Abstract
Free full text

Starvation-Survival Processes of a Marine Vibrio †
Abstract
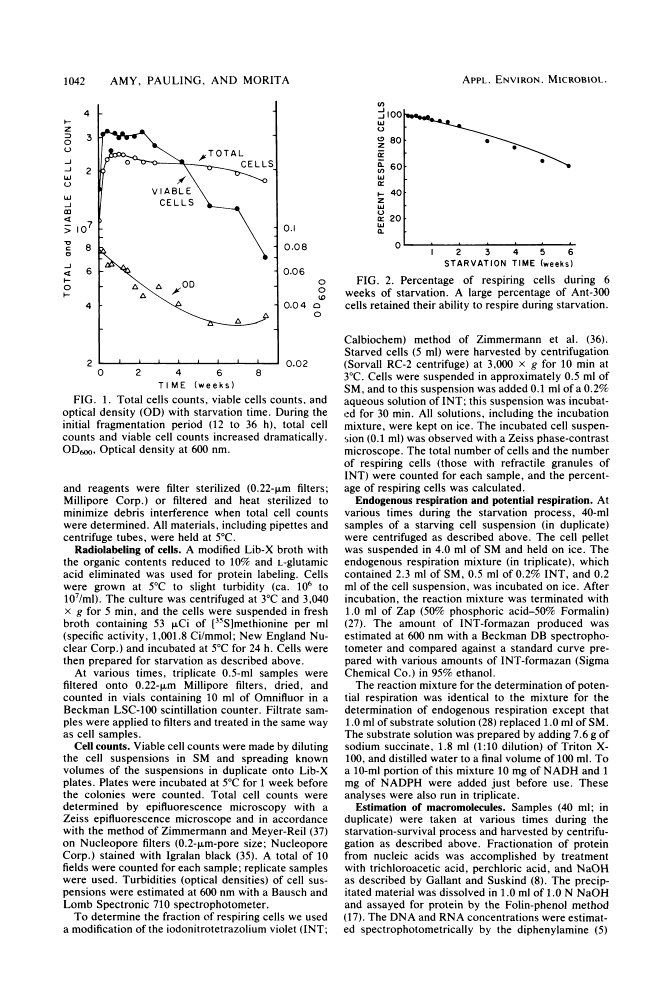
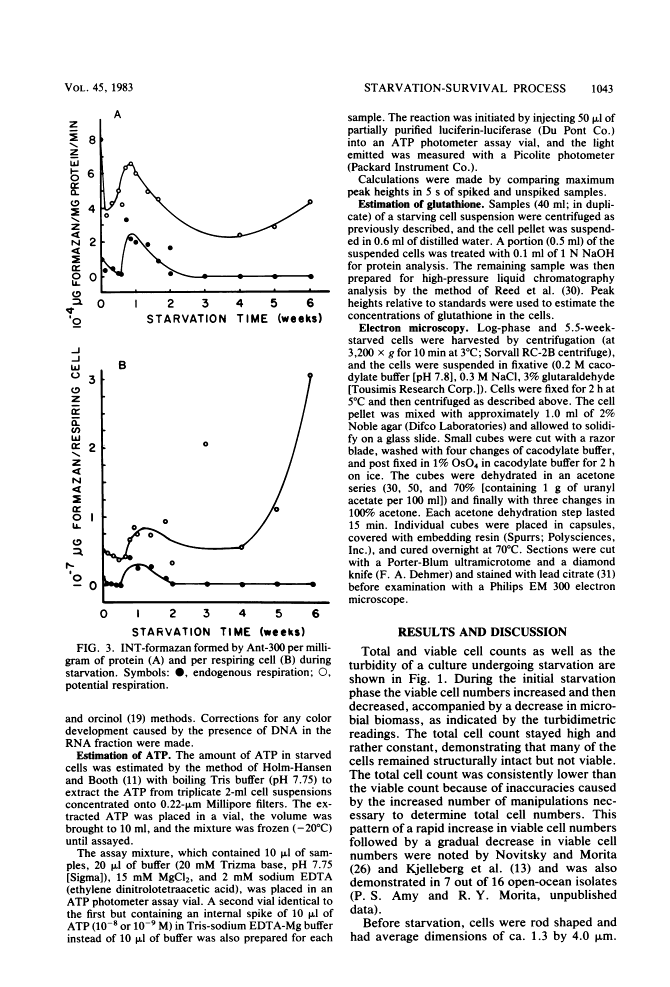
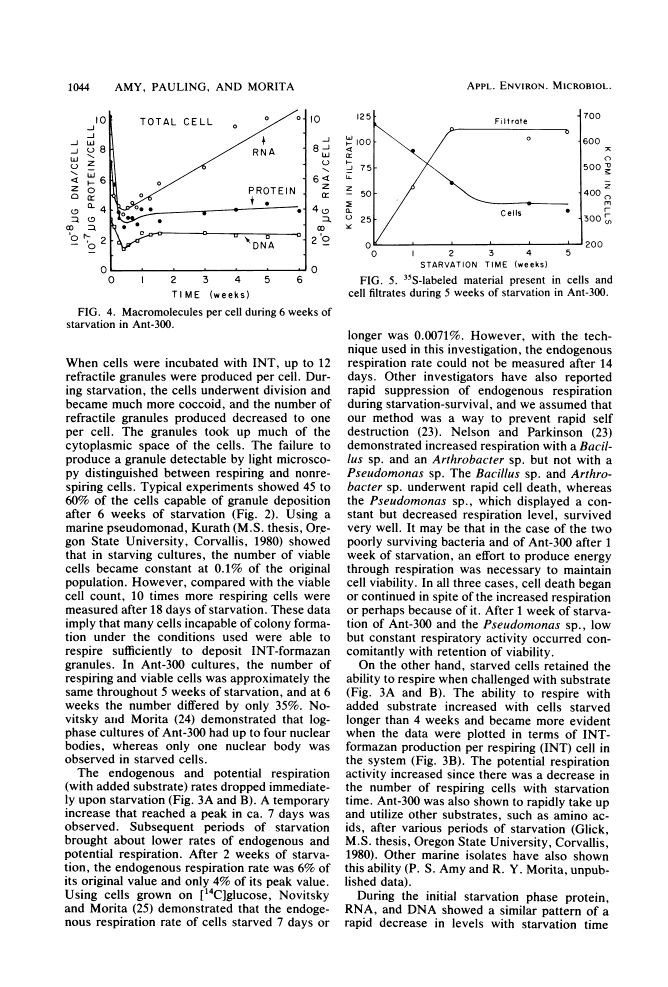
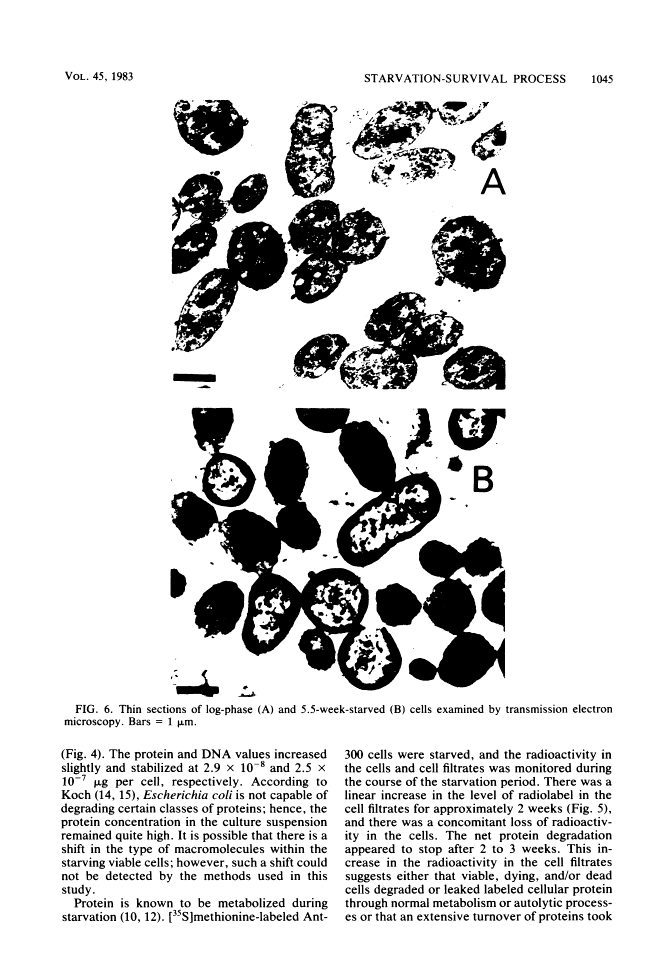
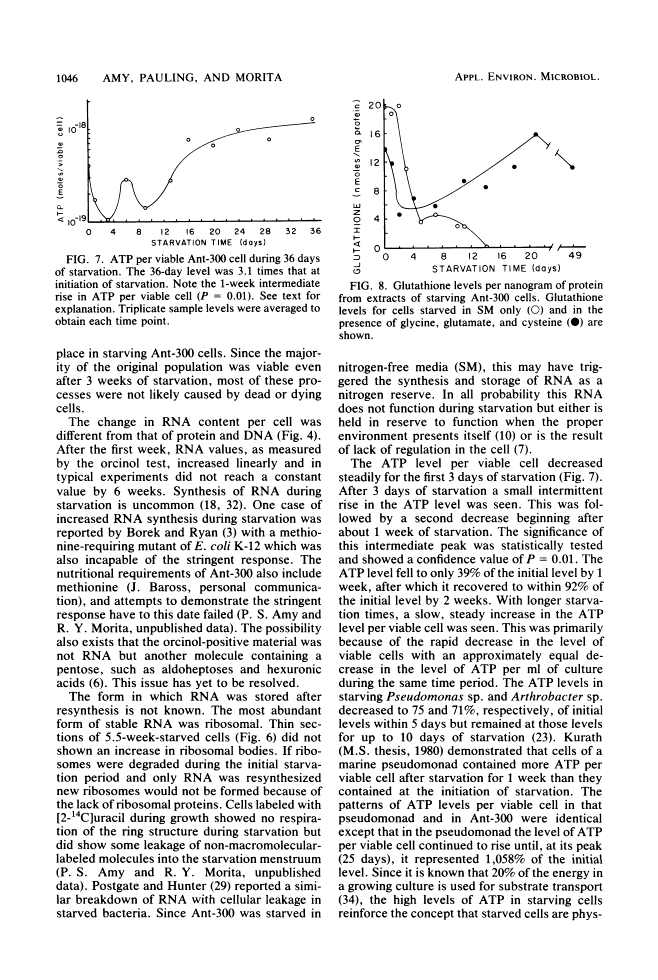
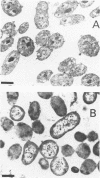
Levels of DNA, RNA, protein, ATP, glutathione, and radioactivity associated with [35S]methionine-labeled cellular protein were estimated at various times during the starvation-survival process of a marine psychrophilic heterotrophic Vibrio sp., Ant-300. Values for the macromolecules were analyzed in terms of total, viable, and respiring cells. Electron micrographs (thin sections) were made on log-phase and 5.5-week-starved cells. On a per-cell basis, the levels of protein and DNA rapidly decreased until a constant level was attained. A second method in which radioactive sulfur was used for monitoring protein demonstrated that the cellular protein level decreased for approximately 2.5 weeks and then remained constant. An initial decrease in the RNA level with starvation was noted, but with time the RNA (orcinol-positive material) level increased to 2.5 times the minimum level. After 6 weeks of starvation, 45 to 60% of the cells remained capable of respiration, as determined by iodonitrotetrazolium violet-formazan granule production. Potential respiration and endogenous respiration levels fell, with an intervening 1-week peak, until at 2 weeks no endogenous respiration could be measured; respiratory potential remained high. The cell glutathione level fell during starvation, but when the cells were starved in the presence of the appropriate amino acids, glutathione was resynthesized to its original level, beginning after 1 week of starvation. The cells used much of their stored products and became ultramicrocells during the 6-week starvation-survival process. Ant-300 underwent many physiological changes in the first week of starvation that relate to the utilization or production of ATP. After that period, a stable pattern for long-term starvation was demonstrated.