Abstract
Free full text

Comparative Opsonic and Protective Activities of Staphylococcus aureus Conjugate Vaccines Containing Native or Deacetylated Staphylococcal Poly-N-Acetyl-β-(1-6)-Glucosamine
Abstract
Staphylococcus aureus and Staphylococcus epidermidis both synthesize the surface polysaccharide poly-N-acetyl-β-(1-6)-glucosamine (PNAG), which is produced in vitro with a high level (>90%) of the amino groups substituted by acetate. Here, we examined the role of the acetate substituents of PNAG in generating opsonic and protective antibodies. PNAG and a deacetylated form of the antigen (dPNAG; 15% acetylation) were conjugated to the carrier protein diphtheria toxoid (DT) and used to immunize animals. Mice responded in a dose-dependent fashion to both conjugate vaccines, with maximum antibody titers observed at the highest dose and 4 weeks after the last of three weekly immunizations. PNAG-DT and dPNAG-DT vaccines were also very immunogenic in rabbits. Antibodies raised to the conjugate vaccines in rabbits mediated the opsonic killing of various staphylococcal strains, but the specificity of the opsonic killing was primarily to dPNAG, as this antigen inhibited the killing of S. aureus strains by both PNAG- and dPNAG-specific antibodies. Passive immunization of mice with anti-dPNAG-DT rabbit sera showed significant levels of clearance of S. aureus from the blood (54 to 91%) compared to control mice immunized with normal rabbit sera, whereas PNAG-specific antibodies were ineffective at clearing S. aureus. Passive immunization of mice with a goat antiserum raised to the dPNAG-DT vaccine protected against a lethal dose of three different S. aureus strains. Overall, these data show that immunization of animals with a conjugate vaccine of dPNAG elicit antibodies that mediated opsonic killing and protected against S. aureus infection, including capsular polysaccharide types 5 and 8 and an untypable strain.
Staphylococcus aureus and coagulase-negative staphylococci (CoNS), principally Staphylococcus epidermidis, are the most frequent causes of hospital acquired bloodstream infections accounting for 40 to 60% of all nosocomial bloodstream infections (52). S. aureus causes diverse infections such as endocarditis, septic arthritis, osteomyelitis, meningitis, skin infections, and abscesses (1, 2, 7, 16, 23, 27) and there appears to be an increase in the recognition of community-acquired S. aureus infections, often involving methicillin-resistant S. aureus strains (16, 27). It is now well appreciated that the emergence of antibiotic resistance among staphylococcal isolates has made the treatment of these infections increasingly difficult, which has amplified the call for new approaches to treat and prevent staphylococcal infections, such as immunotherapy.
Ongoing efforts to design vaccines for S. aureus have targeted various virulence factors of this organism, including capsular polysaccharides (CP) (i.e., CP serotypes 5 and 8) (8, 9), cell wall-associated proteins (i.e., clumping factor A, fibronectin binding proteins, and collagen binding protein) (11, 36, 48), toxins (i.e., alpha-toxin, enterotoxins, and toxic shock syndrome toxin 1) (6, 14, 15, 21, 33), and the surface-associated polysaccharide, poly-N-acetyl-β-(1-6)-glucosamine (PNAG) (25, 26, 29). PNAG is synthesized by enzymes encoded in the intercellular adhesin (ica) locus (12), which occurs not only in most clinical isolates of S. aureus but also in the majority of clinical isolates of CoNS (28, 31, 34, 53), making PNAG an attractive vaccine candidate. Interestingly, a genetic locus termed pga has been identified in a number of gram-negative bacteria (51), and for Escherichia coli, a polysaccharide chemically identical to PNAG has been isolated and characterized (51).
The basic chemical constituents of PNAG were initially described by Mack et al. (24) and referred to as the polysaccharide intercellular adhesin, but more recent studies showed differences between PNAG isolated from S. aureus strain MN8m (18, 25) and the polysaccharide intercellular adhesin preparation of Mack et al. (24). PNAG is a high-molecular-weight (high-MW) (100 to 500), highly acetylated (95 to 100% N-acetylation) polymer of β-1-6-linked glucosamine residues that plays a key role in biofilm formation for both S. aureus and CoNS (28, 47), as well as being a key virulence factor for S. epidermidis in animal models of infection (38-40). Previous work has demonstrated the protective efficacy of PNAG in rabbits against S. epidermidis catheter-related infections (20) and endocarditis (45), where it was referred to as the capsular polysaccharide/adhesin. Purified PNAG (mistakenly identified as poly-N-succinyl glucosamine) also elicited protective efficacy in mice against renal infections due to S. aureus, following both active and passive immunization (29). Further studies showed that only the highest-molecular-weight forms of PNAG were immunogenic in laboratory animals and that rabbit antibodies specific to this surface-associated antigen were able to promote the opsonophagocytosis of various staphylococcal strains in vitro (25). However, it took fairly high doses (100 μg/animal) of purified PNAG to elicit antibodies in mice, and rabbits responded to this antigen only when immunized along with strong adjuvants (25).
In an effort to improve the immunogenicity of PNAG, we investigated means to conjugate the polysaccharide to protein carriers and the role of the acetate substituents in generating protective antibody. In this work, we report the synthesis of two conjugate vaccines using the S. aureus surface-associated polysaccharide PNAG, as well as a deacetylated derivative of PNAG termed dPNAG, conjugated to the carrier protein diphtheria toxoid (DT) (10). PNAG and dPNAG are chemically related but differ mainly in their degree of N-acetylation, 95 to 100% for PNAG and 15% for dPNAG, which also lacks O-linked succinate due to the deacetylation procedure. We compared the immunological properties of PNAG-DT and dPNAG-DT conjugate vaccines in mice and rabbits and the opsonophagocytic activity in rabbit antisera against several staphylococcal strains in vitro. We also evaluated the ability of rabbit antibodies raised to either PNAG-DT or dPNAG-DT to reduce levels of S. aureus injected into the blood of mice, following passive administration of antibodies raised to both of the conjugate vaccines. Finally, as these initial studies indicated that dPNAG-DT was more effective than PNAG-DT at inducing opsonic killing and reductions in bacterial levels in the blood of bacteremic mice, confirmatory immune protection studies were carried out using a goat antiserum raised to dPNAG-DT in a model of lethal S. aureus infection in mice.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus MN8m strain that overproduces PNAG due to a 5-bp deletion in the ica promoter (17) was used for the preparation of PNAG. The following strains were used in the in vitro opsonophagocytic assay: S. aureus MN8 (capsular type 8), Reynolds (capsular type 5), and 502 (capsular type 5), and S. epidermidis M187. Two S. aureus strains deleted for the ica locus, MN8Δica and Newman Δica, were also used in the opsonophagocytic assay; their construction has been described previously (17). The murine bacteremia studies were performed with S. aureus COL (capsular type 5), strain Becker (capsular type 8), and strain 10833 (a derivative of strain Newman that does not produce CP5). The recently sequenced S. aureus strain 476 (13), which does not produce CP5 or CP8, was used for the murine lethality studies.
