Abstract
Free full text

Met/Hepatocyte Growth Factor Receptor Ubiquitination Suppresses Transformation and Is Required for Hrs Phosphorylation
Abstract
The Met receptor tyrosine kinase (RTK) regulates epithelial remodeling, dispersal, and invasion and is deregulated in many human cancers. It is now accepted that impaired down-regulation, as well as sustained activation, of RTKs could contribute to their deregulation. Down-regulation of the Met receptor involves ligand-induced internalization, ubiquitination by Cbl ubiquitin ligases, and lysosomal degradation. Here we report that a ubiquitination-deficient Met receptor mutant (Y1003F) is tumorigenic in vivo. The Met Y1003F mutant is internalized, and undergoes endosomal trafficking with kinetics similar to the wild-type Met receptor, yet is inefficiently targeted for degradation. This results in sustained activation of Met Y1003F and downstream signals involving the Ras-mitogen-activated protein kinase pathway, cell transformation, and tumorigenesis. Although Met Y1003F undergoes endosomal trafficking and localizes with the cargo-sorting protein Hrs, it is unable to induce phosphorylation of Hrs. Fusion of monoubiquitin to Met Y1003F is sufficient to decrease Met receptor stability and prevent sustained MEK1/2 activation. In addition, this rescues Hrs tyrosine phosphorylation and decreases transformation in a focus-forming assay. These results demonstrate that Cbl-dependent ubiquitination is dispensable for Met internalization but is critical to target the Met receptor to components of the lysosomal sorting machinery and to suppress its inherent transforming activity.
Growth factor receptor tyrosine kinases (RTKs) are involved in a variety of cellular processes, including proliferation, differentiation, migration, and survival. RTK activation is tightly controlled through several levels of regulation to achieve an appropriate biological response. The inappropriate activation of RTKs is associated with the development and progression of many human malignancies. Of the 58 transmembrane RTKs identified to date, deregulation of 30 has been associated with human tumors (7). RTK deregulation can occur through receptor amplification, point mutations, and chromosomal translocations, leading to elevated and ligand-independent RTK activation. More recently, the uncoupling of the Met/hepatocyte growth factor (HGF) RTK from ligand-induced ubiquitination and down-regulation revealed another mechanism leading to deregulation and oncogenic activation of RTKs (46, 48).
Met, the receptor for the hepatocyte growth factor/scatter factor (HGF/SF), is predominantly expressed in epithelia in vivo, and in epithelial cell lines in vitro. Its ligand, HGF/SF, is expressed by mesenchymal cells and promotes dissociation of epithelial cells in culture, increasing their motility and invasiveness, and acts as a mitogen for primary epithelia (6). Many of these are processes that are normally regulated by Met and HGF during development, and reflect the ability of HGF to promote cell survival, cell migration and to activate the intrinsic invasive morphogenic programs of different epithelial cells in three-dimensional matrix cultures (6). Deregulated Met signaling is associated with tumor progression and metastasis in a variety of tumors (21). Activating mutations in Met were first identified in both hereditary and sporadic papillary renal carcinomas (57). Many missense mutations in Met have now been reported in a variety of human cancers (32, 39).
Activation of the Met receptor through HGF binding promotes tyrosine phosphorylation of its intracellular domain and the recruitment of signaling protein complexes required for the activation of downstream signaling pathways and the generation of a biological response (12, 13, 15, 41, 45). In addition to the recruitment of positive effectors, acute HGF stimulation of the Met receptor leads to receptor internalization into clathrin-coated vesicles and its down-regulation by as yet incompletely understood mechanisms (19).
Following ligand stimulation, internalized receptors are subject to two distinct fates: either to recycle back to the plasma membrane or to be degraded via the lysosomal pathway (63). The rapid removal of growth factor receptors from the cell surface and their subsequent targeting to lysosomal degradative compartments provides a mechanism of down-regulation that is important to prevent the sustained activation of downstream signaling pathways, which could potentially lead to cellular transformation. Following clathrin-mediated internalization, ligand-activated RTKs, including the Met receptor, are eventually delivered to sorting endosomes (19, 40). At this stage, RTKs can be sorted into intralumenal vesicles of multivesicular bodies that subsequently fuse with lysosomes (20, 53). This event terminates RTK signaling by sequestering their signaling-competent intracellular domain and prevents RTKs from recycling to the cell surface.
In the last few years, genetic and biochemical studies have begun to elucidate the molecular mechanisms through which plasma membrane proteins are recognized for degradation in the lysosome. Studies in Saccharomyces cerevisiae have shown that monoubiquitination of several cell surface proteins is required for their internalization and trafficking to the vacuole for degradation (20, 59, 64). Although multiple signals are used in the regulation of the internalization of plasma membrane proteins in mammalian cells (8), several studies now support a role for monoubiquitination in the trafficking and targeting of the epidermal growth factor receptor (EGFR) for lysosomal degradation (16, 17, 24, 35, 44).
Monoubiquitinated receptors are thought to be recognized by multiple proteins of the endocytic pathway that contain ubiquitin-interacting domains (UBA, ubiquitin-interacting motif, UEV, and CUE) (20, 25, 53). One of these, Hrs (HGF-regulated tyrosine kinase substrate), appears to be the first protein recruited to endosomes and becomes enriched in regions of the endosome containing a specialized bilayered clathrin coat (51, 52, 56). Hrs is believed to be involved in the retention of ubiquitinated receptors within the bilayered clathrin coat and in the recruitment of ESCRT (endosomal sorting complex required for transport) complexes (1, 4, 5, 26, 36). Hrs becomes tyrosine phosphorylated upon activation of several RTKs (27). Hrs phosphorylation requires RTK internalization (65) as well as an intact ubiquitin-interacting motif in Hrs (66), supporting a role for Hrs in retaining ubiquitinated RTKs at the limiting membrane of endosomes.
The recruitment of the Cbl family of ubiquitin-protein ligases to RTKs is important for ligand-induced degradation of several RTKs, among them the EGFR, the platelet-derived growth factor receptor, the colony-stimulating factor 1 receptor and the Met/HGF receptor (33, 35, 43, 46, 47). Cbl is thought to mediate multi-monoubiquitination of RTKs rather than polyubiquitination, through the demonstration that antibodies specific for polyubiquitination fail to recognize ubiquitinated forms of the EGF, platelet-derived growth factor, or Met receptors (9, 17, 44). When overexpressed, Cbl can positively regulate endocytosis of the EGF and Met receptors, possibly through its ability to act as an adaptor for endophilin, an enzyme that may be involved in membrane curvature (35, 49, 61). Ubiquitination of the EGFR by Cbl is required for directing the receptor to internal vesicles of late endosomes and for subsequent lysosomal degradation of the EGFR (10, 16, 24, 68). Moreover, the fusion of monoubiquitin to a truncated EGFR is sufficient, in the absence of other signals, to target this receptor for constitutive internalization, and trafficking to late endosomes, supporting a role for monoubiquitination as a signal in mammalian cells for the trafficking of the EGFR (17).