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber RT. Dissolved organic carbon from deep waters resists microbial oxidation. Nature. 1968 Oct 19;220(5164):274–275. [Abstract] [Google Scholar]
- BOREK E, RYAN A. Studies on a mutant of Escherichia coli with unbalanced ribonucleic acid synthesis. II. The concomitance of ribonucleic acid synthesis with resumed protein synthesis. J Bacteriol. 1958 Jan;75(1):72–76. [Europe PMC free article] [Abstract] [Google Scholar]
- Boylen CW, Ensign JC. Intracellular substrates for endogenous metabolism during long-term starvation of rod and spherical cells of Arthrobacter crystallopoietes. J Bacteriol. 1970 Sep;103(3):578–587. [Europe PMC free article] [Abstract] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. [Europe PMC free article] [Abstract] [Google Scholar]
- Gallant JA. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. [Abstract] [Google Scholar]
- GALLANT J, SUSKIND SR. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. [Europe PMC free article] [Abstract] [Google Scholar]
- Geesey GG, Morita RY. Capture of arginine at low concentrations by a marine psychrophilic bacterium. Appl Environ Microbiol. 1979 Dec;38(6):1092–1097. [Europe PMC free article] [Abstract] [Google Scholar]
- GRONLUND AF, CAMPBELL JJ. NITROGENOUS SUBSTRATES OF ENDOGENOUS RESPIRATION IN PSEUDOMONAS AERUGINOSA. J Bacteriol. 1963 Jul;86:58–66. [Europe PMC free article] [Abstract] [Google Scholar]
- Christian RR, Hanson RB, Newell SY. Comparison of methods for measurement of bacterial growth rates in mixed batch cultures. Appl Environ Microbiol. 1982 May;43(5):1160–1165. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch AL. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. [Abstract] [Google Scholar]
