Abstract
Free full text

The Yeast FACT Complex Has a Role in Transcriptional Initiation†
Associated Data
Abstract
A crucial step in eukaryotic transcriptional initiation is recognition of the promoter TATA by the TATA-binding protein (TBP), which then allows TFIIA and TFIIB to be recruited. However, nucleosomes block the interaction between TBP and DNA. We show that the yeast FACT complex (yFACT) promotes TBP binding to a TATA box in chromatin both in vivo and in vitro. The SPT16 gene encodes a subunit of yFACT, and we show that certain spt16 mutations are synthetically lethal with TBP mutants. Some of these genetic defects can be suppressed by TFIIA overexpression, strongly suggesting a role for yFACT in TBP-TFIIA complex formation in vivo. Mutations in the TOA2 subunit of TFIIA that disrupt TBP-TFIIA complex formation in vitro are also synthetically lethal with spt16. In some cases this spt16 toa2 lethality is suppressed by overexpression of TBP or the Nhp6 architectural transcription factor that is also a component of yFACT. The Spt3 protein in the SAGA complex has been shown to regulate TBP binding at certain promoters, and we show that some spt16 phenotypes can be suppressed by spt3 mutations. Chromatin immunoprecipitations show TBP binding to promoters is reduced in single spt16 and spt3 mutants but increases in the spt16 spt3 double mutant, reflecting the mutual suppression seen in the genetic assays. Finally, in vitro studies show that yFACT promotes TBP binding to a TATA sequence within a reconstituted nucleosome in a TFIIA-dependent manner. Thus, yFACT functions in establishing transcription initiation complexes in addition to the previously described role in elongation.
One of the critical steps in the formation of a preinitiation complex is the assembly of the TATA-binding protein (TBP)-TFIIA-TFIIB complex at the TATA site at promoters. In vitro studies suggest sequential recruitment of TBP followed by TFIIA and TFIIB, with transcriptional coactivators simulating TBP-TFIIA-TFIIB complex formation in vivo (33). For example, some DNA-binding factors, including TBP, are unable to access a binding site within a nucleosome, but Swi/Snf, one of a number of chromatin remodeling complexes that use ATP to change chromatin structure (5), enhances factor binding in chromatin (15, 24, 31, 61).
The yeast FACT complex (yFACT; facilitator of chromatin transactions) also changes chromatin structure, but its activity does not require ATP (20) and does not result in DNA movement relative to the nucleosomal histone core (43). Human FACT was first identified as a factor that stimulates RNA polymerase II elongation through chromatin templates (38). Human FACT promotes the displacement of one H2A-H2B dimer from a nucleosome, and the resulting partial nucleosome is less inhibitory to the elongating RNA polymerase (6). Genetic studies in Saccharomyces cerevisiae suggest that yFACT may be able to partially disrupt nucleosomes as well as restore the nucleosome to its normal state (21).
The human FACT complex is composed of two proteins, p140 and SSRP1 (39), whose yeast homologs are Spt16 (or Cdc68) and Pob3, respectively. SSRP1 contains a DNA-binding motif of the HMGB family, but this motif is absent in Pob3 and is instead provided by a separate small protein, Nhp6. Spt16 and Pob3 form a stable heterodimeric SP complex that weakly associates with Nhp6 (11, 20). A role for yFACT in transcriptional elongation is supported by biochemical studies showing that yFACT associates with known elongation factors (30, 50, 51), by chromatin immunoprecipitation (ChIP) and immunolocalization studies showing association of FACT with elongating RNA polymerase II (36, 45), as well as genetic interactions with mutations affecting elongation factors (21, 51). However, Drosophila FACT associates with the GAGA factor, stimulating chromatin remodeling at the promoter (49), and Spt16 inactivation in yeast results in reduced binding of TBP and TFIIB at promoters (36). These results suggest a role for FACT in transcription initiation in addition to the known role in elongation.
The Nhp6 subunit of yFACT has roles in both transcriptional initiation and elongation, and we recently identified TBP point mutants that are viable in a NHP6 strain but lethal in the nhp6a/b mutant (19). Many of these TBP mutants are also lethal when combined with mutations affecting the Swi/Snf ATP-dependent chromatin remodeling factor or the Gcn5 histone acetyltransferase (9). Additionally, mutations in TFIIA that affect its interaction with TBP are also lethal with nhp6a/b, swi2, and gcn5 (9). These results suggest that Nhp6, Swi/Snf, and Gcn5 enhance assembly of the TBP-TFIIA-DNA complex. In this report we present evidence suggesting that yFACT also facilitates interaction of TBP with TFIIA on nucleosomal DNA and therefore has a direct role in initiation of transcription.
MATERIALS AND METHODS
Strains and media.
All yeast strains used are listed in Table S1 in the supplemental material and are isogenic with W303 (59). Standard genetic methods were used for strain construction (48). Cells were grown in yeast extract-peptone-dextrose (YEPD) medium (48) at 30°C, except where other temperatures are noted, or in synthetic complete medium (48) with 2% glucose and supplemented with adenine, uracil, and amino acids, as appropriate, to select for plasmids. 5-Fluoroorotic acid (5-FOA) medium was prepared as described previously (10).
Plasmids.
The plasmids used are listed in Table S2 in the supplemental material. Plasmid M4806 was constructed by moving a 4.2-kb SalI fragment with TOA1 and TOA2 from pSH346, provided by Steve Hahn, into YEplac195 (23). Plasmid M4761 was constructed by moving a 937-bp BamHI-SacI fragment with NHP6A from plasmid M4221 (9) into YEplac195 (23).
In vitro binding experiments.
Spt16-Pob3 was overexpressed in yeast and purified to apparent homogeneity as described previously using standard chromatographic methods (63) or using a cleavable histidine tag/Ni affinity procedure that produces a similarly pure preparation. Both preparations produce identical results. No ATP is added to the reactions, and yFACT activity is unaffected by the addition of apyrase, ruling out a contribution to the activity from potential contamination with ATP-dependent factors such as remodelers. Recombinant Nhp6 with the native protein sequence was purified from bacteria as described previously (44). Plasmids PH-MLT(+3), PH-MLT(+3)-Mu, PH-MLT(0), and PH-MLT(+6) (24) were PCR amplified using oligonucleotides CCCGGATCCCCCGGGGTTACAAG and GGGCCCGGGTTCGTGATACGAGC, and the resulting PCR products were radiolabeled with polynucleotide kinase and [γ-32P]ATP. Following restriction digestion with BamHI to remove the label at one end, the 157-nucleotide templates were assembled into mononucleosomes by slow dialysis from a high-salt solution in the presence of histone octamers (20). Partial DNase I digestion was performed as described previously (43). The purified Spt16-Pob3, Nhp6, TBP, and TFIIA proteins were used in binding reactions at the following concentrations: 58 nM Spt16-Pob3, 5 uM Nhp6, 3.1 uM TBP, and 0.85 uM TFIIA.
