Abstract
Free full text

Polη, Polζ and Rev1 together are required for G to T transversion mutations induced by the (+)- and (−)-trans-anti-BPDE-N2-dG DNA adducts in yeast cells
Abstract
Benzo[a]pyrene is an important environmental mutagen and carcinogen. Its metabolism in cells yields the mutagenic, key ultimate carcinogen 7R,8S,9S,10R-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide, (+)-anti-BPDE, which reacts via its 10-position with N2-dG in DNA to form the adduct (+)-trans-anti-BPDE-N2-dG. To gain molecular insights into BPDE-induced mutagenesis, we examined in vivo translesion synthesis and mutagenesis in yeast cells of a site-specific 10S (+)-trans-anti-BPDE-N2-dG adduct and the stereoisomeric 10R (−)-trans-anti-BPDE-N2-dG adduct. In wild-type cells, bypass products consisted of 76% C, 14% A and 7% G insertions opposite (+)-trans-anti-BPDE-N2-dG; and 89% C, 4% A and 4% G insertions opposite (−)-trans-anti-BPDE-N2-dG. Translesion synthesis was reduced by ~26–37% in rad30 mutant cells lacking Polη, but more deficient in rev1 and almost totally deficient in rev3 (lacking Polζ) mutants. C insertion opposite the lesion was reduced by ~24–33% in rad30 mutant cells, further reduced in rev1 mutant, and mostly disappeared in the rev3 mutant strain. The insertion of A was largely abolished in cells lacking either Polη, Polζ or Rev1. The insertion of G was not detected in either rev1 or rev3 mutant cells. The rad30 rev3 double mutant exhibited a similar phenotype as the single rev3 mutant with respect to translesion synthesis and mutagenesis. These results show that while the Polζ pathway is generally required for translesion synthesis and mutagenesis of the (+)- and (−)-trans-anti-BPDE-N2-dG DNA adducts, Polη, Polζ and Rev1 together are required for G→T transversion mutations, a major type of mutagenesis induced by these lesions. Based on biochemical and genetic results, we present mechanistic models of translesion synthesis of these two DNA adducts, involving both the one-polymerase one-step and two-polymerase two-step models.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAH) are a class of common environmental pollutants that are produced by the incomplete combustion of organic materials. Benzo[a]pyrene is a widely studied PAH compound due to its potent carcinogenic activity in animal models. The chemically unreactive benzo[a]pyrene is metabolized in cells, forming reactive diol epoxide derivatives that can bind covalently to DNA. Unrepaired benzo[a]pyrene DNA adducts can lead to mutations that may eventually result in cancer. The reactive and mutagenic metabolites of benzo[a]pyrene are the (+)-7R,8S,9S,10R-anti-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide, (+)-anti-BPDE and the (−)-7R,8S,9S,10R enantiomer, (−)-anti-BPDE (1,2). DNA damage occurs mainly by the binding of the C10 position of anti-BPDE to the N2 position of guanine, thus forming the four stereoisomeric bulky adducts 10S (+)-trans-anti-BPDE-N2-dG, 10R (+)-cis-anti-BPDE-N2-dG, 10R (−)-trans-anti-BPDE-N2-dG and 10S (−)-cis-anti-BPDE-N2-dG (2,3). In vitro, the reaction of (+)-anti-BPDE with DNA yields predominantly the (+)-trans-anti-BPDE-N2-dG adduct, while the reaction of (−)-anti-BPDE generates mainly the (−)-trans-anti-BPDE-N2-dG adduct (4). In cells, the major benzo[a]pyrene DNA adduct is (+)-trans-anti-BPDE-N2-dG (2).
The structures of the four anti-BPDE-N2-dG adducts in DNA have been solved by NMR spectroscopy (5). In a duplex DNA containing a (+)- or (−)-trans-anti-BPDE-N2-dG adduct, the pyrenyl residues are not intercalated between adjacent base pairs. Instead, in the case of the (+)-trans-anti-BPDE-N2-dG adduct, the aromatic pyrenyl residue stacks primarily over an adjacent sugar ring in the complementary strand in the minor groove, and is oriented toward the 5′ end of the modified strand. In the case of the (−)-trans-anti-BPDE-N2-dG adduct, the pyrenyl residue stacks mainly over a sugar ring in the complementary strand in the minor groove, and is oriented toward the 3′ end of the modified strand (5–7). Thus, despite their identical chemical structure, the stereoisomeric (+)- or (−)-trans-anti-BPDE-N2-dG adducts adopt different configurations in duplex DNA as a result of the different absolute configurations of substituents about the four chiral carbon atoms. This difference, however, does not appear to affect the efficiency of their removal by human nucleotide excision repair in vitro (8).
