Abstract
Free full text

Maintenance of Intracellular pH and Acid Tolerance in Rhizobium meliloti
Abstract
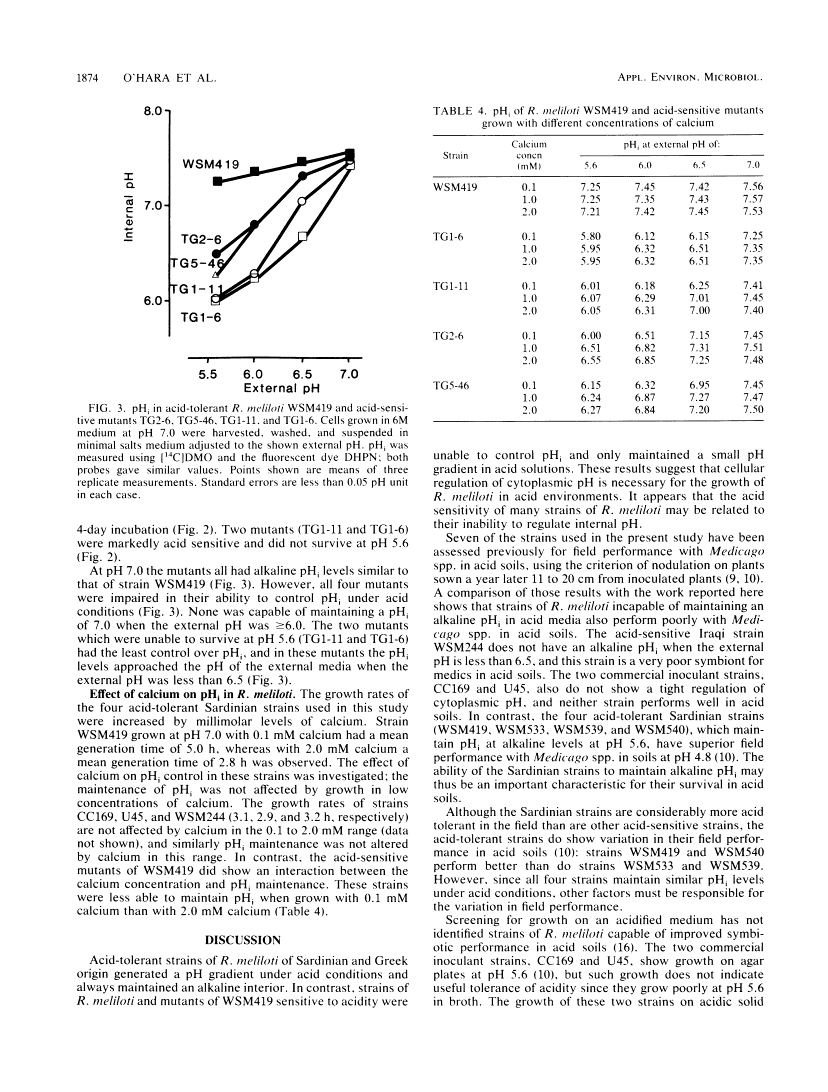
The development and function of the Rhizobium meliloti-Medicago sp. symbiosis are sensitive to soil acidity. Physiological criteria that can be measured in culture which serve to predict acid tolerance in soil would be valuable. The intracellular pH of R. meliloti was measured using either radioactively labeled weak acids (5,5-dimethyloxazolidine-2,4-dione and butyric acid) or pH-sensitive fluorescent compounds; both methods gave similar values. Six acid-tolerant strains (WSM419, WSM533, WSM539, WSM540, WSM852, and WSM870) maintained an alkaline intracellular pH when the external pH was between 5.6 and 7.2. In contrast, two Australian commercial inoculant strains (CC169 and U45) and four acid-sensitive strains from alkaline soils in Iraq (WSM244, WSM301, WSM365, and WSM367) maintained an alkaline intracellular pH when the external pH was ≥6.5, but had intracellular pH values of ≤6.8 when the external pH was ≤6.0. Four transposon Tn5-induced mutants of acid-tolerant strain WSM419, impaired in their ability to grow at pH 5.6, showed limited control over the intracellular pH. The ability to generate a large pH gradient under acid conditions may be a better indicator of acid tolerance in R. meliloti under field conditions than is growth on acidic agar plates.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer JE. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. [Abstract] [Google Scholar]
- Bhandari B, Nicholas DJ. Proton motive force in washed cells of Rhizobium japonicum and bacteroids from Glycine max. J Bacteriol. 1985 Dec;164(3):1383–1385. [Europe PMC free article] [Abstract] [Google Scholar]
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. [Europe PMC free article] [Abstract] [Google Scholar]
- McGivan JD. Mechanism of the stimulation of serine and alanine transport into isolated rat liver cells by bicarbonate ions. Biochem J. 1979 Sep 15;182(3):697–705. [Europe PMC free article] [Abstract] [Google Scholar]
- Gober JW, Kashket ER. H+/ATP stoichiometry of cowpea Rhizobium sp. strain 32H1 cells grown under nitrogen-fixing and nitrogen-nonfixing conditions. J Bacteriol. 1984 Oct;160(1):216–221. [Europe PMC free article] [Abstract] [Google Scholar]
- Hedges RW. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. [Abstract] [Google Scholar]
- Kashket ER. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. [Europe PMC free article] [Abstract] [Google Scholar]
- Kashket ER. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. [Abstract] [Google Scholar]
- Kobayashi H, Murakami N, Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [Abstract] [Google Scholar]
- Kobayashi H, Unemoto T. Streptococcus faecalis mutants defective in regulation of cytoplasmic pH. J Bacteriol. 1980 Sep;143(3):1187–1193. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurtz I, Balaban RS. Fluorescence emission spectroscopy of 1,4-dihydroxyphthalonitrile. A method for determining intracellular pH in cultured cells. Biophys J. 1985 Sep;48(3):499–508. [Europe PMC free article] [Abstract] [Google Scholar]
- Lowendorf HS, Baya AM, Alexander M. Survival of Rhizobium in Acid soils. Appl Environ Microbiol. 1981 Dec;42(6):951–957. [Europe PMC free article] [Abstract] [Google Scholar]