Chromatin immunoprecipitations.
Chromatin immunoprecipitations were performed as described previously (3) using a polyclonal anti-TBP sera generously provided by Tony Weil, except that the wash buffers included 1% Sarkosyl and 0.1% sodium dodecyl sulfate (46). Real-time PCR and calculations were performed as described previously (19), using the Intergenic V primer set (29) as the internal control. The sequences of the PCR primers are as follows: ELP3, TGCCGCTTTCATTGTTTA and TGTTGTTCCCGAGGTTAAAG; SER3, GAGTAATTACTTTGTTGGAAGG and AGTAAAATCTTCATATCACCCG; and Intergenic V, GGCTGTCAGAATATGGGGCCGTAGTA and CACCCCGAAGCTGCTTTCACAATAC.
RESULTS
Genetic interaction between Spt16 and TBP.
We have identified TBP mutants that support viability in an otherwise wild-type strain but are lethal in the absence of Nhp6 (19). As Nhp6 is part of the yFACT complex, along with Spt16 and Pob3 (11, 20), we wanted to determine whether the viability of these TBP mutants is reduced when SPT16 is also mutated. We constructed a strain in which both the SPT15 gene (encoding TBP) and the SPT16 gene are disrupted. (To avoid confusion, we will refer to the SPT15 gene by the protein name, TBP.) The genes encoding TBP and Spt16 are both essential for viability, so the strain is kept alive by a YCp-URA3 plasmid containing the wild-type genes for both TBP and Spt16. This strain was transformed with two plasmids, YCp-TRP1 plasmids with various TBP mutants and YCp-LEU2 plasmids with various Spt16 mutants. Cells that lose the YCp-URA3-TBP-Spt16 plasmid can grow on 5-FOA. Thus, after transformation with the mutant TBP and Spt16 plasmids, the ability to grow on 5-FOA reflects the ability of these TBP and Spt16 mutants to sustain growth in the absence of the wild-type genes.
We tested 16 TBP mutants for synthetic lethality in combination with seven spt16 mutations. The various spt16 alleles showed subtle differences in the pattern of synthetic lethalities with TBP mutants (Table (Table1).1). The spt16-11 (T828I, P859S) allele shows the strongest effects, with eight TBP mutants showing synthetic lethality or a significant growth defect (Fig. (Fig.1A1A and Table Table1).1). Four of the TBP mutants, E93G, K138T/Y139A, G147W, and G174E, showed very strong synthetic defects in most of the spt16 mutants. Our previous genetic analysis of these mutants showed some interesting common features among these same four mutants (9). These TBP mutants are lethal in a gcn5 strain, and the TBP gcn5 synthetic lethality for all four can be suppressed by overexpression of TFIIA. Similarly, these four TBP mutants are all lethal in a swi2 mutant, and TFIIA overexpression suppresses the TBP swi2 synthetic lethality for three of these TBP mutants. All four TBP mutants are lethal in a nhp6a/b strain, and for three of the TBP mutants, this synthetic lethality can be suppressed by a spt3 mutation. These TBP mutants that cannot be tolerated along with mutation of SPT16 are therefore generally sensitive to changes that lead to decreased probability of transcription initiation.
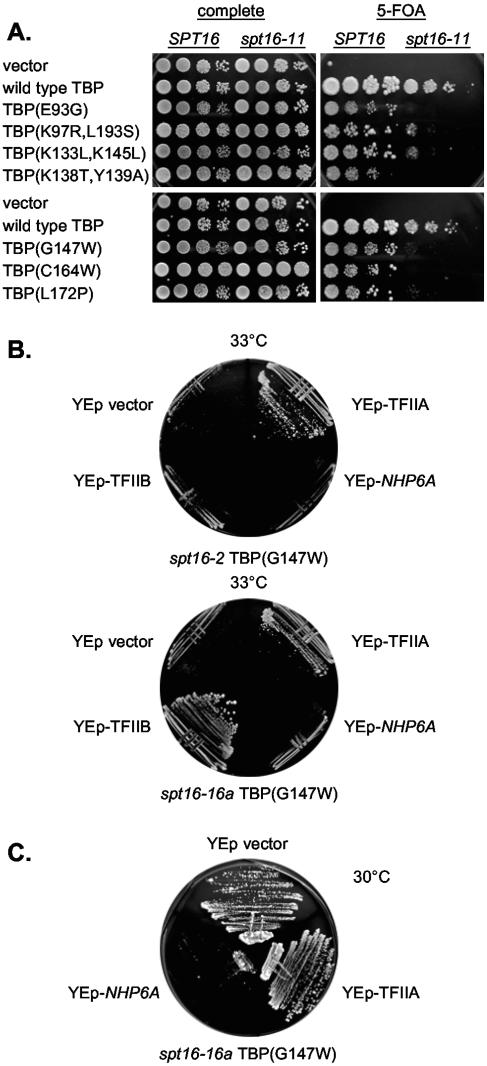
spt16 synthetic lethality with TBP mutants. A. Strain DY8552 (spt15Δ spt16Δ + YCp-URA3-TBP-Spt16) was transformed with two plasmids, a YCp-TRP1 plasmid with a TBP mutant and a YCp-LEU2 plasmid with either wild-type SPT16 or spt16-11, and dilutions were plated on complete or 5-FOA medium at 33°C for 3 days. B. Strains DY8969 and DY9452 were transformed with either YEp-TFIIA, YEp-TFIIB, YEp-NHP6A, or the vector and were plated on selective medium at 33°C for 3 days. C. Strain DY8969 was transformed with either YEp-TFIIB, YEp-NHP6A, or the vector, and dilutions were plated on selective medium at 30°C for 2 days.
TABLE 1.