We have previously demonstrated that the specific uncoupling of the Met receptor from Cbl-dependent ubiquitination promotes cell transformation by this receptor (46, 47). Moreover, several mechanisms have been identified that reduce Cbl-mediated ubiquitination of RTKs through enhanced Cbl degradation or sequestration, leading to cell transformation (3, 31, 69, 70). In addition, mutations in RTKs or Cbl proteins that impair Cbl-mediated ubiquitination of RTKs have been observed in multiple tumors (48). Together, this evidence provides support that the uncoupling of RTKs from ubiquitination may play an important role in tumorigenesis (2, 40, 48, 50). However, the molecular consequences of diminished RTK ubiquitination, at the level of receptor trafficking and signaling, as well as the biological outcomes, remain poorly understood.
Here, we provide a mechanistic understanding for oncogenic activation of the Met RTK through uncoupling from ubiquitination. We demonstrate that a ubiquitination-deficient Met receptor mutant (Met Y1003F) shows increased stability and signaling of downstream pathways, including the Ras-mitogen-activated protein kinase (MAPK) pathway, and oncogenic activation in vivo. We show that the Met Y1003F receptor mutant is internalized and can reach Hrs containing endosomes in a manner similar to the wild-type Met receptor, yet is unable to induce tyrosine phosphorylation of Hrs. The fusion of monoubiquitin to the Met Y1003F receptor rescues Hrs phosphorylation, decreases the stability of the Met Y1003F mutant, and suppresses Met receptor signaling and transforming activity.
MATERIALS AND METHODS
Reagents and antibodies.
Antibody 144 was raised against a carboxy-terminal peptide of the human Met protein (54). Hemagglutinin (HA) antibody was purchased from BABCO (Richmond, CA). Phosphotyrosine (4G10), Met DO-24 and DL-21, and Gab1 antibodies are from Upstate Biotechnology (Lake Placid, NY). Anti-c-Cbl (SC-170) and antiubiquitin (P4D1) are from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and Met antibody AF276 is from R&D Systems (Minneapolis, MN). Green fluorescent protein antibody and Alexa 488-conjugated secondary antibodies are from Molecular Probes (Eugene, OR). Total and phosphorylated protein-specific Erk1/2 (pThr202/pTyr204), MEK1/2 (pSer217/221), and Akt1 (pSer473) are from Cell Signaling Technology (Mississauga, Ontario, Canada). H-Ras and EEA1 antibodies were purchased from BD Biosciences (Mississauga, Ontario, Canada). HA monoclonal antibody was purchased from Covance (Berkeley, CA). Hrs antibodies used for immunoprecipitation were previously described (18) and the Hrs antibody used for immunofluorescence was a kind gift of H. Stenmark. Odyssey blocking buffer, IRDye800 anti-rabbit and Cy5.5 anti-mouse secondary antibodies for use with the Odyssey Infrared Imaging System (Li-COR Biosciences Lincoln, NE) were purchased from Rockland (Gilbertsville, PA). U0126 inhibitor was purchased from Promega (Madison, WI), LY294002 was purchased from BIOMOL Research Labs (Plymouth Meeting, PA). HGF was a kind gift from George Vande Woude (Van Andel Research Institute, Grand Rapids, MI) and recombinant colony-stimulating factor 1 (CSF-1) was provided by the Genetics Institute (Boston, MA).
Quantitative real-time PCR.
Total RNA was extracted from T47D cells using TRIzol reagent (Invitrogen Life Technologies) following the manufacturer's protocol; 5 μg of RNA was reverse transcribed with oligo(dT) (Invitrogen Life Technologies), and the cDNA was amplified using LightCycler-FastStart reaction mix SYBR Green I (Roche Molecular Biochemicals) and Rotor-Gene 3000 light cycler (Corbett Research, Sydney, Australia). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control to normalize mRNA levels. Primer sequences were as follows: GAPDH, sense, 5′-ACCACAGTCCATGCCATCAC-3′, and antisense, 5′-TCCACCACCCTGTTGCTGTA-3′; Met, sense, 5′-GTTTGTCCACAGAGACTTGGCTG-3′, and antisense, 5′-AGTTCAGAAAAGGATGGGCG-3′. The mean threshold cycle (Ct) value for each transcript was normalized by dividing it by the mean Ct value for the GAPDH transcript for that sample. Normalized transcript levels were expressed relative to sample obtained from T47D expressing wild-type Met.
In vivo tumorigenesis assays.
For tumorigenesis assays, NIH 3T3 fibroblast cells were infected with retroviruses expressing either nothing, wild-type Met or Met Y1003F and selected with 10 μg/ml G418 for 2 weeks. The obtained cell populations were injected subcutaneously (5 × 105 cells per 100 μl) into 4- to 5-week-old female nude mice (CD1 nu/nu; Charles River Breeding Laboratories). Tumors were measured periodically and mice were sacrificed prior to the tumors reaching 1 cm3 or undergoing ulceration.
Cell culture, transfections, and focus formation assays.
T47D breast epithelial cells, Madin-Darby canine kidney (MDCK) cells and HEK 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). MDCK cell lines expressing wild-type CSF-Met and CSF-Met Y1003F were established as described previously (46). T47D cell lines expressing wild-type Met and Met Y1003F were generated by retroviral infection. Following infection, cells were selected for 3 weeks in 4 μg/ml G418. Selected cell populations were then sorted by fluorescence-activated cell sorting (FACS) to select cells expressing Met at their cell surface using a MoFlo flow cytometer (Dako Cytomation, Fort Collins, CO).
Cells were labeled with anti-Met (1:50) (R&D Systems) for 1 h and with Alexa 488 donkey anti-goat (1:250) for 30 min in FACS buffer (DMEM containing 1% heat-inactivated FBS, 1 mM CaCl2 and 1 mM MgCl2) and then incubated for 5 min in FACS buffer containing 5 mM EDTA; 105 to 2 × 105 sorted cells were collected and plated in DMEM containing 10% FBS. Transient transfections in T47Ds were performed via electroporation. Cells were trypsinized, resuspended in phosphate-buffered saline (PBS), and electroporated at 0.30 kV and 0.975 μF. Transient transfections in HEK 293 cells were performed using Lipofectamine Plus reagent according to the manufacturer's instructions (Invitrogen Life Technologies).
The focus formation assays were performed as described previously (13). For T47D phase contrast pictures, 8 × 105 cells were seeded in 60-mm dishes and 16 h later were treated where indicated with 1.5 nM HGF for 24 h in the presence of serum. For the MDCK scatter assay, 104 cells/well were seeded in 12-well plates and 18 h later were treated where indicated with CSF (50 ng/ml), U0126 (20 μM), LY294002 (25 μM) in the presence of serum for 24 h. Phase contrast images were then taken with a Zeiss Axiovision 135 microscope with a 25× objective (Carl Ziess Canada Ltd, Toronto, Ontario, Canada). Image analysis was carried out using Northern Eclipse version 6.0 (Empix Imaging, Mississauga, Ontario, Canada).
Immunoprecipitation and Western blotting.
Following stimulation with HGF, T47D cells were harvested in TGH lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin). HEK 293 transfections were harvested 22 h posttransfection with radioimmunoprecipitation assay (RIPA) buffer (0.05% sodium dodecyl sulfate [SDS], 50 mM Tris, pH 8.0, 150 nM NaCl,1% Nonidet P-40, 0.05% sodium deoxycholate) or modified RIPA buffer (0.1% SDS, 25 mM Tris, pH 8.2, 50 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate) supplemented with 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 1 mM sodium fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin.