Reagents.
Isopropyl β-d-thiogalactopyranose (IPTG) was obtained from Invitrogen Corp. (Carlsbad, CA). Sephacryl S-200 and Superose 6 prep-grade gels used to purify DT and conjugate vaccines, respectively, were purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The endotoxin removing gel Detoxi-Gel was obtained from Pierce (Rockford, IL) and cyanylating agent 1-cyano-4-dimethylaminopyridinium tetrafluroborate (CDAP) was purchased from Sigma Chemical Co. (St Louis, MO). Purified sodium cyanoborohydride (NaCNBH3) was obtained from Matreya, Inc. (Pleasant Gap, PA). Fetal bovine serum (FBS) HyClone, (Logan, Utah) and complement (baby rabbit complement) Accurate Chemical and Scientific (Westbury, N.Y.) were used in the opsonophagocytic assay.
Purification of PNAG and preparation of dPNAG.
The method of purification of PNAG from the S. aureus MN8m strain and the chemical characterization of the PNAG antigen used in this study have been previously described (25). One fraction of this material with an average MW of ~100, corresponding to the previously designated PNAG-II fraction (25), was found to have a degree of substitution of the amino groups with acetate of ~95%. This material was then used to prepare dPNAG. Native PNAG was dissolved to a concentration of 2 mg/ml in 5 M NaOH and incubated at 37°C for 18 h. After this time, the sample was neutralized with an equal volume of 5 M HCl, dialyzed overnight against distilled water, and lyophilized. Analysis of the size of the deacetylated polymer showed no significant change in its elution profile when chromatography was carried out with a molecular sieve column. The degree of residual acetylation was measured by 1H-nuclear magnetic resonance as previously described (25), and the resultant antigen was determined to have ~15% of the amino groups substituted with acetate.
Purification of DT.
DT containing a six-His tag was expressed from the plasmid pET22b-DT-51E148K carried by E. coli BL21 and kindly provided by John Collier, Boston, MA (10). E. coli was grown in Luria-Bertani (LB) broth in an 8-liter fermentor at 37°C until the optical density at 650 nm (OD650) reached 1. The temperature was reduced to 28°C, IPTG was added to 1 mM, and growth was continued for 3 h. Periplasmic proteins were extracted by first resuspension of the pelleted cells in 0.4 culture volumes of 20% sucrose, 1 mM EDTA, and 30 mM Tris-HCl, pH 8.0. After 10 min at room temperature, the mixture was centrifuged, and the pelleted cells were resuspended in the same volume of ice-cold 5 mM MgSO4. After 10 min on ice, cells were pelleted by centrifugation, and DT was purified from the supernatant fluid by affinity chromatography on an Ni2+-chelate column. The protein was further purified by size exclusion chromatography on Sephacryl S-200. Endotoxin was removed using Detoxi-Gel (Pierce).
Coupling of PNAG to DT.
Native PNAG (average MW, ~100) containing >95% acetate substituents was covalently coupled to purified DT with the organic cyanylating agent CDAP to activate the polysaccharide hydroxyl groups. CDAP-activated PNAG was subsequently coupled to DT without the need for additional spacer molecules.
(i) Activation of PNAG with CDAP.
Purified PNAG (10 mg) was first dissolved in 5 M HCl (150 μl), neutralized with an equal volume of 5 M NaOH, and then diluted to 1 ml with 0.1 M borate buffer, pH 9.2. CDAP was made up at 100 mg/ml in acetonitrile and stored at −20°C (stable for up to 1 month). A total of 200 μl of the CDAP stock solution was slowly pipetted into the PNAG solution, and the reaction was allowed to proceed for 2 min.
(ii) Coupling of CDAP-activated PNAG with DT.
DT (5 mg) was immediately added to the CDAP-activated PNAG, and the mixture was reacted at room temperature for 3 h with stirring. After this time, the high-MW conjugate was separated from uncoupled components by gel filtration chromatography with a Superose 6 prep-grade column. Fractions containing PNAG-DT conjugate were identified on the basis of the earlier elution of both polysaccharide and protein compared with the elution of the nonconjugated components, pooled, dialyzed against 20 mM HEPES buffer-50 mM NaCl (pH 8), and stored frozen at −20°C.
Coupling of dPNAG to DT.
DT was covalently coupled to purified dPNAG by reductive amination. Aldehyde groups were first introduced onto the surface of DT by treatment of this protein with glutaraldehyde. Activated DT was subsequently reacted with dPNAG through its free amino groups in the presence of the reducing agent sodium NaCNBH3.
(i) Activation of DT with glutaraldehyde.
DT (10 mg) was dissolved in 0.1 M carbonate buffer, pH 10, and glutaraldehyde was added to a final concentration of 1.25% (vol/vol). This mixture was incubated at room temperature for 2 h, and the glutaraldehyde-activated DT was dialyzed against phosphate-buffered saline (PBS), pH 7.4, and concentrated to 10 mg/ml by ultrafiltration. Analysis of the glutaraldehyde-treated DT after treatment showed no detectable self-polymerization, following chromatography on a Superose 6 size-exclusion column.
(ii) Coupling of glutaraldehyde-activated DT to dPNAG.
Purified dPNAG (10 mg) was dissolved in 5 M HCl (0.25 ml), neutralized with an equal volume of 5 M NaOH, and diluted to 2 ml with PBS. dPNAG (10 mg) was mixed with 1 ml of a 10 mg/ml solution of activated DT in PBS, and the pH of the reaction was adjusted to 7.5. Purified NaCNBH3 (200 mg) was added to the mixture, and the reaction was allowed to proceed in the dark for 14 h at 37°C with mixing. After this time, the high-MW PNAG-DT conjugate was purified from uncoupled reagents by gel filtration chromatography on a Superose 6 prep-grade column. Fractions containing dPNAG-DT conjugate were identified as described above for the PNAG-DT conjugate, pooled, dialyzed against 20 mM HEPES buffer-50 mM NaCl (pH 8), and stored frozen at −20°C.
Chemical analysis of conjugate vaccines.
Conjugate vaccines were analyzed for their content of polysaccharide by the hexosamine assay described by Smith and Gilkerson (44) with N-acetylglucosamine as the standard and for protein with the Bradford assay (4) with DT as the standard.
Production of rabbit and goat antisera.
Antibodies to purified PNAG-DT or dPNAG-DT were raised in New Zealand white rabbits by subcutaneous immunization with two 10-μg doses of conjugated polysaccharide emulsified for the first dose in complete Freund's adjuvant and for the second dose in incomplete Freund's adjuvant, followed 1 week later by three intravenous (i.v.) injections of antigen in saline, each spaced 3 days apart. Rabbits were bled every 2 weeks, and sera were tested by enzyme-linked immunosorbent assay (ELISA). In addition, a goat was immunized with dPNAG-DT, following essentially the same protocol as for the rabbits but increasing to 50 μg (each) the doses of conjugated dPNAG used per injection.
Immunization of mice.