Genetic interactions between TBP and Spt16 mutantsa
| TBP mutant | Effect with spt16 allele and substitution(s)b
| Note(s)c | ||||||
|---|---|---|---|---|---|---|---|---|
| spt16-11 (T828I, P859S) | spt16-2 (G132D) | spt16-9A (G836S, P838S) | spt16-12 (A417T, G568S, R569K, P599L) | spt16-22 (A417V) | spt16-24 (T434I) | spt16-16A (R204W, A273V, C290V, D318V) | ||
| E93G | Synthetic lethal | Growth defect 33° | Growth defect | Growth defect | Growth defect 33° | Growth defect 33° | Growth defect 30° 33° | a, b, c |
| K97R, L193S | Growth defect 33° | No effect | No effect | No effect | ND | No effect | No effect | b, d, e |
| I103T, K239Stop | No effect | Growth defect 33° | Growth defect | No effect | No effect | No effect | No effect | b, d, e |
| L114F | No effect | No effect | No effect | No effect | No effect | No effect | No effect | b, c, f, g |
| E129G | No effect | No effect | No effect | No effect | No effect | No effect | No effect | b, d, e |
| K133R | No effect | Growth defect 33° | No effect | No effect | No effect | No effect | No effect | b |
| K133L, K145L | Growth defect 33° | No effect | No effect | No effect | No effect | No effect | Synthetic lethal 33° | b, e, h, i |
| K138T, Y139A | Synthetic lethal | Synthetic lethal | Synthetic lethal | Synthetic lethal | Synthetic lethal | Synthetic lethal | Growth defect | a, b, c, g, h, i |
| G147W | Synthetic lethal 33° | Growth defect 33° | Growth defect 33° | Growth defect 33° | Growth defect 33° | Growth defect 33° | Growth defect 33° | a, b, c |
| C164W | Growth defect 33° | No effect | No effect | No effect | Growth defect | No effect | No effect | b |
| L172P | Synthetic lethal 33° | No effect | Growth defect | No effect | No effect | No effect | No effect | b, c, d, g |
| G174E | Synthetic lethal | Synthetic lethal | Synthetic lethal | Synthetic lethal | Synthetic lethal | ND | No effect | b, d, e, j |
| E186M | No effect | No effect | No effect | No effect | No effect | No effect | No effect | b, d, k |
| F227L | No effect | No effect | No effect | No effect | No effect | No effect | No effect | b |
| F237L | No effect | No effect | No effect | No effect | No effect | No effect | Growth defect 33° | b, g, h, k |
| K239T | No effect | No effect | No effect | No effect | No effect | No effect | No effect | b, l |
These four TBP mutations also have interesting effects on the binding of TBP to other factors. TBP interacts with Spt3, and the TBP(G174E) substitution causes reduced coimmunoprecipitation of TBP with Spt3 (18). (We show below that spt3 mutations also affect a number of spt16 phenotypes.) The TBP(K138T/Y139A) double substitution mutant was identified as an activation-defective TBP mutant that lost the ability to interact with TFIIA in vitro (53). Despite this defect, the TBP(K138T/Y139A) mutant is viable; we suggest that other factors present in vivo, but not in vitro, facilitate formation of the TBP-TFIIA-DNA complex and consequently overcome this defect. Thus, the observed lethality of TBP(K138T/Y139A) with a Spt16 mutant could indicate that yFACT facilitates the TBP-TFIIA interaction. The G147W substitution is on the upper surface of TBP, near K138/Y139, and E93G is on the lower surface of TBP, in close contact with TFIIA in the cocrystal structure (57). These three mutants are in positions that likely affect TBP-TFIIA interaction.
These TBP mutants could be defective in interacting with TFIIA, and the synthetic defects could reflect a role for yFACT in promoting assembly of the TBP-TFIIA-DNA complex. Overexpression of TFIIA might then allow these TBP mutants to interact with TFIIA despite the spt16 mutation. In fact, TFIIA overexpression did suppress the spt16 TBP lethality for 9 of 12 mutant combinations tested, and overexpressing TFIIB suppressed it in one instance (Fig. (Fig.1B1B and Table Table2),2), strongly suggesting that the spt16 mutation exacerbates the defective TBP-TFIIA interaction. Nhp6 overexpression did not suppress any synthetic lethality, but it strongly inhibited growth of several mutant combinations (Fig. (Fig.1C1C and Table Table2).2). Nhp6 may enhance TBP binding more globally (9), and thus excess Nhp6 could be inhibitory because it promotes formation of TBP-containing complexes at nonproductive sites. These results support a role for Spt16 in facilitating formation of the TBP-TFIIA complex in vivo.
TABLE 2.
Multicopy suppression of TBP spt16 synthetic lethalitya
| Strain | TBP mutant | Spt16 mutant | Effect with plasmid
| ||
|---|---|---|---|---|---|
| YEp-TFIIA | YEp-TFIIB | YEp-NHP6A | |||
| DY9446 | TBP(E93G) | spt16-2 | Suppression | No effect | No effect |
| DY9447 | TBP(E93G) | spt16-11 | Suppression | No effect | Inhibition |
| DY9450 | TBP(E93G) | spt16-16a | Suppression | No effect | No effect |
| DY9448 | TBP(E93G) | spt16-22 | Suppression | No effect | Inhibition |
| DY9449 | TBP(E93G) | spt16-24 | Suppression | No effect | No effect |
| DY9451 | TBP(K133L,K145L) | spt16-16a | Inhibition | No effect | No effect |
| DY9452 | TBP(G147W) | spt16-2 | Suppression | No effect | No effect |
| DY9453 | TBP(G147W) | spt16-9a | Suppression | No effect | No effect |
| DY8970 | TBP(G147W) | spt16-12 | Suppression | ND | No effect |
| DY8969 | TBP(G147W) | spt16-16a | Suppression | Suppression | No effect |
| DY8971 | TBP(G147W) | spt16-22 | Suppression | No effect | Inhibition |
| DY8972 | TBP(G147W) | spt16-24 | Suppression | ND | No effect |
| DY9456 | TBP(L172P) | spt16-11 | No effect | No effect | No effect |
| DY9454 | TBP(F237L) | spt16-16a | No effect | No effect | No effect |
Genetic interaction between Spt16 and TFIIA.
