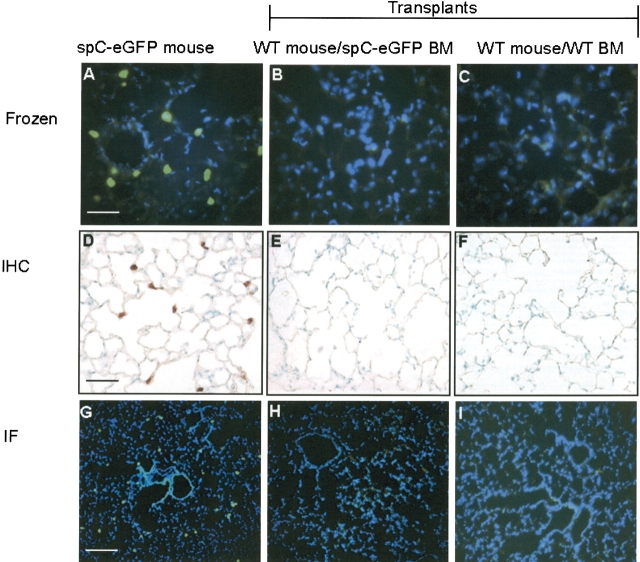
Lack of alveolar engraftment by histology after transplantation with SP-C–eGFP bone marrow. From 2 wk to 3 mo after transplantation with SP-C–eGFP marrow, lungs of recipient mice were examined histologically. Lungs were analyzed for endogenous eGFP fluorescence in frozen, unfixed sections (A–C, ×400 magnification; size bar = 50 μm). In paraffin-embedded sections, we performed eGFP immunoperoxidase IHC (D–F, × 400 magnification; size bar = 50 μm) and eGFP immunofluorescent staining (IF) (G–I, × 100 magnification; size bar = 200 μm). By all methods, eGFP+ type II cells were detectable in lung sections from the donor SP-C–eGFP mouse (A, D, G). In mice transplanted with SP-C–eGFP bone marrow, eGFP+ cells were not seen (B, E, H; n = 8). Negative-control mice were transplanted with wild-type bone marrow (C, F, I; n = 6). Representative images here were taken from mice 1 mo after transplantation.
Marrow Engraftment Assessment by Deconvolution Microscopy
In view of the discrepancy of our results regarding marrow-derived pneumocytes, we re-examined lung sections from mice transplanted with actin-eGFP-bone marrow. Using thinner (2 μm) lung sections with immunohistochemistry and immunofluorescent staining, single cells that were both eGFP+ and pro–SP-C+ were still observed (data not shown). We next reanalyzed these sections by deconvolution microscopy. As shown in Figure 6, standard fluorescent microscopy images show a cell with apparent co-expression of donor-specific (eGFP) and lung-differentiated markers (pro–SP-C). After deconvolution and three-dimensional reconstruction, however, the pro–SP-C signal is seen to reside just outside of the donor-derived eGFP+ cell. Similar findings were observed in our analysis of double positive cells from over 100 lung sections. The distance between the pro–SP-C and eGFP signals was as little as 300 nm, and was not always apparent until a three dimensional image had been viewed from multiple different angles. In contrast, deconvolution and three-dimensional reconstruction on lung sections from both the actin-eGFP and SP-C–eGFP donor mice demonstrated clear co-localization of the eGFP and pro–SP-C signals (Figure 7).
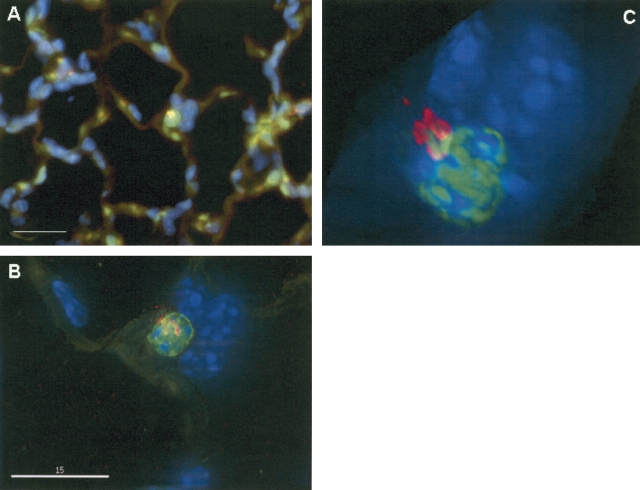
Comparison of conventional and deconvolution microscopy. Using deconvolution microscopy, we reanalyzed lung sections from mice transplanted with actin-eGFP marrow, that had been thought to show alveolar epithelial engraftment by marrow cells. (A) Viewed under conventional widefield fluorescent microscopy at ×1,000 magnification, the cell in the center appears to co-localize eGFP (green) and pro-SP-C (red) proteins. Size bar = 20 μm. (B) Same section viewed under deconvolution microscopy, size bar = 15 μm. (C) Three-dimensional reconstructed image which has been rotated slightly for better visualization. Fluorescent red signal from pro-SP-C actually is outside the eGFP+ cell.