Lysates were incubated with the indicated antibody for 2 h at 4°C with gentle rotation. Proteins collected on either protein A- or G-Sepharose were washed three times in their respective lysis buffers, resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to a nitrocellulose membrane. Membranes were blocked in 3% bovine serum albumin in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM EDTA, 0.1% Tween 20) for 1 h and incubated with primary and secondary antibodies in TBST for 2 h and 1 h, respectively. After four washes with TBST, bound proteins were visualized with an ECL detection kit (Amersham Biosciences). Analyses with the Odyssey infrared imaging system were performed according to the manufacturer's instructions. To detect Met receptor ubiquitination, cells from a 10-cm dish were lysed in 200 μl boiling buffer (2% SDS, 1 mM EDTA). Lysates were boiled for 10 min and diluted to 1 ml with a buffer containing 2.5% Triton X-100, 12.5 mM Tris, pH 7.5, 187.5 mM NaCl, and proteasomal inhibitors (66).
Internalization assays using trypsin and flow cytometry.
Cells were seeded in 60-mm dishes and the following day were stimulated with 3 nM HGF. Stimulations were terminated by placing cells on ice and rinsing them with ice-cold DMEM. On ice, cells were acidified for 10 min with cold DMEM at pH 4.0 containing 1% bovine serum albumin. For the trypsin assays, cells were rinsed once with ice-cold PBS and then incubated with 2 ml of 1 mg/ml trypsin in PBS (pH 7.4) for 30 min on ice. The reaction was stopped by adding 2 ml of 5 mg/ml soybean trypsin inhibitor in ice-cold PBS. Cells were collected, centrifuged for 5 min at 200 g at 4°C, washed once with 1 ml of 5 mg/ml soybean trypsin inhibitor in PBS (ice-cold), and lysed in TGH lysis buffer.
For flow cytometry analysis, cells were washed once in ice-cold FACS buffer (PBS containing 1% heat-inactivated FBS, 1 mM CaCl2 and 1 mM MgCl2). On ice, cells were then incubated with Met antibody (AF206) in ice-cold FACS buffer for 45 min, washed three times with FACS buffer, incubated with donkey anti-goat Alexa 488 in FACS buffer for 20 min, washed three times, and incubated 5 min in 0.5 ml FACS buffer containing 5 mM EDTA. The cells were scraped and analyzed with the FACScan flow cytometer (BD Biosciences).
Confocal Immunofluorescence microscopy.
Cells were seeded at 2 × 105 on glass coverslips (Bellco Glass Inc. Vineland, NJ) in 24-well plates (Nalge NUNC, Rochester, NY) and 16 h later were serum starved for 2 h prior to HGF treatment (1.5 nM) where indicated. Coverslips were washed once with PBS and then fixed with 3% paraformaldehyde (PFA; Fisher Scientific) in PBS for 20 min. Coverslips were then washed four times in PBS and residual PFA was removed with three 5-minute washes with 100 mM glycine in PBS. Cells were permeabilized with 0.3% Triton X-100/PBS and blocked for 30 min with blocking buffer (5% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS). Coverslips were incubated with primary and secondary antibodies diluted in blocking buffer for 1 h and 40 min, respectively, at room temperature. Cells stained for Hrs were permeabilized with 0.05% saponin and fixed with 3% PFA as described previously (60). Coverslips were mounted with Immu-mount (Thermo-Shandon, Pittsburgh, PA). Confocal images were taken using a Zeiss 510 Meta laser scanning confocal microscope (Carl Ziess, Canada Ltd, Toronto, Ontario, Canada) with 100× objective and 2× zoom. Image analysis was carried out using the LSM 5 image browser (Empix Imaging, Mississauga, Ontario, Canada).
Generation of the Met-ubiquitin chimera receptors and other plasmids.
We first inserted a XmaI site just before the stop codon in the human Met receptor cDNA using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) with the 5′-CCTCCTTCTGGGAGACATCAGCCCGGGGCTAGTACTATGTCAAAGC-3′ primer and its reverse complement. Next we amplified by PCR a yeast ubiquitin cDNA that had the seven lysine residues mutated to arginine residues (generous gift of Linda Hicke) using the forward primer 5′-CGATTCCCGGGGTATGCAGATCTTCGTC-3′ (XmaI site underlined) and reverse primer 5′-GAACTGCGGCCGCTAAACACCTCTTAGTCTTAAGACAAG-3′ (NotI site and V76 underlined). The PCR product was fused in frame with Met cDNA using XmaI and NotI. The Hrs-HA construct has been described previously (28, 66). The glutathione S-transferase (GST)-Grb2 and GST-MBD constructs have been described previously (37, 45). GFP-Rab7 was a generous gift from Robert Lodge.
RESULTS
Loss of Cbl-mediated ubiquitination results in sustained tyrosine phosphorylation and enhanced stability of the Met RTK.
To study the in vivo relevance of loss of ubiquitination of the Met receptor, we established populations of T47D epithelial breast cancer cells expressing either the wild-type Met receptor or a Met receptor mutant (Y1003F) (Fig. (Fig.1B).1B). The Y1003F mutant does not associate with the Cbl TKB domain (Fig. (Fig.1A)1A) and is only weakly ubiquitinated in transient assays (46, 47). Unlike the majority of epithelial cells, T47D cells do not express detectable levels of endogenous Met receptor (58), providing a unique epithelial cell model in which to analyze the consequence of loss of Cbl ubiquitination. Stable cell populations were established that express wild-type Met and Met Y1003F mutant receptors (Fig. (Fig.1B).1B). The level of expression of the Met Y1003F mutant receptor in stable cell populations is systematically higher than that of the wild-type Met receptor even though Met RNA levels are similar (Fig. (Fig.1B1B and andCC).
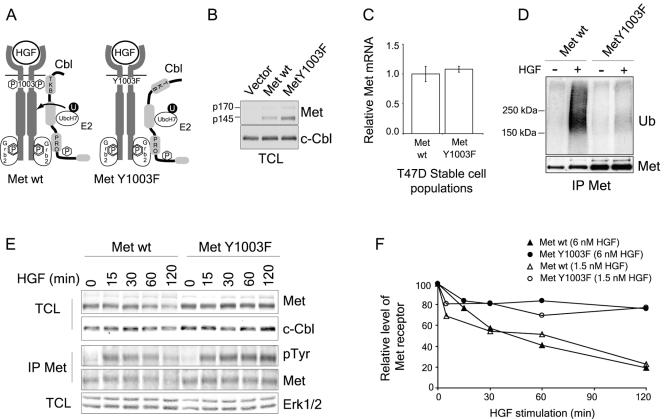
The Met Y1003F receptor mutant is poorly ubiquitinated and its degradation is delayed upon HGF stimulation. (A) Schematic representation of Cbl recruitment to the Met receptor. The Y1003F substitution abrogates binding of the Cbl TKB domain to the Met receptor. The Met Y1003F receptor mutant still recruits and tyrosine phosphorylates Cbl via the Grb2 adaptor. (B) Expression levels of the Met wild-type and Y1003F receptors in retrovirally infected stable T47D cell populations. After selection with neomycin for 2 weeks, cells expressing Met at their cell surface were sorted using FACS. Total cell lysates (TCL) were blotted with Met (antibody 144) and c-Cbl antibodies. The p170 band represents the uncleaved Met precursor and the p145 band represents the processed α chain of the receptor. (C) Met RNA expression levels from T47D cell populations were determined using quantitative real-time PCR. The graph represents the mean ± standard deviation of triplicate samples. (D) T47D cells were stimulated with 3 nM HGF for 2 min and lysed immediately in boiling 2% SDS. Lysates were boiled for 10 min, diluted to 0.4% SDS, 2% Triton and then Met receptor proteins were immunoprecipitated with antibody 144 and blotted with ubiquitin antibodies, stripped and reblotted with Met antibodies. (E) T47D cells were stimulated with either 6 nM HGF (top two panels) or 1.5 nM HGF (bottom three panels) for the indicated amount of time. Lysates were treated as indicated. (F) Met protein levels were quantified using the ImageJ 1.63 software and were corrected using Cbl (6 nM HGF) and Erk2 (1.5 nM HGF) protein levels.