To determine directly whether Spt16 and TFIIA contribute to the same pathway, we next determined whether a spt16-11 mutation is synthetic lethal with TFIIA mutants. TFIIA is composed of two subunits, Toa1 and Toa2, and we tested Toa2 mutants which disrupt formation of the TBP-TFIIA complex in vitro (40). We constructed two strains with a toa2 gene disruption, with either wild-type SPT16 or spt16-11. TOA2 is an essential gene, so these strains contained YCp-URA3-TOA2(wild type) for viability. These strains were transformed with YCp-LEU2 plasmids with the wild-type TOA2 gene or a mutant toa2 gene, or the plasmid vector. Growth on 5-FOA medium was assessed to determine the ability of these transformants to grow without wild-type TOA2. The toa2 mutants shown in Fig. Fig.2A2A are lethal in the spt16-11 strain but viable in the otherwise wild-type strain. Interestingly, the same toa2 mutants that are lethal with spt16-11 also showed synthetic lethality with swi2 and gcn5 (9). The TFIIA residues affected in these toa2 mutants make important contacts with TBP, and the lethality with spt16-11, swi2, or gcn5 suggests that yFACT, Swi/Snf, and the Gcn5 histone acetyltransferase may all be involved in the same pathway stimulating formation of the TBP-TFIIA-DNA complex.
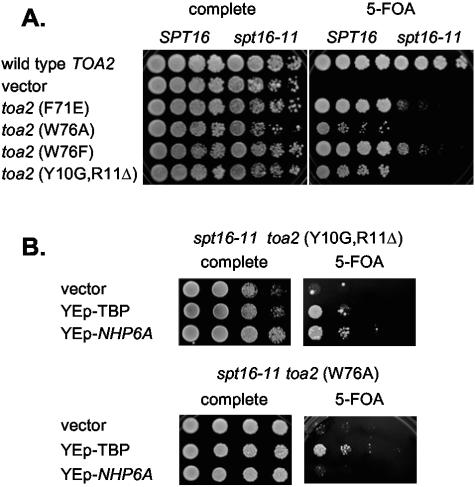
spt16 synthetic lethality with TFIIA mutants. A. Strains DY8541 (toa2Δ) and DY8699 (spt16-11 toa2Δ) transformed with the indicated TFIIA mutant plasmids were plated on complete or 5-FOA plates and incubated at 33°C for 3 days. Two other toa2 mutants (Y69F and F71R) were viable in the spt16-11 mutant (data not shown). B. Strain DY8700 (spt16-11 toa2Δ) was transformed with a YCp-LEU2 plasmid with the indicated toa2 mutant and a multicopy URA3 plasmid with either TFIIA, NHP6A, or the vector, and dilutions were plated on complete or 5-FOA medium at 33°C [toa2(Y10G,R11Δ)] or 30°C [toa2(W76A)].
Some of these spt16-11 toa2 synthetic lethalities can be suppressed by overexpression of TBP or Nhp6. The spt16-11 toa2(Y10G,R11Δ) synthetic lethality is suppressed by YEp-NHP6A, while weaker suppression by YEp-TBP is seen. In contrast, the spt16-11 toa2(W76A) synthetic lethality is only suppressed by YEp-TBP (Fig. (Fig.2B).2B). These results support the idea of a role for the yFACT complex in TBP-TFIIA-DNA complex formation.
Deletion of SPT3 suppresses spt16 phenotypes.
Spt3 and Spt8 are components of the SAGA transcriptional coactivator complex (54). Spt3 physically interacts with TBP, and Spt8 makes direct contact with TFIIA (18, 62). We have observed that disruption of either SPT3 or SPT8 suppresses synthetic lethalities along with transcriptional and growth defects associated with mutations affecting Nhp6, Gcn5, and TBP (9, 65). This suggests that Spt3 and Spt8 act in opposition to these transcription initiation factors at some genes important for growth. In support of this, SPT3 and SPT8 have been shown to negatively regulate expression of specific promoters (7, 65). Based on these observations, we constructed spt16-11 spt3Δ and spt16-11 spt8Δ double mutant strains and found that spt3Δ or spt8Δ suppress the temperature sensitivity of an spt16 mutant (Fig. (Fig.3A).3A). This mutual suppression suggests that Spt16 and Spt3/8 oppose each other while regulating TBP binding to promoters in vivo.
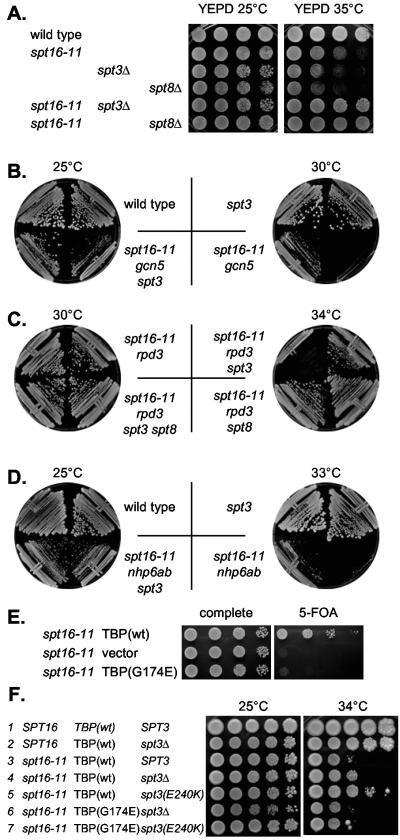
Suppression of spt16-11 by spt3. A. Dilutions of strains DY3398 (wild type), DY8788 (spt16-11), DY8980 (spt3), DY8981 (spt8), DY8977 (spt16-11 spt3), and DY8978 (spt16-11 spt8) were incubated on YEPD medium for 2 days at either 25°C or 35°C. B. Disruption of SPT3 suppresses the spt16-11 gcn5 synthetic lethality. Strains DY150 (wild type), DY6220 (spt3), DY8154 (spt16-11 gcn5), and DY9071 (spt16-11 gcn5 spt3) were grown on YEPD plates at 25°C for 4 days or at 30°C for 2 days. C. Disruption of SPT3 or SPT8 suppresses the spt16-11 rpd3 synthetic lethality. Strains DY8941 (spt16-11 rpd3), DY8946 (spt16-11 rpd3 spt3), DY8948 (spt16-11 rpd3 spt8), and DY8950 (spt16-11 rpd3 spt3 spt8) were grown on YEPD plates at 30°C for 3 days or at 34°C for 3 days. D. Disruption of SPT3 suppresses the spt16-11 nhp6ab synthetic lethality. Strains DY150 (wild type), DY8808 (spt16-11 nhp6ab), DY6220 (spt3), and DY8985 (spt16-11 nhp6ab spt3) were grown on YEPD plates at 25°C for 4 days or at 33°C for 2 days. E. spt16-11 is synthetic lethal with TBP(G174E).Strain DY8552 (spt15Δ spt16Δ + YCp-URA3-TBP-Spt16) was transformed with a YCp-LEU2-spt16-11 plasmid and a YCp-TRP1 plasmid with either TBP(wild type [wt]), TBP(G174E), or the empty YCp-TRP1 vector, and dilutions were plated on complete or 5-FOA medium at 33°C for 2 days. F. Spt3(E240K) suppresses spt16-11 TBP(G174E) synthetic lethality. Strains DY150 (wild type), DY6220 (spt3), DY8107 (spt16-11), DY8903 (spt16-11 spt3Δ), DY9036 [spt16-11 Spt3(E240K)], DY9038 [spt16-11 TBP(G174E)], and DY9040 [spt16-11 TBP(G174E) Spt3(E240K)] were plated on complete or 5-FOA medium at 25°C for 3 days or at 34°C for 4 days.