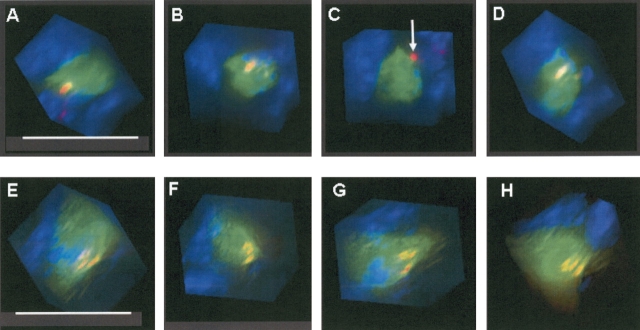
Three-dimensional reconstruction by deconvolution microscopy. (A–D) Different rotational views taken of a presumptive marrow-derived type II cell from a mouse transplanted with actin-eGFP bone marrow. Though the eGFP and pro–SP-C signals initially appear merged, C reveals that the pro–SP-C protein actually resides outside the eGFP+ cell (white arrow). The distance between the pro–SP-C protein and the outer edge of the eGPP+ cell was ~ 300 nm. By contrast, rotational viewing in the SP-C–eGFP mouse (E–H) shows co-localization in all images. For each cell analyzed by deconvolution, 60 rotational views were examined to evaluate for co-localization. Size bar = 15 μm.
DISCUSSION
Our initial data supported the possibility that marrow cells could repopulate the lung as type II cells, though at low frequency. Using basic enzyme histochemistry, immunohistochemistry, and immunofluorescent staining, we appeared to identify marrow-derived cells whose granular, compact morphology, location in the corner of alveoli, and punctate pro–SP-C staining suggested a type II epithelial cell phenotype. By using more rigorous methodology, however, these findings were found to represent false positives with no evidence of engraftment. Previous positive engraftment results in lung may have similarly resulted from flawed methods, and should be carefully re-examined using a rigorous set of standards. This includes use of donor mice that express reporter genes under cell-specific promoters, and evaluation by deconvolution or confocal microscopy.
We are cognizant of the fact that we cannot discount the possibility that the observations reported here are only relevant for the experimental system employed in this work. We chose, however, to perform these experiments during the early postnatal period, a time of ongoing alveolarization. A role for the marrow cell in populating the alveolar epithelium should, thus, be evident at this time. One lesson from these studies is the necessity of using multiple complementary techniques to evaluate engrafted cells.
The importance of using multiple detection methods is emphasized by our cytospin findings. By immunostaining of cytocentrifuge preparations, double-labeled lung cells from transplanted mice, thought to be marrow-derived type II pneumocytes, were visualized instead as small eGFP+ cells with an atypical homogenous pro–SP-C stain. By PAP staining we did not detect lamellar bodies within these cells. This overall staining pattern was rare, was not readily apparent in histologic sections, and may have resulted from fixation and permeabilization of the lung suspension. Although necessary for intracellular antibody staining, fixation and permeabilization increase background, eliminate the ability to exclude dead cells that fluoresce or bind antibody indiscriminately, and may lead to nonspecific intracellular trapping of antibody conjugates. Alternatively, a small number of cells with nonspecific staining could result from the uptake of the pro–SP-C protein by other cell types (i.e., leukocytes) during proteolytic tissue digestion.
We believe that false positives resulting from tissue fixation and antibody staining artifacts can be avoided through use of donor animals that express fluorescent reporter genes under cell-specific promoters. In this regard, after we transplanted irradiated neonates with bone marrow from the SP-C–eGFP mouse, cells with intrinsic green fluorescence were expected to indicate both donor marrow origin and type II cell phenotype. The fact that no eGFP+ cells were identified by flow cytometry or histology was in distinct contrast to our results using a donor mouse with ubiquitous eGFP expression, and offers further support that marrow cells did not engraft as type II cells.
If using donor mice that express reporter genes ubiquitously, rigorous methodology should be employed to avoid the misinterpretation of dual fluorescent signals that arise from two overlapping cells. The potential for this type of error is especially high with studies focused on alveolar cells due to the intricate and closely apposed vascular system that branches and bifurcates extensively. Because of this, contiguous circulating blood cells may give rise to a false positive signal. Further, inflation and fixation procedures may cause, in part, the close apposition of alveolar macrophages to type II cells in tissue sections.
Using a conventional wide-field microscope, fluorescent light emitted by a specimen is collected and magnified through the objective lens to yield a specimen image. Identification of co-localized fluorescent structures with this method assumes that both fluorescent signals originate from the same plane. In fact, light from in-focus structures, situated at a depth corresponding to the focal plane of the objective lens, has been estimated to contribute as little as 1–2% to the viewed image (16). The remaining incoming light may consist of blur artifact from objects above or below the focal plane.