Following stimulation with 3 nM HGF, the wild-type Met receptor is robustly ubiquitinated, whereas the Met Y1003F receptor mutant shows only low levels of ubiquitination (Fig. (Fig.1D).1D). Consistent with a role for ligand-induced ubiquitination in Met degradation, after 2 h of stimulation, the steady-state levels of the wild-type Met receptor are decreased by 75%, whereas the levels of the Met Y1003F receptor mutant are decreased by only 20% (Fig. (Fig.1E1E and andF).F). In addition to enhanced stability, tyrosine phosphorylation of the Met Y1003F receptor mutant is sustained up to 2 h following HGF stimulation, whereas tyrosine phosphorylation of the wild-type Met receptor is decreased within 30 min (Fig. (Fig.1E),1E), indicating that the Met Y1003F receptor, which is poorly ubiquitinated, induces prolonged signaling of downstream pathways.
Met Y1003F receptor mutant is transforming in vitro and in vivo.
The enhanced stability of the Met Y1003F mutant is reflected by its enhanced biological activity. T47D cell lines stably expressing the Met Y1003F receptor but not the wild-type Met receptor display loss of epithelial organization, a hallmark of epithelial cell transformation that is enhanced by HGF stimulation (Fig. (Fig.2A).2A). Furthermore, NIH 3T3 cells expressing a Met Y1003F receptor, when subcutaneously injected into nude mice, formed solid tumors (0.10 cm3) with short latencies (20 days), which reached a volume of 0.70 cm3 within 35 days. In contrast, control NIH 3T3 cells or cells expressing a wild-type Met receptor formed only a small mass of 0.10 cm3 by 35 days postinjection (Fig. (Fig.2B2B and andC).C). Although the cell populations injected into nude mice expressed similar amounts of Met proteins, the tumors that developed contained higher levels of Met Y1003F than wild-type Met proteins (Fig. (Fig.2C).2C). We conclude that the specific uncoupling of the Met receptor from Cbl-mediated ubiquitination is sufficient to increase both the in vitro and in vivo tumorigenicity of the Met receptor.

NIH 3T3 fibroblast cells expressing the ubiquitination-deficient Met Y1003F receptor are tumorigenic. (A) Met Y1003F-expressing cells have an altered morphology. Phase contrast pictures of T47D cells unstimulated or stimulated for 24 h with 1.5 nM HGF. (B) NIH 3T3 cell populations expressing either wild-type Met or Met Y1003F were injected subcutaneously into nude mice. The results represent the mean tumor volume obtained from eight measurements and are representative of two independent experiments. (C) Pictures of representative tumors as well as Met protein expression in the NIH 3T3 cell populations injected and in excised tumors. The tumors were lysed in TGH buffer and total cell lysates (TCL) were blotted with Met (DL-21) antibodies. Then, the membrane was stained with Coomassie brilliant blue.
Met Y1003F receptor preferentially promotes sustained activation of the Ras-MAPK pathway.
Several studies have indicated that recruitment of Cbl is required for the ubiquitination and ligand-induced down-regulation of the Met and EGF receptors (34, 35, 46, 47). However, reports on Met or EGF receptor mutants, uncoupled from Cbl-mediated ubiquitination, have failed to address the consequence of loss of ubiquitination on receptor signaling. To understand at the mechanistic level why the Met Y1003F receptor mutant is tumorigenic and promotes enhanced epithelial scattering, we examined its cellular signaling and trafficking in T47D cell populations stably expressing the Met wild-type or Y1003F receptor.
Signaling downstream from the Met receptor is mediated through the recruitment of the adaptor proteins Grb2 and Shc, which couple the Met receptor to the Ras signaling pathway, and the scaffold protein Gab1. Gab1 couples the Met receptor to the MAPK pathway as well as to phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ, Crk, and the tyrosine phosphatase SHP2 (29, 30, 41, 42). Consistent with the sustained phosphorylation of the Met Y1003F receptor, tyrosine phosphorylation of downstream signals, such as Gab1 and c-Cbl, are prolonged in cells expressing the Met Y1003F receptor (Fig. (Fig.3A).3A). Furthermore, Ras activation, as measured by an in vitro Ras binding assay, is transient (1 h) downstream from the wild-type Met receptor, while it is sustained downstream of the Met Y1003F receptor (4 h, Fig. Fig.3B).3B). Activation of signaling pathways downstream from Ras, MEK1/2, and Erk1/2, as detected using phosphorylation site-specific antibodies, showed a similar trend (Fig. (Fig.3B3B).
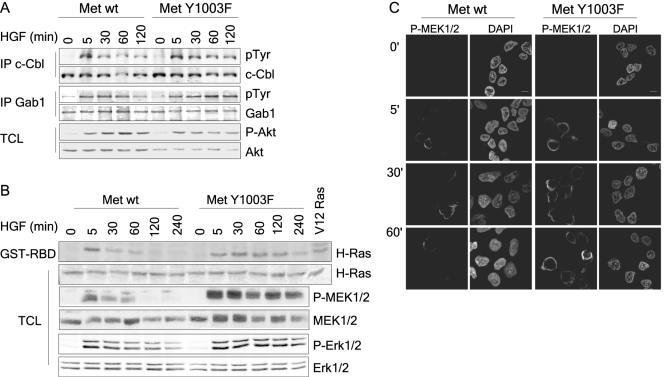
Activation of the Ras-MAPK but not the PI3K pathway is sustained downstream from Met Y1003F. (A) T47D cells were stimulated with 1.5 nM HGF for the indicated times and lysed in TGH buffer. In the upper two panels, c-Cbl proteins were immunoprecipitated and immunoblotted with phosphotyrosine antibodies. Membranes were stripped and blotted with Cbl antibodies. In the middle two panels, Gab1 proteins were immunoprecipitated and blotted with both antiphosphotyrosine, 4G10, and Gab1 antibodies using the Odyssey Infrared Imaging System (LI-COR). In the bottom two panels, lysates were blotted with either phospho-Ser473 or total Akt antibodies as indicated using the Odyssey system. (B) T47D cells were stimulated with 1.5 nM HGF and lysed as above. Upper two panels: Ras activation was determined using an in vitro binding assay with the GST-RBD fusion protein, with HEK293T cells expressing V12 H-Ras as a positive control. Panels were blotted with an H-Ras antibody using the Odyssey System. In the bottom four panels, cell lysates were blotted with phospho-Ser217/221 MEK1/2, total MEK1/2, phospho-Thr202/Tyr204 Erk1/2, and total Erk1/2 antibodies as indicated using the Odyssey system. (C) T47D cells were plated on coverslips, serum starved for 16 h, and stimulated with 1.5 nM HGF at 37°C for the indicated time points. Coverslips were fixed in 3% paraformaldehyde (PFA), and stained with phospho-Ser217/221 MEK1/2 (first panel). Cell nuclei were visualized using 4′,6′-diamidino-2-phenylindole (DAPI) (second panel). Confocal images were taken with a 100× objective and 2× zoom. The bar represents 5 μm.