Deletion of SPT3 suppresses the synthetic lethality between spt16-11 and other transcription factors.
We have shown that a spt16-11 mutation shows a strong synthetic growth defect when combined with gcn5 or rpd3 mutations (21). Since deletion of the SPT3 gene suppresses other spt16 defects, we asked whether a spt3Δ mutation would also suppress these synthetic growth defects. The spt16-11 gcn5 double mutant grows poorly at 25°C and is lethal at 30°C, but both of these defects are suppressed by deletion of the SPT3 gene (Fig. (Fig.3B).3B). The Gcn5 histone acetyltransferase and the Rpd3 histone deacetylase act in opposition, and thus it is surprising that spt16-11 shows synthetic growth defects with both gcn5 and rpd3. The spt16-11 rpd3 strain is lethal at 34°C, but a spt3Δ mutation suppresses this synthetic lethality (Fig. (Fig.3C).3C). In interpreting these results, we note that the Gcn5 and Rpd3 factors have been implicated primarily as regulators acting at promoter regions. Moreover, histone acetylation promotes in vivo binding by TBP (47) and in vitro binding by TBP and TFIIA to nucleosomal templates (9). We propose that at some promoters both the yFACT complex and histone acetylation influence DNA binding by TBP and TFIIA and that either the proper level of acetylation or the ability to remove this modification at the appropriate time is an important component of the effect of acetylation.
The Nhp6 architectural transcription factor is required for the Spt16-Pob3 complex to bind to nucleosomes in vitro (20). An nhp6ab strain, with deletions of both of the genes that encode Nhp6, NHP6A and NHP6B, is viable, as is the spt16-11 mutant. However, combining these viable mutations in the spt16-11 nhp6ab strain results in synthetic lethality at 33°C (20). However, a spt3Δ mutation also suppresses the synthetic lethality of the spt16-11 nhp6ab double mutant (Fig. (Fig.3D).3D). Nhp6 has been shown to stimulate formation of the TBP-TFIIA-DNA complex in vitro (9), and the suppression of spt16-11 nhp6ab synthetic lethality by spt3Δ supports the idea that yFACT is involved in regulating TBP-TFIIA-DNA complex formation.
The spt16 phenotype depends on a functional interaction between TBP and Spt3.
The Spt3 component of the SAGA complex has been shown to interact with TBP, and the TBP(G174E) mutant shares phenotypes with spt3 mutations (18). Additionally, allele-specific suppression between TBP and Spt3 mutations suggests a direct interaction between TBP and Spt3 (18). TBP binding to the GAL1 promoter is lost in either a TBP(G174E) or a Spt3(E240K) mutant (32). However, TBP binding to GAL1 is restored in the TBP(G174E) Spt3(E240K) double mutant, demonstrating a functional interaction between TBP and Spt3. We have shown that Spt3 inhibits TBP binding to the HO promoter (66). Additionally, the reduced HO expression caused by a gcn5 mutation can be suppressed by either a TBP(G174E) or a Spt3(E240K) mutant. The presence of both TBP(G174E) and Spt3(E240K) reduces HO expression in the gcn5 mutant to the same level as observed with wild-type TBP and Spt3, again demonstrating the functional interaction between TBP(G174E) and Spt3(E240K), in this case to maintain repression of a hypoacetylated promoter.
Based on these results, we wanted to test whether the phenotypes of the spt16-11 strain are dependent on the functional interaction between Spt3 and TBP, using the TBP(G174E) and Spt3(E240K) mutants. Isogenic strains were constructed that differ at SPT16, TBP, and SPT3. However, we were unable to isolate a spt16-11 TBP(G174E) SPT3 strain in our crosses, suggesting that this combination was lethal. A plasmid shuffle experiment shows that spt16-11 and TBP(G174E) are synthetically lethal, showing no growth on 5-FOA (Fig. (Fig.3E),3E), while the TBP(G174E) mutant grows well in an SPT16 strain (65). However, introduction of either a spt3Δ gene disruption or the spt3(E240K) mutation allows the spt16-11 TBP(G174E) strain to grow (Fig. (Fig.3F,3F, lines 6 and 7). The spt16-11 strain grows poorly at a semipermissive temperature of 34°C. This defect is suppressed by a spt3Δ gene disruption, and the suppression is even stronger with the spt3(E240K) mutation (Fig. (Fig.3F,3F, compare lines 3, 4, and 5). Importantly, when the allelic interaction is restored with Spt3(E240K) in the presence of TBP(G174E), the strain once again becomes sensitive to growth at a higher temperature (Fig. (Fig.3F,3F, compare lines 5 and 7). We conclude that the ability of Spt3 to bind to TBP is required for Spt3 to perform the activity that opposes yFACT action with TBP.
spt16 and spt3 mutations affect TBP binding in vivo.
ChIP experiments have shown that mutations in Spt16 and Spt3 both affect TBP binding to promoters in vivo. Inactivation of Spt16 results in reduced binding of TBP and TFIIB to promoters (36). Spt3 is required for TBP binding to some promoters (8, 17). In light of these results, and our results showing mutual suppression between spt16-11 and spt3 (Fig. (Fig.3A),3A), we decided to examine whether this suppression is also seen in terms of TBP binding.