BPDE DNA adducts are mutagenic in a variety of prokaryotic and eukaryotic cellular systems (9–15). However, the molecular mechanism of BPDE-induced mutagenesis is not well understood in eukaryotes. In cells, base damage-induced mutagenesis is mainly mediated through error-prone translesion synthesis. Key steps of translesion synthesis include copying damaged sites of the template by specialized polymerases during DNA replication. Conceptually, copying the lesion site may be divided into two distinct steps: nucleotide insertion opposite the lesion and extension synthesis from opposite the lesion. Recent studies indicate that Polζ and the Y family polymerases are important translesion polymerases in eukaryotes [reviewed in Refs (16–20)]. In the yeast Saccharomyces cerevisiae, the Y family consists of Polη and Rev1 (21). Mammals contain two additional members of the Y family polymerases: Polκ and Polι (16,21).
Translesion synthesis can be error-free or error-prone. Whereas error-prone translesion synthesis results in mutagenesis, error-free translesion synthesis suppresses mutagenesis. In vitro, Polκ effectively performs error-free translesion synthesis in response to (+)- and (−)-trans-anti-BPDE-N2-dG DNA adducts (22–26), whereas human Polη is capable of error-prone nucleotide insertion opposite these lesions (23,27–29). In vivo, Polκ indeed plays an important role in suppressing BPDE-induced mutagenesis (30), and is required for recovery from the BPDE-induced S-phase checkpoint (31). In yeast cells, there is evidence supporting a role for Polη in error-pone translesion synthesis following exposure of DNA to (±)-anti-BPDE (15). The majority of mutations induced by (±)-anti-BPDE in yeast, however, is generated through the Polζ mutagenesis pathway that includes the Rev1 protein (15). Similarly, mutagenesis following cellular exposure to racemic (±)-anti-BPDE also requires Polζ and Rev1 in cultured human cells (32,33).
The treatment of plasmid DNA or cells with racemic (±)-anti-BPDE yields several different types of DNA adducts. Therefore, it is difficult to define the roles of Polη, Polζ and Rev1 in BPDE-induced mutagenesis with DNA adducts of defined structure based on the treatment of DNA or cells with racemic (±)-anti-BPDE. In order to understand BPDE mutagenesis at a more defined molecular level, we examined in vivo translesion synthesis of two site-specific (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells. In this report, we show that while the Polζ pathway is generally required for translesion synthesis and mutagenesis of the (+)- and (−)-trans-anti-BPDE-N2-dG DNA adducts, Polη, Polζ and Rev1 together are required for G→T transversion mutations, a major type of mutagenesis induced by these lesions. Based on in vitro biochemical and in vivo genetic results, we present mechanistic models of translesion synthesis and mutagenesis of these two DNA adducts.
MATERIALS AND METHODS
Materials
T4 DNA ligase, the T4 gene 32 protein and T4 polynucleotide kinase were obtained from Enzymax (Lexington, KY). Yeast lytic enzyme (70 000 U/g) was purchased from MP Biomedicals (Irvine, CA). The Wizard PCR Preps DNA Purification Resin was from Promega (Wisconsin, WI). The Thermo Sequenase kit was obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Oligonucleotides containing a site-specific (+)-trans-anti-N2-dG or a (−)-trans-anti-BPDE-N2-dG adduct was prepared as described previously (34–36). Its sequence is 5′-CTCGATCGCTAACGCTACCATCCGAATTCGCCC-3′, where the modified guanine is underlined. Other DNA oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA).
000 U/g) was purchased from MP Biomedicals (Irvine, CA). The Wizard PCR Preps DNA Purification Resin was from Promega (Wisconsin, WI). The Thermo Sequenase kit was obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Oligonucleotides containing a site-specific (+)-trans-anti-N2-dG or a (−)-trans-anti-BPDE-N2-dG adduct was prepared as described previously (34–36). Its sequence is 5′-CTCGATCGCTAACGCTACCATCCGAATTCGCCC-3′, where the modified guanine is underlined. Other DNA oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA).
Yeast strains
Yeast strains used were the wild-type BY4741 (MATa his3 leu2 met15 ura3) and the isogenic BY4741Δrad30 (rad30 deletion mutant), BY4741Δrev1 (rev1 deletion mutant), BY4741Δrev3 (rev3 deletion mutant) and BY4741Δrev3Δrad30 (rev3 rad30 double deletion mutant). BY4741 was purchased from ATCC (Manassas, VA). BY4741Δrad30 (lacking Polη) was purchased from Research Genetics (Huntsville, AL). BY4741Δrev1, BY4741Δrev3 (lacking Polζ) and BY4741Δrev3Δrad30 were constructed as described previously (15,37).