HGF stimulation induces a robust but relatively transient activation of MEK1/2 (1 h) and Erk1/2 (decreased by 60 min) in T47D cells expressing a wild-type Met receptor, whereas activation of these proteins is sustained for 4 h in cells expressing the Met Y1003F receptor mutant (Fig. (Fig.3B).3B). Indirect immunofluorescence of activated MEK1/2 in T47D cell populations, using phosphorylation-specific antibodies, revealed a sustained and perinuclear signal in Met Y1003F-expressing cells compared to a transient and perinuclear signal in cells expressing the wild-type receptor (Fig. (Fig.3C),3C), demonstrating that the subcellular localization of the pMEK1/2 signal is not detectably different following sustained activation. Alternatively, in these cells, PI3K-dependent activation of Akt/PKB, as detected using a phospho-Ser473 antibody, is similar downstream from both the wild-type and Y1003F receptors (Fig. (Fig.3A),3A), revealing a selective enhancement of the Ras signaling pathway.
The requirement of the Ras-MAPK and PI3K-dependent signals for biological activity of the Met Y1003F receptor were examined in T47D and MDCK epithelial cells. These cells undergo an epithelial-mesenchymal like transition following stable expression of the Met Y1003F receptor (Fig. (Fig.2A2A and and4)4) (46). Treatment of fibroblast-like MDCK and T47D cells expressing Met Y1003F with a pharmacological inhibitor of MEK1/2 (U0126) induces a reversal in cell morphology, and cells begin to form tight colonies 24 h posttreatment, in the presence or absence of ligand (Fig. (Fig.44 and data not shown). In contrast, minimal changes in the fibroblast-like morphology of the Met Y1003F-expressing cells were observed after treatment with an inhibitor of PI3K (LY294002) (Fig. (Fig.44 and data not shown). This demonstrates a specific requirement for the Ras-MAPK pathway in the maintenance of the epithelial scattered phenotype induced by the Met Y1003F receptor mutant.
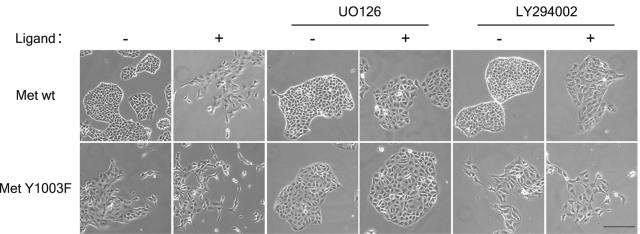
Ras-MAPK is required for Met Y1003F increased biological activity. MDCK cells stably expressing either wild-type CSF-Met or CSF-Met Y1003F, where indicated, were treated with CSF (2.7 nM), UO126 (20 μM) and LY294002 (25 μM) for 24 h. After 24 h, phase-contrast pictures of live cells were taken with a Zeiss Axiovision 135 microscope under a 10× objective. The bar represents 100 μm.
Wild-type and Y1003F Met receptors internalize with similar kinetics.
The enhanced stability of the Met Y1003F receptor may indicate that this receptor is retained on the cell surface. Although several reports support the ability of ubiquitination to promote the internalization of cell surface receptors in yeast (20), whether ubiquitination of RTKs is directly involved in their internalization has only been examined for EGFR and remains controversial (10, 16, 24, 34, 62, 68). To address the requirement for Met receptor ubiquitination for its internalization, we compared internalization of the Met wild-type and Y1003F receptors in T47D cells, using trypsin and flow cytometry-based assays. Following HGF stimulation, the amount of internalized, trypsin-protected, receptors increases at a similar rate for Met wild-type and Y1003F (Fig. (Fig.5A).5A). In addition, using flow cytometry, we observed that the wild-type and Y1003F Met receptors are internalized at similar rates following stimulation (Fig. (Fig.5B).5B). In agreement with this, by immunofluorescence microscopy, both wild-type and Y1003F Met mutant receptors internalize and colocalize with the early endosome marker EEA1, 5 min post-HGF treatment, and this colocalization is enhanced at 15 and 30 min (Fig. (Fig.5C).5C). These results demonstrate that there is no significant difference between the rate of internalization and subcellular localization of wild-type Met and Met Y1003F receptors.
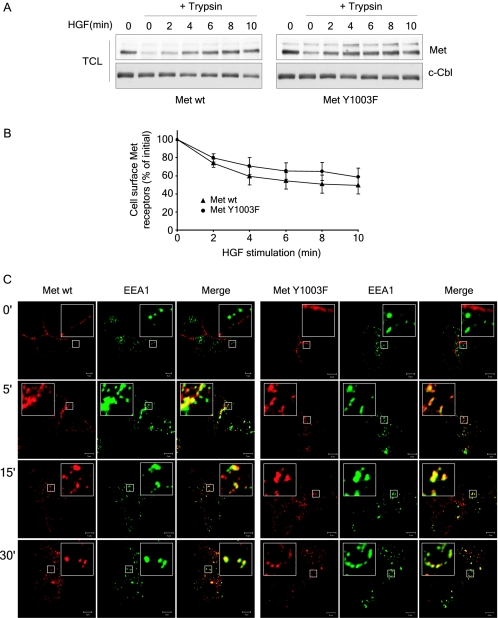
Ubiquitination-deficient Met receptor mutant is internalized. (A) T47D cells were stimulated with 3 nM HGF at 37°C and then transferred on ice. Cells were acidified for 10 min and then treated with trypsin for 30 min. Trypsin was inhibited before cells were lysed. Lysates were blotted with Met (DL-21) and c-Cbl antibodies. (B) T47D cells were stimulated with 3 nM HGF at 37°C and then transferred on ice. Cells were acidified for 10 min and then labeled with Met AF276 antibody as described under Materials and Methods. The mean fluorescence intensity per cell was measured by flow cytometry and the percentage of Met receptors remaining at the cell surface over time is plotted. The graph represents the mean ± standard deviation of three independent experiments. (C) Both wild-type Met and Met Y1003F internalize and traffic to early endosomes. T47D cells were plated on coverslips, and 16 h later stimulated with 1.5 nM HGF at 37°C for the indicated times. Coverslips were fixed in 3% PFA and stained with Met AF276 (red) and EEA1 (green) antibodies. Confocal images were taken with a 100× objective and 2× zoom. Yellow staining represents colocalization between Met and EEA1. The bar represents 5 μm.
Met RTK ubiquitination is required for Hrs phosphorylation but not for trafficking to Hrs-positive endosomes.