To study the immunogenicity of PNAG and dPNAG-DT conjugates, groups of 10 mice (Swiss-Webster; female; 5 to 7 weeks of age) were immunized subcutaneously at day 0 and boosted at weeks 1 and 2 with 0.15-, 0.75-, or 1.5-μg doses of conjugated PNAG or dPNAG in PBS. Control groups received a mixture of unconjugated polysaccharide and protein in PBS in the same ratio as in the conjugate vaccine. Blood was withdrawn weekly for a month, and specific antibody titers were determined by ELISA.
ELISA.
PNAG- and dPNAG-specific antibodies were measured in sera obtained from immunized mice, rabbits, and a goat by ELISA as described previously (25). PNAG fraction II was used to sensitize the ELISA plate. A similar protocol was used to detect the responses to DT, using plates sensitized with 5 μg DT/ml in phosphate buffer, pH 7.4.
Phagocyte-dependent killing assays.
White blood cells (WBC) were prepared from fresh human blood collected from healthy adult volunteers. Twenty-five milliliters were mixed with an equal volume of dextran-heparin buffer and incubated at 37°C for 1 h. The upper layer containing the leukocytes was collected, the cells were pelleted by centrifugation, and hypotonic lysis of the remaining erythrocytes was accomplished by resuspension of the cell pellet in 1% NH4Cl and incubation for 10 min at room temperature. WBC were then washed and resuspended with RPMI with 15% FBS (RPMI-FBS). With trypan blue staining to differentiate dead from live leukocytes, the final WBC count was adjusted to 5 × 106 WBC per ml. The complement source (1 ml of baby rabbit serum diluted 1:15 in RPMI-FBS) was adsorbed at 4°C for 30 min with continual mixing with bacteria resuspended from a pellet containing ~109 CFU of the target S. aureus or S. epidermidis strain. After adsorption, the complement solution was centrifuged and filter sterilized. The test sera were absorbed at 4°C for 30 min with bacteria resuspended from a pellet containing ~109 CFU of the PNAG-negative S. aureus MN8 Δica strain (17) to remove antibodies not directed to the PNAG antigen. The bacteria were removed by centrifugation, and the test sera were filter sterilized. The bacterial strains to be evaluated for phagocyte-dependent killing activity of antibody in immune sera were grown overnight in tryptic soy broth (TSB) with 1% glucose, adjusted to an OD650 of 0.4 (~2 × 108 CFU/ml), and a 1:100 dilution in RPMI-FBS was made for use in the killing assay.
The actual phagocytic killing assay was performed by mixing 100 μl (each) of the WBC suspension, target bacteria, dilutions of test sera, and the complement source. The reaction mixture was incubated on a rotor rack at 37°C for 90 min; samples were taken at time zero and after 90 min. A 10-fold dilution was made in TSB with 0.5% Tween to inhibit bacterial aggregation, and samples were plated onto tryptic soy agar plates. Tubes lacking any serum and tubes with normal rabbit serum (NRS) were used as controls, as were tubes containing serum and complement but lacking WBC to control for potential aggregation of bacteria by the antibody, which would reduce the apparent CFU counts at the end of the assay. At the concentrations of antisera used in the opsonic killing assay, there was no reduction in CFU of >10% in samples lacking phagocytes but containing antibody and complement, indicating little agglutinating activity of the antibody to PNAG or dPNAG antigens. The percentage of killing was calculated by determining the ratio of the number of CFU surviving in the tubes with bacteria, leukocytes, complement, and sera to the number of CFU surviving in tubes lacking sera but containing bacteria, complement, and leukocytes. Killing rates of >30% were considered biologically significant, as this level represents the upper limit of the percentage reduction in CFU that occurs in the opsonic killing assay with normal sera lacking antibody to S. aureus, due to normal variation in CFU counts in this assay.
For inhibition studies, rabbit antiserum was diluted 1:10 and incubated for 90 min at 4°C with an equal volume of a solution containing 0.8 to 100 μg/ml of either native PNAG or deacetylated PNAG. Subsequently, the antiserum was centrifuged, and the supernatant was used in the opsonophagocytic assay as described above. Inhibition of >40% was considered biologically significant, as this represented twice the upper limit of nonspecific inhibition seen in control tubes containing irrelevant polysaccharide antigens as inhibitors.
Murine bacteremia model.
Groups of eight mice (Swiss-Webster; female; 5 to 7 weeks of age) were immunized intraperitoneally (i.p.) with 0.4 ml of NRS or immune sera raised to PNAG-DT or dPNAG-DT. In preliminary studies, we determined that serum titers peaked 48 h after the i.p. injection (data not shown). Thus, after 48 h, each group was challenged i.v. with a dose of 3.1 × 105 to 2.5 × 107 CFU of S. aureus (depending on the strain) in 0.2 ml of PBS. The strains used had not been recovered from previously infected mice to promote animal adaptation. Mice were sacrificed 2 h after bacterial challenge, blood samples were withdrawn, and the number of bacteria were expressed as the number of CFU per milliliter of blood. Comparisons between immune and control groups were by unpaired t tests.
Murine intraperitoneal infection and dissemination.
Groups of eight mice (Swiss-Webster; female; 5 to 7 weeks of age) were immunized i.p. 24 h before and 24 h after infection with 0.2 ml of normal goat serum (NGS) or immune goat serum raised to dPNAG-DT. Preliminary studies indicated this regimen of antibody injection maintained the highest levels of local antibody in the peritoneum. S. aureus strains were grown overnight on tryptic soy agar plates and resuspended in PBS to an OD650 of 1.2, corresponding to approximately 4 × 1010 to 8 × 1010 CFU/ml, depending on the strain. Mice were challenged i.p. with a dose of 2 × 109 to 5 × 109 CFU in 0.2 ml of PBS and monitored twice daily. Moribund mice were sacrificed as per criteria described in an approved animal studies protocol and counted as dead along with any animal deaths that occurred between monitoring periods. Survival was compared by chi-square analysis with the continuity correction. Animal maintenance and experimental protocols were approved by the Harvard Medical Area Institutional Animal Care and Use Committee.
RESULTS
Preparation of PNAG and dPNAG-DT conjugate vaccines.
PNAG and dPNAG were coupled to DT in two steps using two different conjugation chemistries that were needed due to the individual chemical properties of each antigen. As most of the amino groups on PNAG were acetylated and not available for conjugation, a small proportion (<5%) of the free hydroxyl groups on the PNAG molecule was instead used for coupling to the protein carrier. Limited activation of the hydroxyl groups on PNAG was achieved with the organic cyanylating reagent CDAP, after which the activated polysaccharide was immediately conjugated to free amino groups on the carrier protein DT.
Use of CDAP with the dPNAG molecule was contraindicated, as the large number of free amino groups on this molecule would react with activated hydroxyl groups, resulting in self-polymerization of the dPNAG. Therefore, we chose to use the free amino groups on the dPNAG molecule to conjugate the polysaccharide to DT by the classic reaction of reductive amination. DT was first derivatized with glutaraldehyde, and then the activated DT was allowed to react overnight with dPNAG in the presence of the reducing agent NaCNBH3. This coupling method uses mild pH and temperature, conditions required to preserve the structure of both protein and polysaccharide.