Four isogenic strains differing at the SPT16 and spt3 loci were grown at 25°C to early log phase and then shifted to 37°C for 3 h, and then TBP binding was analyzed by ChIP. Following cross-linking, immunoprecipitation with anti-TBP antibody, and reversal of cross-links, TBP binding to various promoters was measured by real-time PCR. After the shift to 37°C, TBP binding to the ELP3 and SER3 promoters was markedly reduced in both the spt16-11 and spt3 mutant strains (Fig. (Fig.4).4). Moreover, TBP binding in the spt16-11 spt3 double mutant is higher than in either single mutant, approaching that seen in the wild type. These results show that the improved growth in the spt16-11 spt3 double mutant compared to the single mutants (Fig. (Fig.3A)3A) is reflected by changes in TBP binding to multiple promoters.
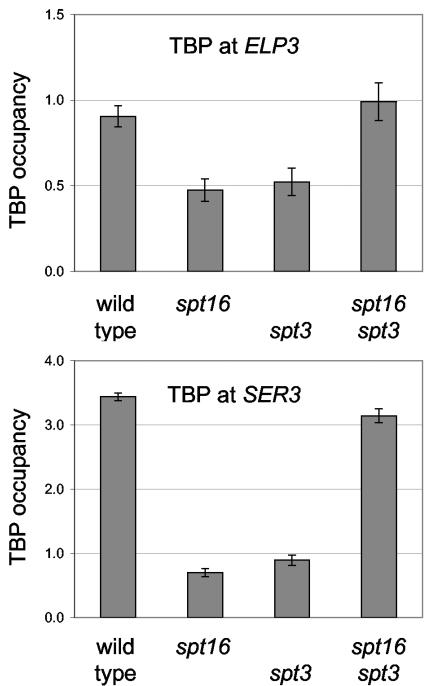
spt16 and spt3 mutations affect TBP binding. TBP occupancy at the ELP3 and SER3 promoters was determined by chromatin immunoprecipitation with polyclonal anti-TBP antisera and quantitative PCR, using cells that had been grown at 25°C and then shifted to 37°C for 3 h. Relative binding is shown, after normalization to an Intergenic V internal control. Error bars reflect variance among replicate PCRs. Strains DY3398 (wild type), DY8788 (spt16-11), DY8980 (spt3), and DY8977 (spt16-11 spt3) were used.
yFACT stimulates TBP-TFIIA binding to a nucleosome in vitro.
We next used purified Spt16-Pob3 and Nhp6 in an in vitro assay to determine whether yFACT could promote formation of TBP-TFIIA-DNA. TBP can bind to DNA containing a TATA element but cannot bind when the TATA is within a nucleosome (24). Importantly, the Swi/Snf chromatin remodeler facilitates TBP binding to a nucleosomal TATA in the presence of TFIIA (24). We used the PH-MLT(+3) template (24), containing a nucleosome positioning sequence that places the TATA element at the center of the positioned histone octamer, to test whether yFACT could promote TBP binding to TATA. The PH-MLT(+3) template was radiolabeled, assembled into a nucleosome, and used for DNase I digestion experiments (Fig. (Fig.5).5). The nucleosome shows the expected periodicity in DNase I protection (lanes 1 to 3), while yFACT enhances sensitivity at specific sites (lanes 4 to 6), particularly in the dyad region, consistent with previous observations (20, 43). DNase I is able to access the region containing the TATA sequence in free nucleosomes (see the TATA region in lanes 1 to 3 in Fig. Fig.5)5) or in nucleosomes bound by yFACT (lanes 4 to 6). However, the region is protected from DNase I digestion when TBP is present, but only when yFACT and TFIIA are both also added (compare the TATA region in lanes 7 to 9 to the same region in adjacent lanes 3 to 6 and 10 to 11 in Fig. Fig.5).5). This protection is specific to the TATA region, as other nearby sequences display constant accessibility to DNase I (Fig. (Fig.5).5). Further, the presence of TBP or TFIIA reverses the enhanced DNase I sensitivity induced by yFACT near the dyad. These changes indicate that TBP binds to its cognate site in the nucleosome only if yFACT and TFIIA are present, demonstrating that the reorganization of the nucleosome by yFACT promotes accessibility of this site for assembling a TBP-TFIIA complex.
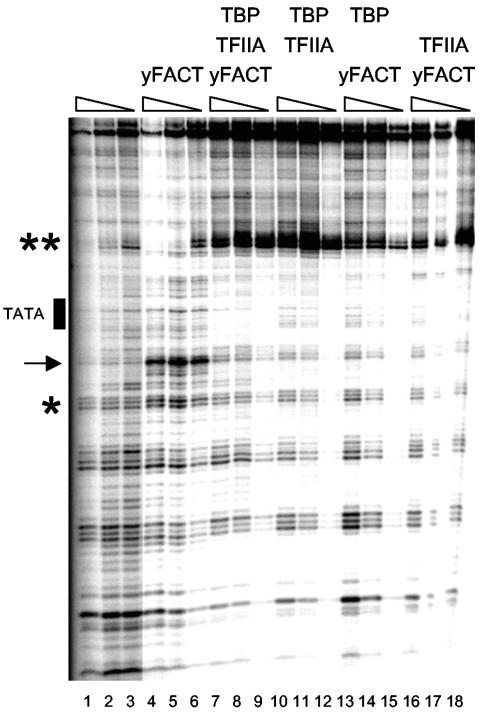
yFACT stimulates TBP and TFIIA binding to a nucleosomal TATA site. The PH-MLT(+3) template with a nucleosome positioning sequence and a TATA element near the dyad (24) was radiolabeled and assembled into nucleosomes, and the structure of these nucleosomes was assessed by partial DNase I digestion followed by electrophoresis and phosphorimager analysis. Each set of three lanes has twofold decreases in the amount of DNase I. Lanes 1 to 3 (nucleosome only), the DNase I digestion pattern shows the 10-bp periodicity of a rotationally phased nucleosome. Lanes 4 to 6, addition of yFACT to the binding reaction results in changes in the pattern of DNase I protection, particularly near the dyad (marked with an arrow), demonstrating that yFACT reorganizes the structure of the nucleosome. The changes in the DNase I digestion pattern of the PH-MLT(+3) sequence due to yFACT are different from those seen with either the 5S or 601 nucleosome positioning sequences previously examined with yFACT (20, 43), but as in those cases increased access to DNase I is observed near the dyad axis. This is consistent with the previous conclusion that the effects of nucleosome reorganization induced by yFACT are focused in specific regions of the nucleosome structure but the specific sites digested are strongly influenced by the DNA sequence. Lanes 7 to 18, with added TBP and/or TFIIA, as indicated. The position of the TATA element is indicated. The regions marked with single or double asterisks are discussed in the text. The single asterisk indicates the sequences that display constant accessibility to DNase I (showing that protection is specific to the TATA region). The double asterisks indicate a site within the nucleosomes in which the combination of TBP and TFIIA strongly enhances DNase I sensitivity.