The enhanced stability of the Met Y1003F receptor could also indicate that the endosomal sorting machinery does not recognize the poorly ubiquitinated Met Y1003F receptor, a process thought to be required for efficient targeting of the receptor to the sorting endosome. Hrs, one member of the endosomal sorting machinery, was first identified as a tyrosine phosphorylated protein downstream from the Met receptor (18, 27). Therefore, as a readout for Met receptor recruitment by endosomal sorting proteins, we examined whether the Met Y1003F receptor mutant can phosphorylate Hrs. Following stimulation of T47D cells with HGF, the endogenous Hrs protein is tyrosine phosphorylated upon activation of a wild-type Met receptor, but is not detectably phosphorylated in cells expressing the Met Y1003F receptor (Fig. (Fig.6A6A).
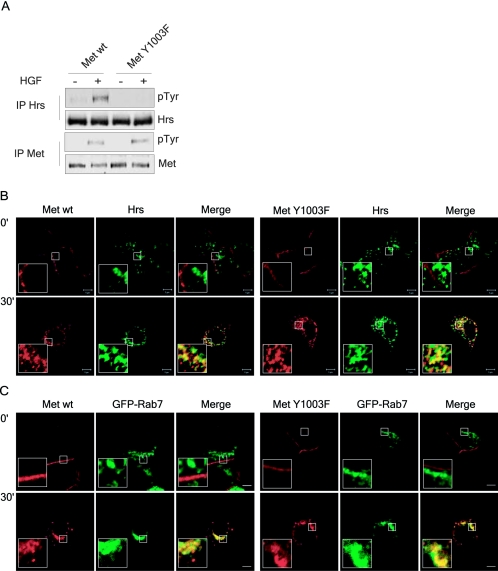
Ubiquitination-deficient Met receptor mutant is unable to induce Hrs tyrosine phosphorylation. (A) T47D cells were serum starved for 16 h and stimulated where indicated with 1.5 nM HGF for 8 min. Cells were then lysed in TGH buffer and Hrs and Met proteins were immunoprecipitated and first blotted with phosphotyrosine antibodies, stripped, and reblotted with Hrs and Met antibodies as indicated. (B and C) Wild-type Met and Met Y1003F both colocalize with endogenous Hrs and transfected GFP-Rab7. T47D cells, nontransfected (B) and transfected (C), were plated on coverslips and 16 h later stimulated with 1.5 nM HGF at 37°C for the indicated times. Coverslips were fixed with 3% PFA and stained with (B and C) Met AF276 (red) and (B) Hrs (green) antibodies. Yellow staining represents colocalization between Met and Hrs or Met and GFP-Rab7. Confocal images were taken with a 100× objective and 2× zoom. The bar represents 5 μm.
To determine if the inability of the Met Y1003F mutant receptor to induce Hrs phosphorylation reflects its inability to reach an Hrs positive endosome, we examined the subcellular localization of both Met wild-type and Y1003F receptors with either endogenous or transiently transfected Hrs. As observed by indirect immunofluorescence, both the Met wild-type and Y1003F receptors traffic to Hrs-positive endosomes (Fig. (Fig.6B6B and data not shown). Moreover, this occurs with similar kinetics, where both the wild-type and Y1003F Met receptors begin to localize with either endogenous or GFP-tagged Hrs as early as 5 min post-HGF stimulation (data not shown) and colocalization increases up to 30 min post-HGF stimulation (Fig. (Fig.6B6B and data not shown). At the latter time point (30 min), both receptors also begin to localize to GFP-Rab7-positive endosomes (a marker of multivesicular bodies/late endosomes) (Fig. (Fig.6C)6C) (11, 67). Hence, although the ubiquitination-deficient Met Y1003F receptor mutant reaches Hrs and Rab7-positive endosomes, it does not induce Hrs tyrosine phosphorylation. This demonstrates that Cbl-mediated ubiquitination of the Met receptor is not essential for its targeting to Hrs- and Rab7-positive endosomes, but is required to induce Hrs tyrosine phosphorylation.
Monoubiquitination of Met Y1003F decreases its stability and rescues Hrs phosphorylation.
The addition of monoubiquitin to the transferrin receptor results in its partial exclusion from the recycling compartment and its retention within late endosomes (51). Similarly, the fusion of ubiquitin to the carboxy terminus of a severely truncated EGFR was sufficient for this receptor to traffic to late endosomes (17). However, the ability of these proteins to interact with components of the cargo sorting pathway and their stability was not examined. To establish whether the addition of monoubiquitin to the Met Y1003F receptor can rescue its targeting to the lysosomal degradative pathway and if this enhances its ability to be recruited to the endosomal sorting complex containing Hrs, we generated Met-ubiquitin receptor chimeras.
We fused a single ubiquitin moiety that cannot form ubiquitin chains, where the seven lysine residues in ubiquitin are replaced with arginine residues (59), to the carboxy terminus of the full-length wild-type and Y1003F Met receptors (Fig. (Fig.7A).7A). Following transient overexpression in HEK 293 cells, where the Met receptor is activated in a ligand-independent manner, both proteins are detected as a discrete band with an antibody raised against ubiquitin (Fig. (Fig.7B).7B). Moreover, both proteins associate with known receptor binding proteins to similar levels. Both the Grb2 adaptor protein and the Met binding domain (MBD) of Gab1 associate with the chimeric receptors in in vitro binding assays, demonstrating that the chimeric receptors are properly folded, tyrosine phosphorylated and can interact with signaling proteins (Fig. (Fig.7B).7B). In stable T47D cell populations, the chimeric receptors are expressed at the cell surface, as determined by flow cytometry (data not shown) and by immunofluorescence microscopy (Fig. (Fig.7C).7C). Upon HGF stimulation, both chimeric receptors internalize and colocalize with EEA1-positive endosomes (Fig. (Fig.7C7C).
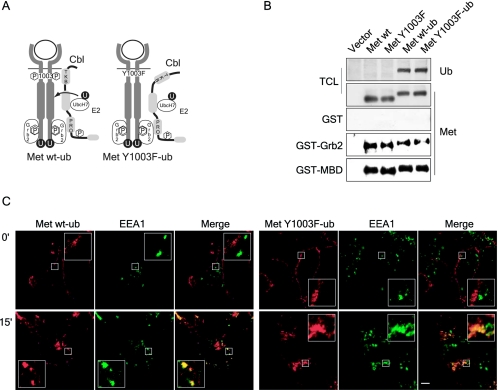
Fusion of monoubiquitin to the carboxy terminus of the Met receptor does not alter its maturation to the cell surface, recruitment of signaling proteins and its HGF-induced internalization. (A) Schematic representation of ubiquitinated wild-type and Y1003 Met chimeric receptors. Met receptor chimeras were generated with a single ubiquitin moiety fused to the carboxy-terminal end of the full-length wild-type and Y1003F Met receptors. The seven lysine residues within the ubiquitin moiety were replaced with arginine residues to prevent polyubiquitination and the carboxy-terminal glycine residue was replaced with a valine residue to prevent conjugation to free amino groups. (B) The chimeric receptors are properly expressed and tyrosine phosphorylated. HEK 293 cells were transiently transfected with the indicated CSF-Met constructs. In the top two panels, lysates were blotted with Met (antibody 144) and ubiquitin antibodies. In the bottom three panels, lysates were incubated with GST alone, GST-Grb2, or GST-MBD (Met binding domain of Gab1). In vitro binding assays and total cell lysates (TCL) were immunoblotted for Met. (C) T47D cells were plated on coverslips and 16 h later stimulated with 1.5 nM HGF at 37°C for 15 min. Coverslips were fixed in 3% PFA and stained with Met AF276 (red) and EEA1 (green) antibodies. Confocal images were taken with a 100× objective and 2× zoom. Yellow staining represents colocalization between Met and EEA1. The bar represents 5 μm.