In deconvolution microscopy, optical sections are obtained throughout the depth of a specimen, with subsequent application of a mathematical algorithm to each section to distinguish out-of-plane blur artifact from in-focus florescence. Blurred light is subtracted out or reassigned to its in-focus location, to yield a deconvolved image. This improved resolution is further enhanced by combining the stacked images to construct a three-dimensional view, which can be rotated and viewed from all angles. With this method we showed that those cells which appeared to co-express eGFP and pro–SP-C were actually composed of an endogenous pro–SP-C+ type II pneumocyte overlaid by a marrow-derived eGFP+ cell. Though the identity of these marrow-derived eGFP+ cells was not further examined, we suspect they could be macrophages because of their abundance in alveoli and their ability to adopt a variety of morphologic shapes. It should be noted that chromatic aberration and other imaging errors may conversely cause co-localized structures to appear spatially separated after deconvolution (17), but this was not observed with lung sections from actin-eGFP and SP-C–eGFP mice.
Previous strategies used to avoid false identification of co-localized cells have included the use of thinner tissue sections, and staining of serial sections with CD45 or CD68 (18). We found, however, that falsely co-localized cells could still be found in 2-μm sections. Moreover, serial sections have been demonstrated to be inadequate for excluding overlapping leukocytes (19). Deconvolution microscopy offers superior resolution to conventional wide-field fluorescent microscopy, and when combined with three-dimensional reconstruction, has been shown to resolve overlapping structures located 100 nm apart (20). Another effective microscopy tool would be confocal microscopy, used by Spyridonidis and colleagues to demonstrate both true colonic epithelial chimerism and false-positives in sex-mismatched transplant patients (19). Instead of relying on computational methods to subtract out blur, this microscopy technique avoids its initial inclusion with a pinhole aperture that blocks light originating outside the focal plane.
In summary, we did not find evidence for a role for the bone marrow in populating the alveolar epithelium in the neonatal lung. Our results highlight the potential danger of establishing lung epithelial engraftment solely based on standard fluorescent microscopy and flow cytometry of fixed cells. Because of positive engraftment results based on these techniques, several investigators have recommended clinical trials using marrow cells to reconstitute the diseased human lung. Our findings question the fundamental rationale and basis for this type of therapeutic strategy.
Acknowledgments
The authors are grateful to Dr. Beth Sullivan for assistance with deconvolution microscopy.
Notes
This work was supported by National Institute of Health Grants RO1-HL-69148 and R21-HL-72205.
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
Articles from American Journal of Respiratory Cell and Molecular Biology are provided here courtesy of American Thoracic Society
Full text links
Read article at publisher's site: https://doi.org/10.1165/rcmb.2005-0129oc
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2715342?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Emerging delivery approaches for targeted pulmonary fibrosis treatment.
Adv Drug Deliv Rev, 204:115147, 06 Dec 2023
Cited by: 2 articles | PMID: 38065244 | PMCID: PMC10787600
Review Free full text in Europe PMC
Stem cell-based therapy for pulmonary fibrosis.
Stem Cell Res Ther, 13(1):492, 04 Oct 2022
Cited by: 19 articles | PMID: 36195893 | PMCID: PMC9530416
Review Free full text in Europe PMC
Mesenchymal stromal cell therapy for acute respiratory distress syndrome due to coronavirus disease 2019.
Cytotherapy, 24(8):835-840, 11 Apr 2022
Cited by: 3 articles | PMID: 35649958 | PMCID: PMC8995321
CXCR4-Overexpressing Umbilical Cord Mesenchymal Stem Cells Enhance Protection against Radiation-Induced Lung Injury.
Stem Cells Int, 2019:2457082, 05 Feb 2019
Cited by: 16 articles | PMID: 30867667 | PMCID: PMC6379846
Bone marrow mesenchymal stem cells tune the differentiation of myeloid-derived suppressor cells in bleomycin-induced lung injury.
Stem Cell Res Ther, 9(1):253, 26 Sep 2018
Cited by: 8 articles | PMID: 30257700 | PMCID: PMC6158827
Go to all (74) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Failure of bone marrow to reconstitute lung epithelium.
Am J Respir Cell Mol Biol, 33(4):328-334, 16 Jun 2005
Cited by: 178 articles | PMID: 15961722 | PMCID: PMC2715341
Circulating vascular progenitor cells do not contribute to compensatory lung growth.
Circ Res, 93(4):372-379, 24 Jul 2003
Cited by: 49 articles | PMID: 12881479
Transplanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice.
Cytotherapy, 5(6):523-533, 01 Jan 2003
Cited by: 45 articles | PMID: 14660048
Engraftment of marrow-derived epithelial cells: the role of fusion.
Proc Am Thorac Soc, 3(8):691-695, 01 Nov 2006
Cited by: 15 articles | PMID: 17065375 | PMCID: PMC2647654
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (2)
Grant ID: R21-HL-72205
Grant ID: R01-HL-69148