The polysaccharide (OD650) and protein (OD595) profiles of the conjugates chromatographed over a Superose 6 column readily allowed for separation of the conjugated PNAG-DT (Fig. (Fig.1A)1A) and dPNAG-DT (Fig. (Fig.1B)1B) from nonconjugated components. As can be seen from the elution profiles, the conjugated protein and polysaccharide start to coelute in the void volume of the column (void volume = 40 ml). This is indicative of the formation of high-MW conjugates. Fractions were pooled, which contained material reactive in both the protein and hexosamine assays and which eluted from the column earlier than any fraction predetermined to contain the free polysaccharide or DT protein. To be sure that the high-MW fractions contained conjugated protein, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis on individual fractions from the Superose 6 column. Fractions containing polysaccharide-conjugated protein did not enter the polyacrylamide gel, due to their high MW (fractions, 40 to 80 ml); neither free polysaccharide nor free DT was detected in the gel after silver staining. On the other hand, fractions containing unconjugated DT were easily identified and excluded from the pools of conjugate vaccines. The ratio of polysaccharide to protein was about 4:1 for the PNAG-DT conjugate (80% polysaccharide, 20% protein) and almost 1:1 in the case of the dPNAG-DT conjugate (44% polysaccharide, 56% protein).
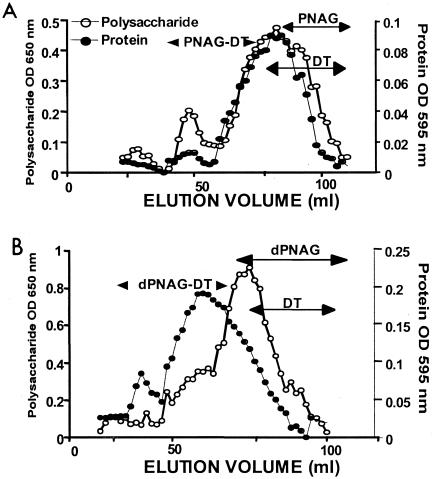
Gel filtration elution profiles of PNAG-DT (A) and dPNAG-DT conjugate vaccines (B) on a Superose 6 prep-grade column. The presence of polysaccharide (open circles) in fractions was monitored by the hexosamine assay (OD650) and the presence of protein (closed circles) in the fractions was monitored by the Bradford assay (OD595). Double-headed arrows indicate the fractions containing clearly conjugated protein and polysaccharide, as well as where the unconjugated polysaccharide (PNAG or dPNAG) and protein (DT) eluted.
Immunogenicity and IgG isotype distribution in sera of mice immunized with PNAG-DT and dPNAG-DT.
Mice vaccinated with the PNAG-DT conjugate developed high antibody titers to the immunizing polysaccharide as determined by ELISA (Fig. (Fig.2A).2A). Mice responded in a dose-dependent fashion with immunoglobulin G (IgG) titers that increased during the weeks postimmunization. Maximum IgG titers (ca. 5,500) were seen in mice vaccinated with 1.5 μg of conjugated PNAG and achieved 4 weeks after the last immunization. Serum samples beyond 4 weeks postimmunization were not obtained. A similar dose-dependent response was observed in mice immunized with the dPNAG-DT vaccine. The highest anti-dPNAG titers (ca. 10,000) were again found in the final serum sample obtained 4 weeks after immunization with 1.5 μg of dPNAG-DT vaccine (Fig. (Fig.2B).2B). Control mice, which were immunized with the same doses of a mixture of unconjugated polysaccharide and protein, did not develop any detectable titers to the polysaccharides (not shown). Interestingly, the titers to DT in the mice were rather low, ranging from 0 in animals given the protein and polysaccharide as a mixture to a maximum of 1,741 in mice immunized with 1.5 μg of the dPNAG-DT conjugate. This finding is consistent with previously described results showing low immunogenicity of carrier proteins such as DT when used in polysaccharide-protein conjugate vaccines (41). Overall, these results demonstrate that the conjugation of PNAG and dPNAG to the carrier protein DT significantly increased the immunogenicity of these polysaccharide antigens and induced modest amounts of antibody to DT.
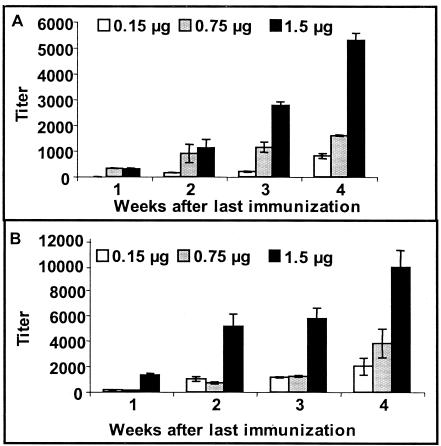
Mean titers of IgG antibodies in sera pooled from 10 mice immunized three times at weekly intervals with the indicated dose of PNAG-DT (A) or dPNAG (B) conjugate vaccines. Sera were collected weekly for 4 weeks starting 1 week after the last dose of conjugate vaccine and were tested by ELISA with the homologous immunizing antigen, either PNAG (A) or dPNAG (B) as a coating antigen. Bars represent means and error bars indicate standard deviations. All preimmune titers were <25.
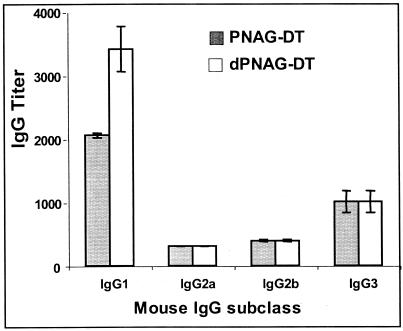
In mice, IgG3 is the predominant isotype made to unconjugated polysaccharide antigens, whereas IgG1 is often the predominant isotype responding to polysaccharide conjugated to protein (3). We investigated the IgG isotype distribution of mice vaccinated with either native or deacetylated conjugate, by determining titers of the antisera with ELISA plates coated with an excess of antigen relative to the amount of binding antibody in the most concentrated sample to ensure that no isotype blocked binding of another. We found that IgG1 was the predominant isotype elicited by both vaccines, followed by a lower titer of IgG3 and still lower and comparable levels of IgG2a and IgG2b (Fig. (Fig.33).
Immunogenicity of conjugate vaccines and antibody specificity in rabbits.