When examined for stability, the Met Y1003F receptor mutant is stable for up to 6 h after stimulation with HGF, whereas the ubiquitinated Met Y1003 chimeric receptor is degraded after 2 h of stimulation (Fig. (Fig.8A),8A), in a manner similar to the wild-type Met protein (Fig. (Fig.1E).1E). Consistent with this, the prolonged activation of the Ras-MAPK signaling pathway observed downstream of the Met Y1003F receptor (4 h, Fig. Fig.8B)8B) is reversed by the addition of monoubiquitin to this receptor. MEK1/2 activation downstream from ubiquitinated Met Y1003 is transient (60 min, Fig. Fig.8B),8B), as it is downstream of the wild-type Met receptor (Fig. (Fig.3B).3B). These data indicate that the fusion of monoubiquitin to the Met receptor is sufficient to reverse the prolonged stability and signaling of the Met Y1003F receptor mutant. This suggests that monoubiquitination is sufficient to engage the Met Y1003F receptor mutant with components of the cargo-sorting pathway.
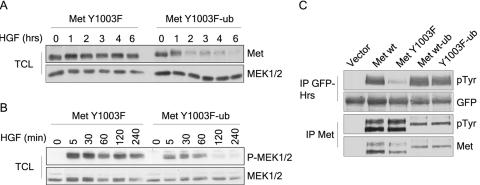
Monoubiquitination of the Met Y1003F receptor leads to its degradation and restores HGF-induced Hrs tyrosine phosphorylation. (A) T47D cells treated with 100 μg/ml cycloheximide were stimulated with 3 nM HGF for the indicated amount of time. Total cell lysates (TCL) (30 μg) were immunoblotted for Met (DL-21) and Erk. (B) T47D cells were stimulated with 1.5 nM HGF for the indicated times and lysed in TGH buffer. Cell lysates were blotted with phospho-Ser217/221 MEK1/2 and total MEK1/2. (C) HEK293 cells were cotransfected with GFP-Hrs and vector or the indicated CSF-Met constructs. GFP-Hrs and Met proteins were immunoprecipitated and blotted with phosphotyrosine antibodies, then stripped and reblotted for GFP and Met, respectively.
To test this, we examined its ability to induce phosphorylation of Hrs in HEK 293 transient transfection assays, where Met is constitutively activated due to overexpression. We observed that while the Met Y1003F receptor is severely reduced in its ability to induce robust tyrosine phosphorylation of Hrs compared with a wild-type Met receptor, the ubiquitinated Met Y1003 chimeric receptor induces robust Hrs phosphorylation (Fig. (Fig.8C).8C). Hence, this demonstrates that monoubiquitination of the Met Y1003F receptor is sufficient to target the receptor for degradation and for it to phosphorylate Hrs, a component of the cargo-sorting machinery.
Monoubiquitination of the Met RTK suppresses its transforming activity.
Since uncoupling the Met receptor from Cbl-dependent ubiquitination results in enhanced stability, signaling, and cell transformation, we examined the ability of the Met-ubiquitin chimeric receptors to transform Rat-1 fibroblasts using a focus-forming assay. The addition of a monoubiquitin moiety to the Met Y1003F receptor mutant decreases its transforming activity by 60% (Fig. (Fig.9).9). Moreover, the addition of monoubiquitin to a wild-type Met receptor reduces its transforming activity in the presence of ligand by 90% (Fig. (Fig.9).9). Thus, monoubiquitination of the Met RTK is sufficient to suppress the oncogenic activity of both the Met wild-type and Y1003F receptors. Altogether, fusion of monoubiquitin to the Met receptor rescues the phenotypes associated with the Y1003F mutation, namely, increased Met stability, prolonged MEK activation, loss of Hrs phosphorylation, and Met oncogenic activation (Fig. (Fig.88 and and9).9). Hence, this demonstrates that these phenotypes result from the loss of ubiquitination of the Met Y1003F receptor and are unlikely to be caused by another mechanism.
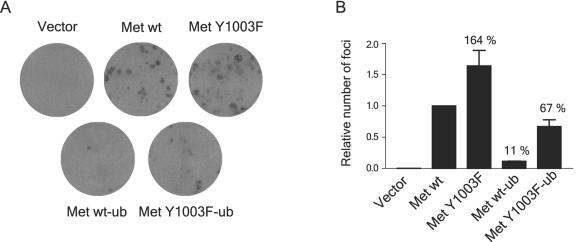
Monoubiquitination of the Met Y1003F receptor mutant is sufficient to reverse cell transformation. Rat-1 fibroblast cells were infected with retroviruses encoding the various CSF-Met constructs and grown for 3 weeks in the presence of 0.3 nM of colony-stimulating factor 1. (A) Representative pictures of cell monolayers. (B) The graph represents the relative number of foci per dish corrected for the PFU of each retrovirus, from two independent experiments.
DISCUSSION
In this study, we provide a mechanistic basis for the oncogenic activation of the Met RTK through its uncoupling from ubiquitination. We demonstrate that the specific uncoupling of the Met RTK from Cbl-mediated ubiquitination (Met Y1003F) promotes oncogenic activation of Met in vivo and in vitro (Fig. (Fig.2).2). Moreover, we show that cell transformation by the Met Y1003F receptor mutant or a ligand-activated wild-type Met receptor can be reversed through the fusion of monoubiquitin to these receptors (Fig. (Fig.9),9), demonstrating the importance of ubiquitination as a mechanism to suppress the inherent oncogenic activity of deregulated RTKs.
When uncoupled from Cbl-mediated ubiquitination, the Met receptor, as shown for the EGFR (16, 23), has a prolonged half-life (Fig. 1D and E), which leads to elevated Met protein levels in the stable cell populations and in the tumors (Fig. (Fig.1B1B and and2C).2C). This supports a previous study where ubiquitination of the Met receptor was shown to correlate with ligand-induced degradation (22). Importantly, the enhanced stability of Met is reversed in stable cell lines following the fusion of monoubiquitin to the carboxy terminus of the Met Y1003F mutant receptor (Fig. (Fig.8A).8A). This provides direct evidence that monoubiquitin is sufficient to target the Met receptor to a degradative pathway. This is in agreement with the observation that monoubiquitination of EGFR is sufficient to target this receptor for degradation (44).