- LAW JH, SLEPECKY RA. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. [Europe PMC free article] [Abstract] [Google Scholar]
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [Abstract] [Google Scholar]
- Nathan CF, Arrick BA, Murray HW, DeSantis NM, Cohn ZA. Tumor cell anti-oxidant defenses. Inhibition of the glutathione redox cycle enhances macrophage-mediated cytolysis. J Exp Med. 1981 Apr 1;153(4):766–782. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson LM, Parkinson D. Effect of starvation on survival of three bacterial isolates from an arctic soil. Can J Microbiol. 1978 Dec;24(12):1460–1467. [Abstract] [Google Scholar]
- Novitsky JA, Morita RY. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. [Europe PMC free article] [Abstract] [Google Scholar]
- Novitsky JA, Morita RY. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. [Europe PMC free article] [Abstract] [Google Scholar]
- POSTGATE JR, HUNTER JR. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. [Abstract] [Google Scholar]
- Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980 Jul 15;106(1):55–62. [Abstract] [Google Scholar]
- REYNOLDS ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. [Europe PMC free article] [Abstract] [Google Scholar]
- SCHAECHTER M. Patterns of cellular control during unbalanced growth. Cold Spring Harb Symp Quant Biol. 1961;26:53–62. [Abstract] [Google Scholar]
- Stouthamer AH. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. [Abstract] [Google Scholar]
- Watson SW, Novitsky TJ, Quinby HL, Valois FW. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. [Europe PMC free article] [Abstract] [Google Scholar]
- Zimmermann R, Iturriaga R, Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.45.3.1041-1048.1983
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/45/3/1041.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/45/3/1041