To investigate further the immunological properties of native and deacetylated conjugate vaccines, two rabbits were immunized with each conjugate, and the antibody titers were determined by ELISA. Both conjugates were very immunogenic in rabbits, which responded with high IgG titers to the immunizing polysaccharide antigens (Table (Table1).1). As with the mice, titers to DT were quite low, ranging between 452 and 831 in the four immune rabbit sera (data not shown). Since PNAG and dPNAG differed mainly in the degree of N-acetylation (>95% in PNAG versus 15% in dPNAG), we determined the epitope specificity of the rabbit antibodies raised to each conjugate by measuring ELISA titers in the different sera to both the native and deacetylated antigens (Table (Table1).1). Rabbits immunized with the highly acetylated PNAG conjugate vaccine had significantly higher antibody titers to PNAG than to dPNAG. On the other hand, rabbits vaccinated with poorly acetylated dPNAG-DT had comparable antibody titers to PNAG and dPNAG (Table (Table1).1). These results indicate that antibodies to PNAG-DT mainly react with acetyl-containing epitopes, whereas antibodies in the dPNAG-DT vaccinated rabbits are primarily directed to epitopes not dependent on the presence of acetate but expressed comparably on both highly and poorly acetylated PNAG.
TABLE 1.
Antibody titers determined by ELISA to purified PNAG and dPNAG antigens in sera of two animals immunized with PNAG-DT or two different animals immunized with dPNAG-DT conjugate vaccines
| Animal | Immunogen | Coating antigen | Titer |
|---|---|---|---|
| Rabbit 1 | PNAG-DT | PNAG | 1,170,000 |
| dPNAG | 23,000 | ||
| Rabbit 2 | PNAG-DT | PNAG | 178,000 |
| dPNAG | 1,150 | ||
| Rabbit 3 | dPNAG-DT | PNAG | 130,000 |
| dPNAG | 92,250 | ||
| Rabbit 4 | dPNAG-DT | PNAG | 137,000 |
| dPNAG | 152,000 | ||
| Goat | dPNAG-DT | PNAG | 148,000 |
| dPNAG | 246,000 |
Phagocyte-dependent killing activity of vaccine-induced antibodies.
We used a phagocytosis assay to compare the phagocyte-dependent killing ability of rabbit antibodies induced by the PNAG-DT conjugate, which were primarily reactive with the highly acetylated molecule, with antibodies elicited by the dPNAG-DT conjugate that primarily recognized nonacetylated or backbone epitopes. As the opsonic killing activity of the two rabbits immunized with PNAG-DT were virtually identical to each other, as was the opsonic killing activity of the two rabbits immunized with dPNAG-DT, results from the individual sera from the two rabbits immunized with the same vaccine were averaged. Additionally, since we grew the bacteria in TSB with 1% glucose to promote in vitro PNAG expression, it is important to emphasize that accurate determinations of phagocyte-dependent killing of S. aureus and S. epidermidis required the incorporation of 0.5% Tween into TSB that was used for the dilutions to be plated for bacterial enumeration. This prevented the otherwise highly adherent S. aureus and S. epidermidis strains from rapidly adhering (within seconds) to the plastic or glass dilution vessels and pipettes, which would have distorted the measurement of the actual CFU surviving in the assay. As can be seen in Fig. 4A to C, S. epidermidis strain M187, S. aureus strain 502, and S. aureus strain MN8 were killed more efficiently when dPNAG-specific antibodies were used than with the antibodies specific to the native PNAG. On the other hand, in the case of the S. aureus strain Reynolds, we found low but comparable levels of killing by antibodies raised by both the PNAG-DT and dPNAG-DT vaccines (Fig. (Fig.4D).4D). As we know from other assays that strain Reynolds makes very low levels of PNAG in vitro (unpublished observations), this may account for the overall low level of killing and lack of difference in efficacy between the two antibody preparations. In no case did preimmune rabbit antisera at a dilution of 1:10 elicit killing rates of >10% of any of the test strains (not shown), nor was there any level of killing of >10% in control tubes which contained bacteria but lacked one or more of the other three components of the opsonic killing assay (not shown).
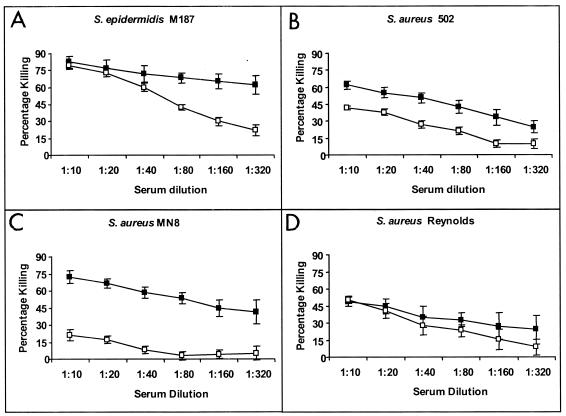
Opsonophagocytic killing of various Staphylococcal strains indicated in each panel by rabbit sera raised to PNAG-DT conjugate vaccine (open symbols) or dPNAG-DT conjugate vaccines (solid symbols). Points represent mean of quadruplicate determinations, two for each of the two rabbits immunized with each vaccine, and error bars indicate standard deviations. Opsonic killing of >30% is considered biologically significant and is statistically significant at P < 0.01; analysis of variance (ANOVA) and Fisher probable least squares difference (PLSD) posthoc, pairwise analysis.
To show the specificity of the opsonic killing antibody for the PNAG antigen, we evaluated antibody-dependent killing using two strains of S. aureus deleted for the ica locus (17), strains MN8Δica and Newman Δica. Of note, the loss of ica in S. aureus leads to increased killing by complement and white cells alone, as has been previously shown for S. epidermidis (42, 43, 50) and confirmed by us for S. aureus (A. Kropec, T. Maira-Litrán, K. Jefferson, M. Grout, S. E. Cramton, F. Goetz, D. A. Goldmann, and G. B. Pier, submitted for publication). Thus, for use with strains MN8Δica and Newman Δica, the level of the complement source had to be reduced to 5% final concentration; we could not use strain 10833Δica, as this strain was killed at a rate of >90% at complement levels that were <3% of final concentration, which is too low for antibody-mediated opsonic killing (Kropec et al., submitted). Notably, strains MN8Δica and Newman Δica express either the CP8 or CP5 capsule, whereas 10833Δica does not, which may account for its high susceptibility to antibody-independent killing. Nonetheless, even at a level of 5% complement, wild-type S. aureus strains were clearly killed by antibody in sera raised to dPNAG and PNAG while there was only minimal killing in NRS, whereas the ica-deleted strains were not killed any better by antisera raised to either conjugate vaccine when compared to background killing by NRS (Fig. (Fig.5).5). Thus, in the absence of an ica locus, the antibodies to native or deacetylated PNAG are ineffective at mediating opsonophagocytic killing.
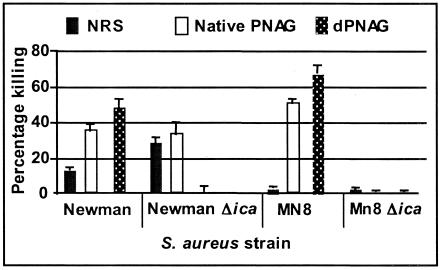
Opsonic killing of native and Δica S. aureus strains MN8 and Newman by 1:10 dilutions of either preimmune serum or antiserum raised to native PNAG-DT or dPNAG DT in the presence of 5% final concentration of complement. Bars represent mean percentages of killing and error bars indicate the standard deviation. Killing of wild-type strains by antisera to either native PNAG or dPNAG was significant at P values of <0.05, compared to killing by NRS.