Although proteasomal inhibitors inhibit Met receptor degradation and appears to promote its recycling to the cell surface (18), it remained unknown whether ubiquitination of the Met receptor itself is important for its internalization and trafficking. We demonstrate here that the inability of the Met Y1003F receptor mutant to become ubiquitinated by Cbl does not alter its ability to internalize or enter the endocytic pathway in response to ligand. Using several approaches, we show that both the Met wild-type and Y1003F receptors are internalized, enter the endocytic pathway, and can traffic to endosomes that contain the cargo sorting protein, Hrs (Fig. (Fig.55 and and6B)6B) and late endosomes containing Rab7 (Fig. (Fig.6C).6C). This indicates that signals other than ubiquitination can promote ligand-induced Met receptor internalization. These may involve Grb2 (23), the adaptor role of Cbl (49, 61), as well as Cbl-mediated ubiquitination of proteins other than Met (62), since Cbl is still recruited to the Met Y1003F receptor indirectly via Grb2 (46).
Monoubiquitination of a severely truncated or full-length EGFR was shown to be sufficient for constitutive internalization when overexpressed in transient assays (17, 44). We observed that in stable cell populations, the constitutively monoubiquitinated Met wild-type and Y1003F chimeric receptors are mainly localized at the cell surface and do not colocalize with EEA1 in the absence of stimulation (Fig. (Fig.7C).7C). Hence, monoubiquitination of the Met RTK is not sufficient for its internalization, which remains ligand-dependent. This may reflect differences between transient overexpression versus stable cell lines, where receptor activation is dependent on ligand. Alternatively, a monoubiquitin moiety on a truncated EGFR lacking its entire cytosolic domain may be recognized in a different manner than a monoubiquitin moiety fused to a full-length Met RTK. This highlights the need to fully understand the interplay between monoubiquitin and other signals involved in internalization, which may be specific for each receptor.
The ubiquitination of lysine residues in Met could control the interaction of Met with endosomal sorting proteins that contain ubiquitin interacting domains, such as Hrs. Although Hrs is phosphorylated following stimulation of cells with HGF or EGF (27), a requirement for the direct ubiquitination of RTKs for Hrs phosphorylation had not been tested. Our data demonstrate that the ability of Met to promote phosphorylation of Hrs is dependent on ubiquitination of the Met receptor. Compared to wild-type Met, the Met Y1003F receptor mutant fails to robustly phosphorylate Hrs (Fig. (Fig.6A).6A). Importantly, this can be rescued through the fusion of monoubiquitin to the carboxy terminus of the Met Y1003F receptor (Fig. (Fig.8C).8C). Hence, our data reveal that both the stability of Met and the phosphorylation of Hrs are dependent on the direct ubiquitination of the Met receptor. Interestingly, recent data from Row et al. have established that the Hrs ubiquitin-interacting motif domain is required for Hrs phosphorylation downstream from the Met receptor (55). Together, this provides support for a model where the interaction of a ubiquitinated Met RTK with the sorting machinery, involving Hrs, is important for the targeting of the Met receptor for efficient lysosomal degradation. Consistent with this, the specific depletion of Hrs with siRNA enhances the stability of the Met receptor (18).
In addition to enhanced stability, the Met Y1003F receptor also has prolonged tyrosine phosphorylation following ligand stimulation. This results in sustained activation of the Ras-MAPK pathway, whereas no significant difference was observed in the activation of Akt, a downstream target of PI3K (Fig. (Fig.3).3). In agreement with this, the transforming and scatter activities of the Met Y1003F receptor are dependent on the activation of MEK1/2 but not PI3K (Fig. (Fig.4).4). Whereas activation of Akt is thought to occur at the plasma membrane, the MAPK pathway can be activated from several subcellular compartments, including endosomes (63). Our results are consistent with the interpretation that the enhanced signaling of the Met Y1003F receptor, in particular to the Ras-MAPK pathway, reflects a receptor that is not efficiently targeted to the sorting machinery through its inability to couple to Hrs, and that instead remains in a signaling-competent endosomal compartment.
A decrease in ubiquitination of both the Met (31, 46, 48) and EGF RTKs (3, 69, 70), mediated through multiple mechanisms, has been associated with their oncogenic activation in cell-based models. Moreover, the observations that a Met receptor mutant identified in human lung cancer is missing exon 14, which contains the Cbl binding site (38), and that several proteins involved in RTK internalization and lysosomal degradation are altered in human tumors and transformed cells (2, 14) strongly support the notion that loss of RTK down-regulation is a common mechanism for oncogenic activation of RTKs in human tumors. Our studies now raise the need to understand how ubiquitination and altered trafficking of other RTKs impacts on their signaling and oncogenic activity.
Acknowledgments
We thank Marisa Ponzo and Naila Chughtai for help with transient transfection assays and Dongmei Zuo and Laurent Sansregret for help with confocal imaging and flow cytometry, respectively. We are grateful to members of the Park laboratory for critical comments on the manuscript and Stephane Laporte for his helpful discussion. We thank Harald Stenmark for providing us with Hrs antibodies, Linda Hicke for the ubiquitin (7KtoR) plasmid, George Vande Woude for HGF, and Robert Lodge for GFP-Rab7.
J.A. is a recipient of an Alexander McFee studentship and P. P. is a recipient of a Terry Fox research studentship from National Cancer Institute of Canada. Sylvie Urbé is a Wellcome Trust Career Development Fellow. M.P. is recipient of a Canadian Institute of Health Research (CIHR) senior scientist award. This research was supported by an operating grant to M.P. from the CIHR.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.25.21.9632-9645.2005
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1265818?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Conserved regulatory motifs in the juxtamembrane domain and kinase N-lobe revealed through deep mutational scanning of the MET receptor tyrosine kinase domain.
Elife, 12:RP91619, 13 Sep 2024
Cited by: 6 articles | PMID: 39268701 | PMCID: PMC11398868
A tripartite organelle platform links growth factor receptor signaling to mitochondrial metabolism.
Nat Commun, 15(1):5119, 15 Jun 2024
Cited by: 0 articles | PMID: 38879572 | PMCID: PMC11180189
Signaling pathways in liver cancer: pathogenesis and targeted therapy.
Mol Biomed, 5(1):20, 31 May 2024
Cited by: 2 articles | PMID: 38816668 | PMCID: PMC11139849
Review Free full text in Europe PMC
Spatiotemporal regulation of the hepatocyte growth factor receptor MET activity by sorting nexins 1/2 in HCT116 colorectal cancer cells.
Biosci Rep, 44(6):BSR20240182, 01 Jun 2024
Cited by: 1 article | PMID: 38836326 | PMCID: PMC11196213
Recording and classifying MET receptor mutations in cancers.
Elife, 13:e92762, 23 Apr 2024
Cited by: 1 article | PMID: 38652103 | PMCID: PMC11042802
Review Free full text in Europe PMC
Go to all (128) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Epidermal growth factor receptor fate is controlled by Hrs tyrosine phosphorylation sites that regulate Hrs degradation.
Mol Cell Biol, 27(3):888-898, 13 Nov 2006
Cited by: 45 articles | PMID: 17101784 | PMCID: PMC1800687
Endosomal dynamics of Met determine signaling output.
Mol Biol Cell, 14(4):1346-1354, 01 Apr 2003
Cited by: 74 articles | PMID: 12686592 | PMCID: PMC153105
A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation.
J Biol Chem, 279(28):29565-29571, 29 Apr 2004
Cited by: 77 articles | PMID: 15123609
Met receptor: a moving target.
Sci Signal, 4(190):pe40, 06 Sep 2011
Cited by: 24 articles | PMID: 21917713
Review