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/45/3/1041
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aem.45.3.1041-1048.1983
Article citations
Marine bacteria Alteromonas spp. require UDP-glucose-4-epimerase for aggregation and production of sticky exopolymer.
mBio, 15(8):e0003824, 03 Jul 2024
Cited by: 0 articles | PMID: 38958440 | PMCID: PMC11325263
Increased intracellular persulfide levels attenuate HlyU-mediated hemolysin transcriptional activation in Vibrio cholerae.
J Biol Chem, 299(9):105147, 09 Aug 2023
Cited by: 2 articles | PMID: 37567478 | PMCID: PMC10509353
Bacterial transcription during growth arrest.
Transcription, 12(4):232-249, 01 Aug 2021
Cited by: 6 articles | PMID: 34486930 | PMCID: PMC8632087
Review Free full text in Europe PMC
A Pure Life: The Microbial Ecology of High Purity Industrial Waters.
Microb Ecol, 76(1):9-18, 15 Feb 2016
Cited by: 3 articles | PMID: 26879941
Review
Starvation-Survival in Haloarchaea.
Life (Basel), 5(4):1587-1609, 12 Nov 2015
Cited by: 7 articles | PMID: 26569313 | PMCID: PMC4695838
Go to all (46) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Survival of a psychrophilic marine Vibrio under long-term nutrient starvation.
Appl Environ Microbiol, 33(3):635-641, 01 Mar 1977
Cited by: 80 articles | PMID: 16345219 | PMCID: PMC170737
Recovery from nutrient starvation by a marine Vibrio sp.
Appl Environ Microbiol, 45(5):1685-1690, 01 May 1983
Cited by: 30 articles | PMID: 6191662 | PMCID: PMC242516
Effect of growth rate and starvation-survival on cellular DNA, RNA, and protein of a psychrophilic marine bacterium.
Appl Environ Microbiol, 55(10):2710-2716, 01 Oct 1989
Cited by: 21 articles | PMID: 16348037 | PMCID: PMC203148
Starvation-Survival Physiological Studies of a Marine Pseudomonas sp.
Appl Environ Microbiol, 45(4):1206-1211, 01 Apr 1983
Cited by: 45 articles | PMID: 16346265 | PMCID: PMC242440