Inhibitory capacity of PNAG and dPNAG in the opsonophagocytic assay.
Since there were low but nonetheless measurable amounts of antibodies to dPNAG epitopes present in the antisera raised to PNAG-DT, we used an inhibition assay to determine if phagocytic killing in the PNAG-DT antisera involved the antibodies that were specific to the dPNAG epitopes. Figure Figure66 shows the comparative ability of PNAG and dPNAG (concentrations of 0.8 to 100 μg/ml in the assay) to inhibit the opsonic killing of four staphylococcal strains by serum raised to PNAG-DT. Our results indicated that for all strains and at every concentration tested, dPNAG was either as effective as PNAG or more effective than PNAG at inhibiting killing mediated by antibodies raised to PNAG. Thus, inhibiting binding of antibodies with specificity for the nonacetylated epitopes on PNAG with dPNAG eliminated almost all phagocytic killing in the antisera raised to PNAG-DT. dPNAG and PNAG antigens both showed the same pattern in inhibiting the phagocyte-dependent killing of S. aureus mediated by antibody to dPNAG (Fig. (Fig.7),7), further validating that the dPNAG-specific epitopes were available on the acetylated PNAG antigen for binding to antibody raised to dPNAG.
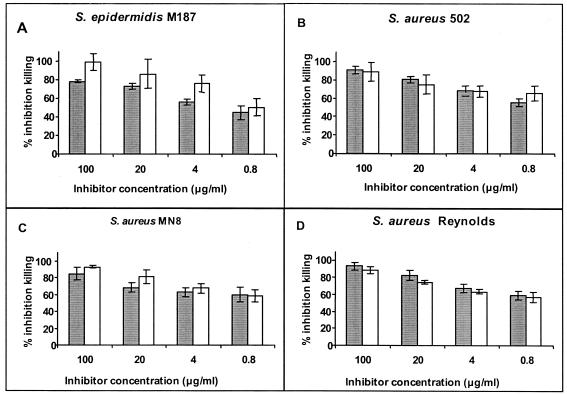
Inhibitory capacity of PNAG and dPNAG in the opsonophagocytic assay. Inhibition of opsonic killing of the staphylococcal strain indicated in each panel by rabbit serum raised to PNAG-DT conjugate mixed with purified PNAG (solid bars) or dPNAG (open bars) antigens at the indicated concentration. Bars represent means of quadruplicate determinations and error bars indicate the standard deviations. Inhibition of >40% is considered biologically significant and is statistically significant at P values of <0.01; ANOVA and Fisher PLSD posthoc, pairwise analysis.
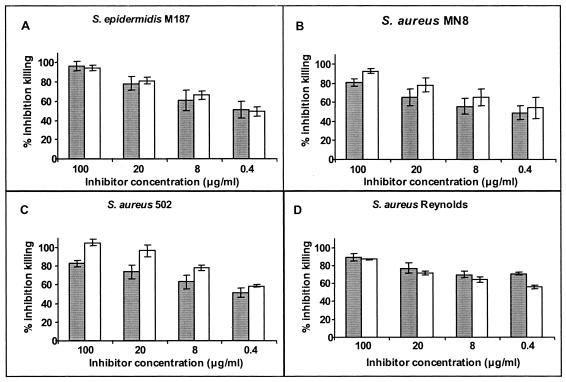
Inhibitory capacity of PNAG and dPNAG in the opsonophagocytic assay against antisera raised to dPNAG-DT. Inhibition of opsonic killing of the staphylococcal strain indicated in each panel by rabbit serum raised to dPNAG-DT conjugate mixed with purified PNAG (solid bars) or dPNAG (open bars) antigens at the indicated concentration. Bars represent means of quadruplicate determinations, and error bars indicate the standard deviations. Inhibition of >40% is considered biologically significant and is statistically significant at P values of <0.01; ANOVA and Fisher PLSD posthoc, pairwise analysis.
Protective efficacy of rabbit PNAG-DT and dPNAG-DT antibodies against bacteremia due to S. aureus in mice.
We next compared the protective activity of antibodies specific to PNAG and dPNAG in vivo using a mouse bacteremia model. Groups of mice were passively immunized i.p. with either NRS or immune sera raised to PNAG or dPNAG conjugates. Two days later, when maximum antibody levels were achieved in blood (data not shown), animals were infected i.v. with S. aureus. Two hours after infection, mice were sacrificed and the levels of bacteria were measured by serial dilution and plating of blood samples; results were expressed as the geometric mean number of bacteria per milliliter of blood.
As shown in Table Table2,2, mice passively immunized with rabbit sera raised to dPNAG-DT were able to clear 54% to 91% of the CFU of four different S. aureus strains compared to the control groups given NRS. We then evaluated the clearance activity of antibody raised to the PNAG-DT conjugate vaccine against S. aureus strains MN8 and 10833, for which we achieved the greatest reductions in CFU/ml blood with antibody to dPNAG-DT. Our results showed that in comparison to CFU levels in the blood of mice given NRS, the number of CFU per milliliter of blood in mice given antibody to PNAG was slightly, but not significantly, increased (120% for 10883 [P > 0.1, t test]; 106% for strain MN8 [P > 0.1, t test]). We could not test the strains deleted for the ica locus in this or any animal model to show specificity of the antibodies, because loss of ica greatly compromises bacterial virulence and there are virtually no PNAG-deficient bacteria in the blood or tissues of nonimmune mice by 2 h post-i.v. injection (Kropec et al., submitted). Overall, the results indicated that antibody to epitopes encompassing acetate residues that are present on the native PNAG was ineffective at promoting clearance of S. aureus from the blood of mice. In addition, the inability of antibody raised to PNAG-DT to reduce bloodstream bacterial levels indicated no protective effect of antibodies present in these sera, due to use of complete Freund's adjuvant in the immunization mixture.
TABLE 2.
Protection elicited by rabbit dPNAG-DT sera against various S. aureus strains in the murine bacteremia model in comparison to NRS
| S. aureus strain | Challenge dose | Mean CFU/ml blood ± SEMa
| % Reduction | P valuea | |
|---|---|---|---|---|---|
| NRS | dPNAG-DT sera | ||||
| 10833 | 2.5 × 106 | 1,925 ± 588 | 446 ± 52 | 77 | 0.04 |
| Becker | 1.8 × 106 | 184 ± 3.5 | 82 ± 2.5 | 55 | 0.034 |
| COL | 3.1 × 105 | 229 ± 2.1 | 105 ± 2 | 54 | <0.001 |
| MN8 | 2.5 × 107 | 9,821 ± 3,545 | 914 ± 167 | 91 | 0.045 |
Protective efficacy of goat antibodies raised to dPNAG-DT in a murine model of lethal S. aureus infection.