- Padan E, Schuldiner S. Intracellular pH regulation in bacterial cells. Methods Enzymol. 1986;125:337–352. [Abstract] [Google Scholar]
- Rink TJ, Tsien RY, Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. [Europe PMC free article] [Abstract] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. [Abstract] [Google Scholar]
- Selvaraj G, Iyer VN. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. [Europe PMC free article] [Abstract] [Google Scholar]
- Shechter E, Letellier L, Simons ER. Fluorescence dye as monitor of internal pH in Escherichia coli cells. FEBS Lett. 1982 Mar 8;139(1):121–124. [Abstract] [Google Scholar]
- Slavík J. Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 1982 Apr 5;140(1):22–26. [Abstract] [Google Scholar]
- Stock JB, Rauch B, Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [Abstract] [Google Scholar]
Associated Data
Articles from Applied and Environmental Microbiology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aem.55.8.1870-1876.1989
Read article for free, from open access legal sources, via Unpaywall:
https://aem.asm.org/content/aem/55/8/1870.full.pdf
Free to read at aem.asm.org
http://aem.asm.org/cgi/content/abstract/55/8/1870
Free after 4 months at aem.asm.org
http://aem.asm.org/cgi/reprint/55/8/1870
Citations & impact
Impact metrics
Citations of article over time
Article citations
Enhance of tomato production and induction of changes on the organic profile mediated by Rhizobium biofortification.
Front Microbiol, 14:1235930, 02 Aug 2023
Cited by: 2 articles | PMID: 37601341 | PMCID: PMC10433389
Analysis of the Interaction between Pisum sativum L. and Rhizobium laguerreae Strains Nodulating This Legume in Northwest Spain.
Plants (Basel), 9(12):E1755, 11 Dec 2020
Cited by: 5 articles | PMID: 33322342 | PMCID: PMC7763339
Aqueous peat extract exposes rhizobia to sub-lethal stress which may prime cells for improved desiccation tolerance.
Appl Microbiol Biotechnol, 102(17):7521-7539, 23 Jun 2018
Cited by: 5 articles | PMID: 29934654
Probiotic activities of Rhizobium laguerreae on growth and quality of spinach.
Sci Rep, 8(1):295, 10 Jan 2018
Cited by: 14 articles | PMID: 29321563 | PMCID: PMC5762915
Analysis and effect of the use of biofertilizers on Trifolium rubens L., a preferential attention species in Castile and Leon, Spain, with the aim of increasing the plants conservation status.
AIMS Microbiol, 3(4):733-746, 22 Aug 2017
Cited by: 0 articles | PMID: 31294185 | PMCID: PMC6604960
Go to all (47) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cloning, characterization, and complementation of lesions causing acid sensitivity in Tn5-induced mutants of Rhizobium meliloti WSM419.
J Bacteriol, 172(9):5173-5179, 01 Sep 1990
Cited by: 13 articles | PMID: 2168374 | PMCID: PMC213178
Survival of Rhizobium in Acid soils.
Appl Environ Microbiol, 42(6):951-957, 01 Dec 1981
Cited by: 16 articles | PMID: 16345909 | PMCID: PMC244139
Acid tolerance in Rhizobium meliloti strain WSM419 involves a two-component sensor-regulator system.
Microbiology (Reading), 142 ( Pt 7):1693-1704, 01 Jul 1996
Cited by: 48 articles | PMID: 8757734
Studies of the Physiological and Genetic Basis of Acid Tolerance in Rhizobium leguminosarum biovar trifolii.
Appl Environ Microbiol, 59(6):1798-1804, 01 Jun 1993
Cited by: 11 articles | PMID: 16348956 | PMCID: PMC182164