The combination of TBP and TFIIA also strongly enhances DNase I sensitivity at a site within these nucleosomes (Fig. (Fig.5).5). This effect occurs away from the TATA site and is independent of yFACT. yFACT therefore only enables TBP-TFIIA effects on the accessibility of the nucleosomal DNA to DNase I at the appropriate TATA site. We used a nucleosome with a mutant TATA to further demonstrate binding specificity (Fig. (Fig.6A).6A). Protection is seen when yFACT, TBP, and TFIIA are incubated with the nucleosome with the wild-type TATA, while no binding is seen with the mutant TATA (compare lanes 5 to 6 to lanes 7 to 8), showing that TBP-TFIIA binding to the nucleosome is TATA sequence specific. More significantly, TBP binding to a nucleosomal TATA requires both yFACT and TFIIA. Imbalzano et al. (24) showed that the orientation of the TATA element relative to the histone core was critical for TBP-TFIIA binding to a nucleosome in the presence of Swi/Snf. Using two additional templates, PH-MLT(0) and PH-MLT(+6), which differ in the rotational position of the TATA sequence relative to the positioning sequence, they showed that only one orientation of the TATA relative to the histone core could be made accessible to TBP binding by Swi/Snf. We found that yFACT stimulates TBP-TFIIA binding to the PH-MLT(0) nucleosome, although not as well as to PH-MLT(+3) (Fig. (Fig.6B),6B), but was unable to stimulate TBP-TFIIA binding to PH-MLT(+6) (data not shown). We conclude that both Swi/Snf and yFACT can stimulate TBP-TFIIA binding to PH-MLT(+3), but only yFACT stimulates binding to the PH-MLT(0) template, suggesting that yFACT may provide accessibility to a broader range of sites within nucleosomes than Swi/Snf.
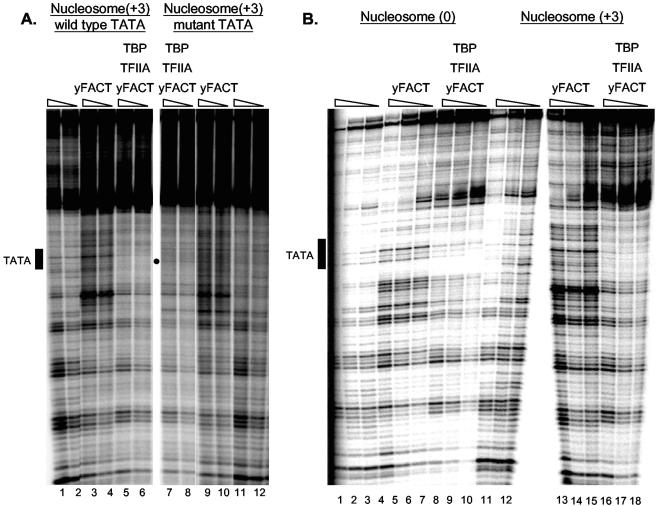
TBP and TFIIA binding to a nucleosome is affected by the integrity and rotational phase of the TATA site. A. An intact TATA box is required for TBP-TFIIA binding. Nucleosomes were assembled onto either the PH-MLT(+3) template with a wild-type TATA or the PH-MLT(+3)-Mu template with a mutated TATA (24), and TBP-TFIIA binding was examined as described for Fig. Fig.5.5. B. The position of the TATA sequence within the nucleosome affects binding. Nucleosomes were assembled onto either the PH-MLT(+3) template or the PH-MLT(0) template which has a 3-nucleotide change in the rotational position of the TATA sequence relative to the histone core (24), and TBP-TFIIA binding was examined as described for Fig. Fig.55.
DISCUSSION
Although there is substantial evidence that yFACT participates in transcriptional elongation (6, 26, 30, 36, 38, 51), SPT16 was first characterized because its overexpression or mutation cause the Spt− phenotype, which results from aberrant TATA site utilization (35). This suggests that Spt16 functions in transcriptional initiation, but a recent report provided an alternative explanation: the Spt− phenotype can be caused by a defect in reestablishing chromatin structure after passage of the elongating polymerase (25). While replacing nucleosomal components may be an important component of the function of Spt16, here we have presented the results of in vitro and in vivo experiments indicating that yFACT directly promotes formation of TBP-TFIIA-DNA complexes on promoters. We find strong genetic interactions between yFACT and basal transcription factors, and biochemical studies show that purified yFACT stimulates TBP-TFIIA binding to a nucleosomal template.
Support for a direct role of yFACT in initiation also derives from our experiments showing genetic interactions between spt16 and spt3 mutations. Unlike yFACT, which also has a role in transcriptional elongation, the available evidence indicates that Spt3 functions solely at promoters (4, 7, 8, 14, 32, 34, 60, 65), and Spt3 interacts with TBP physically and genetically (18). The suppression of spt16 defects by a spt3 gene deletion is consistent with the idea that yFACT stimulates TBP binding to certain promoters while Spt3 usually functions as a TBP inhibitor. In contrast, our ChIP experiments suggest Spt3 promotes TBP binding, as TBP occupancy at selected promoters is reduced in the spt3 mutant. These results are not inconsistent, as Spt3 can either stimulate or inhibit TBP binding, depending on the promoter (7, 8, 17, 65). In any case, we see mutual suppression in both experiments, with reduced growth at 35°C and reduced TBP binding in the spt16 and spt3 single mutants and suppression of both effects in the spt16 spt3 double mutant. Finally, ChIP experiments show that yFACT binds both to promoters and to coding regions (36), and Drosophila FACT associates with the GAGA factor, stimulating chromatin remodeling at promoters (49). This large set of interactions, many of which depend on allele-specific interactions among proteins known to interact directly, strongly implicates yFACT directly in promoting transcription initiation. The ability of yFACT to promote TBP binding in vitro using purified components demonstrates that this activity is direct.