Since dPNAG-DT appeared to elicit killing and protective antibodies whereas antibodies elicited by native PNAG were not protective, we used this conjugate vaccine to immunize a goat to obtain large amounts of dPNAG-specific antibodies. Three bleeds were obtained from the goat at 2, 6, and 10 weeks after the final immunization, and the antisera were tested for opsonic killing activity. We found the three serum samples mediated an average killing rate of 48%, 60%, and 50% of S. aureus MN8, whereas preimmune NGS had no phagocyte-dependent killing activity. We next tested the protective efficacy of goat antibody to dPNAG against a lethal i.p. challenge of mice with several S. aureus strains. Two of the strains used in the above-described bacteremia model, COL and Becker, displayed insufficient virulence in the mice after injection by the i.p. route to yield a lethal outcome (<25% lethality at a dose of ≥5 × 109 CFU/mouse), so we instead used strain M476, a recently sequenced S. aureus isolate that was found to have a lethal effect in 100% of mice following injection of 5 × 109 CFU. Mice were passively immunized i.p. with NGS or anti-dPNAG goat sera 24 h before and 24 h after a high dose of S. aureus was administered i.p.; morbidity and mortality were recorded for 5 days. As can be seen in Table Table3,3, dPNAG-vaccinated mice were highly protected (62.5 to 100% survival) against a lethal dose of three S. aureus strains compared with control groups given NGS (0 to 18.7% survival [P ≤ 0.01, chi-square]). To ensure that antibodies present in the goat immune serum that were potentially cross-reactive with S. aureus antigens due to the use of complete Freund's adjuvant during immunization did not account for the protection seen, we tested the protective efficacy against challenge with S. aureus MN8 of a goat antiserum raised to an irrelevant peptide antigen. This serum was produced using the same immunization schedule with the same adjuvants as was used for the dPNAG-DT conjugate. All (100%) of the animals in groups given either NGS or a comparable dose of the irrelevant immune sera became moribund or died within 72 h of infection, indicating no contribution to protection from antibody to adjuvant components.
TABLE 3.
Protection against lethality caused by various S. aureus strains by a goat antiserum raised to the dPNAG-DT conjugate vaccine in comparison to NGS
| S. aureus strain | Challenge dose | No. of survivors/total (%)
| P valuea | |
|---|---|---|---|---|
| NGS | dPNAG-DT serum | |||
| MN8 | 2 × 109 | 0/32 (0) | 29/32 (91) | <0.001 |
| 476 | 5 × 109 | 3/16 (18.7) | 11/16 (68.7) | 0.01 |
| 10833 | 5 × 109 | 2/16 (12.5) | 15/16 (94) | <.001 |
DISCUSSION
Bacteria use numerous strategies to avoid innate and acquired host defenses and maintain their capacity to cause serious infections. One well-known strategy is illuminated by the poor immune response of human infants and young children to polysaccharide antigens, which are major protective antigens for many bacterial pathogens. However, by conjugating polysaccharides to protein carriers this immunologic barrier can be broken, and effective conjugate vaccines to Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis have been developed (3, 19, 54). Another strategy pathogens use to avoid host immune effectors is to elicit high levels of poorly protective antibodies, which can have this property based on low antibody affinity, production of an inappropriate antibody isotype, or specificity for nonprotective epitopes (32, 37, 46). In the case of the PNAG antigen, it appears that both poor overall immunogenicity of the native polysaccharide and a preferential induction of poorly opsonic, poorly protective antibody induced by highly acetylated, native PNAG could contribute to ineffective immune responses to this antigen. By both deacetylating PNAG and conjugating this polysaccharide to DT we were able to elicit phagocyte-dependent killing and protective antibodies.
For three of four staphylococcal strains, the antisera raised to dPNAG-DT mediated higher levels of phagocyte-dependent killing than antisera raised to PNAG-DT. However, the killing activity in the antisera to PNAG-DT for all four staphylococcal strains evaluated was completely inhibited by purified dPNAG antigen, indicating that antibodies with specificity to the deacetylated form of the antigen are key for mediating killing, even when the native form of PNAG is used to elicit the antibodies. Since the titers to dPNAG in the rabbit antiserum raised to native PNAG were quite low, it may be that the killing achieved with antisera to PNAG-DT was a result of a synergistic interaction between the antibodies specific to the native and deacetylated epitopes and that inhibiting the dPNAG-specific antibodies was sufficient to abrogate the synergistic effect leading to phagocyte-dependent killing activity in this serum. The remaining antibody specific to the native PNAG could not mediate phagocyte-dependent killing on its own. Overall, it is clear that the dPNAG antigen contained epitopes against which phagocyte-dependent opsonic killing antibody was directed.
The protection assays also supported the conclusion that antibodies reactive with the nonacetylated, backbone portion of the PNAG antigen were able to mediate clearance of bacteria from the blood and protection against a high-dose lethal infection. Evaluation of the contribution of both O- and N-linked acetyl groups to opsonic and protective antibody directed to other bacterial polysaccharide antigens has led to the conclusion that the role of acetylation in inducing protective antibodies is antigen specific. For example, Fattom et al. (9) found that antibodies to native and de-O-acetylated CP5 were both effective at mediating opsonic killing of a variety of S. aureus strains, and that native, O-acetylated CP5 conjugated to Pseudomonas aeruginosa exotoxin A elicited populations of antibodies directed to both highly and poorly O-acetylated CP5. In contrast, and more consistent with our findings using PNAG, Michon et al. (30) showed that a de-O-acetylated version of N. meningitides group C capsular polysaccharide was immunogenic in humans and superior to a highly O-acetylated group C capsular polysaccharide at inducing bactericidal antibody. In the case of P. aeruginosa alginate (also called mucoid exopolysaccharide) opsonic, protective antibodies are directed to the O-acetylated residues on the mannuronic acid components of this polysaccharide (35), and nonopsonic, nonprotective antibodies are directed to non-O-acetylated epitopes. Thus, there are examples with different bacterial capsular polysaccharides indicating that the acetate substituents either are of no consequence, interfere with eliciting protective antibody, or are required for generating protective antibody.
A key problem associated with preparation of glycoconjugate vaccines is choosing an appropriate, simple, and clinically acceptable method of conjugation chemistry that will introduce neither toxic components or neoepitopes likely to cross-react with host antigens. Native PNAG is a large, almost fully N-acetylated glucosamine polymer in which the only functional groups available for conjugation are the hydroxyl groups. Cyanlylation with CNBr is widely used as a method of activating hydroxyl groups. However, activation can be much more effective if performed with other cyanylating reagents such as CDAP. In comparison to CNBr, CDAP is easier to use, can be employed at a milder pH, and has fewer side reactions (5, 22). We used CDAP to activate PNAG before it was reacted with the carrier protein DT. Both reactions were carried out at room temperature in 0.1 M borate buffer, pH 9.2, for a total time of 3 h. These conjugation conditions were mild enough to preserve the N-acetyl groups on PNAG, which require much stronger conditions (high alkalinity, high temperature, and longer periods of incubation) to be removed. On the other hand, dPNAG was covalently linked to DT by using the classic reductive amination reaction, as cyanylation was contraindicated due to the potential of self-polymerization between activated hydroxyls and free amino groups. To conjugate the dPNAG molecule to the DT carrier, aldehyde groups were first introduced onto the surface of DT by incubation of the protein with glutaraldehyde, which was added in excess to the protein solution to prevent the self-polymerization of DT. Glutaraldehyde-activated DT was subsequently reacted with dPNAG in the presence of NaCNBH3 for 14 h in PBS at pH 7.5. Although the reductive amination reaction has been reported to be more efficient at higher pHs, we carried out this reaction at several pHs (10, 9, 8, and 7.5) and found pH 7.5 to be optimal (data not shown). Additionally, increasing the incubation time from 14 h to 3 days or 6 days did not improve the yield of this reaction (data not shown). Overall, we have defined clearly acceptable and useful means to conjugate both native PNAG and dPNAG to carrier proteins for immunologic studies.
Although we had somewhat different ratios of polysaccharide to protein in the two conjugates both were nonetheless capable of eliciting high titers of antibodies to the polysaccharide contained in the conjugate. Indeed, the rabbit antisera raised to native PNAG-DT had higher titers to the immunizing antigen than to dPNAG in animals immunized with the dPNAG-DT conjugate vaccine. As the primary goal of this study was to compare the killing and protective antibodies with specificities to different epitopes on PNAG, the conjugates we produced clearly achieved this purpose and showed that antibody to nonacetylated epitopes are effective at mediating opsonic killing and protection. We cannot exclude the possibility that a different ratio of native PNAG to protein might have induced a different population of antibodies that could have killing and protective properties comparable to those elicited by the dPNAG-DT vaccine. Also, we did not explore whether a PNAG molecule with an intermediate amount of acetylation between native PNAG and dPNAG might have highly desirable immunogenic properties. Of note, Vuong et al. (49) recently identified the product of the icaB gene in the ica locus of S. epidermidis as an N-deacetylase, removing the N-linked acetates from fully acetylated PNAG. In the absence of IcaB-dependent partial deacetylation of PNAG (referred to as PIA) there was no PNAG retained on the S. epidermidis cell surface, and all of the highly acetylated antigen was found in culture supernates. As a result, the S. epidermidis strain studied became susceptible to antibody-independent killing by phagocytes and complement and had significantly reduced virulence in a foreign-body infection model (50). We have found that icaB of S. aureus provides an identical function in this species (N. Cerca, K. Jefferson, and G. B. Pier, unpublished observations), suggesting that one reason that antibodies to the dPNAG epitopes are effective mediators of protection is that they bind preferentially to the low-acetate PNAG molecules most closely associated with the cell surface. Overall, while additional studies may be indicated to determine the optimal ratio of either PNAG or dPNAG to carrier protein for induction of protective antibody in humans, our study indicates that poorly acetylated forms of the vaccine are clearly capable of inducing the desired antibody.
In summary, conjugation of PNAG and dPNAG to DT significantly increased the immunogenicity of both of these antigens. Mice injected with either PNAG-DT or dPNAG-DT conjugate vaccines responded with high titers of antibodies to the immunizing polysaccharides in a dose-dependent fashion, as opposed to control groups given mixtures of these components, wherein antibody titers to the polysaccharide antigens did not develop. Critically important, we showed that de-N-acetylation of PNAG to a level of ~15% was needed to induce the most effective killing and protective antibodies. As highly acetylated PNAG is likely the form of this antigen naturally expressed by most isolates of S. aureus and CoNS, it appears that the immunodominance of the nonprotective epitopes on this molecule may contribute to the avoidance of host immune effectors by PNAG-expressing bacteria. Furthermore, as recent evidence suggests that not only staphylococci, but a variety of gram-negative pathogens including E. coli, Yersinia pestis, Bordetella pertussis, and others have a genetic locus related to the ica locus and may be capable of producing PNAG, which has been definitively shown for E. coli (51), the immunochemical properties of this antigen resulting in the immunodominance of nonprotective antibodies may be beneficial to the virulence of a variety of pathogens. It will be of great interest to pursue further the role of antibodies to different epitopes on the PNAG molecule in mediating immunity to the multitude of bacterial pathogens that appear to be able to synthesize this antigen.
Acknowledgments
This work was supported by National Institutes of Health grant R01 AI46706.
We thank Martha Grout for conducting opsonic killing assays, Sarah Cramton for provision of the S. aureus MN8Δica strain used to adsorb the antisera in the opsonic assays, Robin Ross for first isolating the S. aureus MN8m PNAG-overproducing strain, John Collier for provision of the recombinant plasmid expressing the DT carrier protein, and Arthur Tzianabos for providing the goat antiserum raised to a control antigen.
REFERENCES
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.73.10.6752-6762.2005
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/73/10/6752.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.73.10.6752-6762.2005
Article citations
A comprehensive synthetic library of poly-N-acetyl glucosamines enabled vaccine against lethal challenges of Staphylococcus aureus.
Nat Commun, 15(1):3420, 24 Apr 2024
Cited by: 2 articles | PMID: 38658531 | PMCID: PMC11043332
A high-throughput sequencing approach identifies immunotherapeutic targets for bacterial meningitis in neonates.
EBioMedicine, 88:104439, 27 Jan 2023
Cited by: 3 articles | PMID: 36709579 | PMCID: PMC9900374
Polysaccharides' Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens.
Int J Mol Sci, 24(4):4030, 17 Feb 2023
Cited by: 17 articles | PMID: 36835442 | PMCID: PMC9965654
Review Free full text in Europe PMC
Molecular Targets for Antibody-Based Anti-Biofilm Therapy in Infective Endocarditis.
Polymers (Basel), 14(15):3198, 05 Aug 2022
Cited by: 3 articles | PMID: 35956712 | PMCID: PMC9370930
Review Free full text in Europe PMC
An Overview of Biofilm Formation-Combating Strategies and Mechanisms of Action of Antibiofilm Agents.
Life (Basel), 12(8):1110, 23 Jul 2022
Cited by: 31 articles | PMID: 35892912 | PMCID: PMC9394423
Review Free full text in Europe PMC
Go to all (125) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Synthesis and evaluation of a conjugate vaccine composed of Staphylococcus aureus poly-N-acetyl-glucosamine and clumping factor A.
PLoS One, 7(9):e43813, 06 Sep 2012
Cited by: 18 articles | PMID: 22970144 | PMCID: PMC3435376
Synthetic {beta}-(1->6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens.
Infect Immun, 78(2):764-772, 30 Nov 2009
Cited by: 69 articles | PMID: 19948836 | PMCID: PMC2812210
Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine.
Infect Immun, 75(7):3406-3413, 30 Apr 2007
Cited by: 75 articles | PMID: 17470540 | PMCID: PMC1932961
Biologic properties and vaccine potential of the staphylococcal poly-N-acetyl glucosamine surface polysaccharide.
Vaccine, 22(7):872-879, 01 Feb 2004
Cited by: 56 articles | PMID: 15040940
Review
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R01 AI046706
Grant ID: R01 AI46706