Genetic analyses show many similarities in the relationships between yFACT and Swi/Snf with TBP-TFIIA, suggesting that each facilitates formation of TBP-TFIIA-TATA complexes. For example, we have shown that mutating yFACT causes synthetic lethality with both TBP and TFIIA mutants, and we previously showed that swi2 is also synthetically lethal with the same TBP and TFIIA mutants (9). Moreover, TFIIA overexpression suppresses the spt16 TBP and swi2 TBP synthetic lethalities, and TBP overexpression suppresses the spt16 TFIIA and swi2 TFIIA synthetic lethalities. Additionally, histone acetylation by Gcn5 is also important for TBP binding. Much like the results with Swi/Snf and yFACT, a gcn5 mutation is synthetically lethal with TBP and TFIIA mutants, and these lethal interactions were suppressed by overexpression of the other partner in the TBP-TFIIA complex (9). Further, acetylation of histones also promotes TBP-TFIIA binding to TATA sites within nucleosomes (9). Thus, chromatin remodeling by Swi/Snf, histone acetylation by Gcn5, and now nucleosome reorganization by yFACT have all been shown to contribute independently to formation of the TBP-TFIIA-DNA complex in a native chromatin context.
The mechanisms used by each of these factors to promote formation of the preinitiation complex are likely to be distinct. Swi/Snf is the archetypical ATP-dependent chromatin remodeling factor and appears to use ATP hydrolysis to translocate DNA relative to the histone octamer (5). This could either place the TATA site in an accessible linker location or on the surface of a repositioned nucleosome such that it is more available for binding. The strong dependence of TBP binding on rotational phasing in the presence of Swi/Snf is most consistent with the latter interpretation.
In contrast, reorganization of nucleosomes by yFACT alters chromatin structure without hydrolyzing ATP and does not involve movement of the histone octamer core relative to the DNA sequence (20, 43). Specific sites become more accessible to DNase I and to some restriction endonucleases in the reorganized nucleosomes, even though they can be recovered subsequently in a largely intact form (43). We have proposed that yFACT promotes an internal rearrangement of the nucleosomal components and that while this may lead to displacement of some components, it is instead normally a rapidly reversible process that leaves the nucleosome unaffected afterwards (21). We propose that this ability to reversibly reorganize nucleosome structure to an alternate form is important during both initiation and elongation by RNA polymerase II. One possibility is that reorganization assists the formation of TBP-TFIIA-TATA complexes required for initiation by making the binding site at least temporarily more accessible, and it also promotes elongation by making nucleosomes less inhibitory to polymerase passage.
yFACT contains Nhp6, Spt16, and Pob3. Mutations in NHP6 are also synthetically lethal with either TBP or TFIIA mutants (19), and thus one might think that spt16 mutants should show similar genetic interactions. However, there are important functional differences between Nhp6 and Spt16-Pob3. Strains with nhp6ab gene disruptions are viable, while SPT16 and POB3 are essential genes. Nhp6 does not bind tightly to Spt16-Pob3 (11, 20), and multiple Nhp6 molecules are needed to allow Spt16-Pob3 binding to a nucleosome (44). Nhp6 bends DNA sharply (42), and we believe that multiple Nhp6 molecules are needed to destabilize the nucleosome to promote binding by other factors such as Spt16-Pob3 (44). Nhp6 is a very abundant protein and has been shown to interact with a number of important chromatin proteins, including Spt16-Pob3, Swi/Snf, RSC, and Ssn6/Tup1 (9, 20, 22, 56). Nhp6 and other HMG proteins have been previously shown to interact with basal transcription factors like TBP (9, 16, 41, 55) but Spt16-Pob3 has not. Thus, while Nhp6 supports the function of Spt16-Pob3 in the context of yFACT, it is not simply a subunit of yFACT but instead has roles in other contexts. In this view, it is not surprising that phenotypes are not always shared among mutants in Nhp6, Spt16, and Pob3. However, Nhp6 and Spt16-Pob3 each function directly during initiation of transcription. Further work is needed to understand the mechanisms by which yFACT facilitates binding of factors to chromatin.
Acknowledgments
We thank Mike Hampsey, Tony Imbalzano, and Paul Lieberman for providing plasmids, Tony Weil for antibody, and Susan Ruone for technical assistance.
This work was supported by grants awarded to T.F. and D.J.S. from the National Institutes of Health.
Footnotes
†Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.25.14.5812-5822.2005
Read article for free, from open access legal sources, via Unpaywall:
https://mcb.asm.org/content/25/14/5812.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/145086821
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/mcb.25.14.5812-5822.2005
Article citations
A fluorescent assay for cryptic transcription in Saccharomyces cerevisiae reveals novel insights into factors that stabilize chromatin structure on newly replicated DNA.
Genetics, 226(4):iyae016, 01 Apr 2024
Cited by: 0 articles | PMID: 38407959
Structure and Dynamics of Compact Dinucleosomes: Analysis by Electron Microscopy and spFRET.
Int J Mol Sci, 24(15):12127, 28 Jul 2023
Cited by: 0 articles | PMID: 37569503 | PMCID: PMC10419094
Establishment and function of chromatin organization at replication origins.
Nature, 616(7958):836-842, 05 Apr 2023
Cited by: 16 articles | PMID: 37020028
Hmo1 Protein Affects the Nucleosome Structure and Supports the Nucleosome Reorganization Activity of Yeast FACT.
Cells, 11(19):2931, 20 Sep 2022
Cited by: 5 articles | PMID: 36230893 | PMCID: PMC9564320
Genome-Wide Analysis of the Peptidase M24 Superfamily in Triticum aestivum Demonstrates That TaM24-9 Is Involved in Abiotic Stress Response.
Int J Mol Sci, 23(13):6904, 21 Jun 2022
Cited by: 0 articles | PMID: 35805912 | PMCID: PMC9266489
Go to all (64) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Role for Nhp6, Gcn5, and the Swi/Snf complex in stimulating formation of the TATA-binding protein-TFIIA-DNA complex.
Mol Cell Biol, 24(18):8312-8321, 01 Sep 2004
Cited by: 28 articles | PMID: 15340090 | PMCID: PMC515044
Chd1 and yFACT act in opposition in regulating transcription.
Mol Cell Biol, 27(18):6279-6287, 09 Jul 2007
Cited by: 30 articles | PMID: 17620414 | PMCID: PMC2099615
TATA-binding protein mutants that are lethal in the absence of the Nhp6 high-mobility-group protein.
Mol Cell Biol, 24(14):6419-6429, 01 Jul 2004
Cited by: 29 articles | PMID: 15226442 | PMCID: PMC434259
Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.
Gene, 833:146581, 18 May 2022
Cited by: 11 articles | PMID: 35597524
Review