Construction of plasmids containing site-specific (+)-trans-anti-BPDE-N2-dG or (−)-trans-anti-BPDE-N2-dG adducts
Plasmids containing site-specific (+)-trans-anti-BPDE-N2-dG or (−)-trans-anti-BPDE-N2-dG adducts were constructed by Enzymax using a previously described method (37). Briefly, a 20mer DNA oligonucleotide, 5′-GTGCCCTCCATGGAAAAATC-3′, was annealed to the single-stranded phagemid pELUf1 vector at its unique NcoI restriction site within the URA3 gene. Following digestion with the NcoI restriction endonuclease, the linearized pELUf1 was annealed with a 62mer DNA scaffold, 5′-CTGUGCCCUCCAUGGGGCGAAUTUGGAUGGUAGCGUTAGCGAUCGAGGAAAAAUCAGTCAAG-3′, and the damaged 33mer oligonucleotide that had been phosphorylated at the 5′ end by T4 polynucleotide kinase. While the mid region of the scaffold is complementary to the damaged oligonucleotide, its ends are complementary to the single stranded pELUf1 ends. The BPDE-modified oligonucleotide was ligated into the pELUf1 vector by T4 DNA ligase at 16°C for 20 h, and the DNA was precipitated in ethanol. Finally, the complementary strand of pELUf1 was synthesized with T4 DNA polymerase in the presence of T4 gene 32 protein and 0.5 mM each of dATP, dCTP, dGTP and dUTP, using the scaffold as the primer. The resulting construct was a double-stranded plasmid containing a site-specific (+)-trans-anti-BPDE-N2-dG or (−)-trans-anti-BPDE-N2-dG adduct, in which the undamaged strand contained U in place of T. Formation of double-stranded plasmid pELUf1-BPDE was confirmed by electrophoresis on a 1% agarose gel.
In vivo translesion synthesis assays in yeast cells
In vivo translesion synthesis assays were performed according to a previously described method (37) with modifications. Briefly, site-specifically damaged pELUf-BPDE plasmid (2 µg) was transformed into yeast cells of various strains by the lithium acetate method (38). Following transformation, yeast cells were collected by centrifugation (20 s at 5000 r.p.m.) in a microcentrifuge. Cells were resuspended in 400 µl of sterile water and were plated onto two YNB minimal agar (0.17% yeast nitrogen base, 0.49% ammonium sulfate, 2% glucose and 2% agar) plates lacking leucine but supplemented with 5 mM 5-fluoroorotic acid (5-FOA), 150 µM methionine and 380 µM uracil to score for colonies containing replicated pELUf1-BPDE. Cells transformed by the vector pELUf1 without the damaged oligonucleotide insert remained URA3 wild-type and thus could not grow on plates containing 5-FOA. After incubation at 30°C for 3–4 days, yeast colonies were counted. In each experiment with each strain, transformation efficiency was determined by a parallel transformation using the undamaged and double-stranded pELUf1. Translesion synthesis was calculated as transformants per µg of the damaged plasmid per 106 transformable cells with the undamaged plasmid (i.e. transformants per µg of the damaged plasmid × 106/transformation efficiency expressed as transformants per µg of the undamaged plasmid). Relative translesion synthesis was obtained by comparing translesion synthesis in various mutant strains to that in the wild-type cells.
Yeast colonies on the 5-FOA plates were individually resuspended in 10 µl of a solution containing 1 mg/ml yeast lytic enzyme in sterile water. After incubation at 37°C for 1.5–2 h, an aliquot of 1 µl was used for PCR amplification of a 670 bp plasmid region containing the original lesion site, using the primers, 5′-CCCGCAGAGTACTGCAATTTGAC and 5′-GAGCGGATAACAATTTCACACAGG. After heating the PCR mixture (20 µl) at 94°C for 4 min, 35 cycles of amplification were performed according to the following conditions: 30 s denaturation at 94°C, 30 s annealing at 65°C, and 45 s extension at 72°C. After the last cycle, the reaction was continued for 7 more min at 72°C. An aliquot of 2 µl PCR products was separated by electrophoresis on a 1% agarose gel containing 0.5 µg/ml ethidium bromide. Amplified DNA was purified by using the Wizard PCR Preps DNA Purification Resin according to the manufacturer's instruction. The precise specificity of translesion synthesis opposite the lesion was determined by sequencing the PCR DNA fragment. Yeast colonies that did not yield PCR products and plasmid clones that did not contain the inserted oligonucleotide sequence were excluded from the calculations. A few transformants by the empty vector pELUf1 escaped selection by the 5-FOA plates, probably due to mutations somewhere in the vector URA3 gene.
RESULTS
The Polζ pathway is the major mechanism for translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells
An in vivo genetic assay slightly modified from that of Zhao et al. (37) was used to examine translesion synthesis of a site-specific (+)- or (−)-trans-anti-BPDE-N2-dG adduct in yeast cells. A 33mer oligonucleotide containing the site-specific adduct was ligated into a single-stranded plasmid, which was subsequently converted into the double-stranded form by in vitro synthesis of the complementary strand using dUTP instead of dTTP. After transformation of the site-specifically damaged plasmid into cells, the complementary strand was degraded as a result of extensive DNA strand cleavage at sites of uracil by the sequential actions of a uracil-DNA glycosylase and an AP endonuclease, converting the plasmid DNA back into the single-stranded form (37). It is thus expected that this assay specifically reflects translesion synthesis without interference by DNA repair and template switching mechanisms (37), both of which require double-stranded DNA. The transformation efficiency was determined by using undamaged and double-stranded plasmids in the same experiment. After normalizing for transformation efficiency, the translesion synthesis efficiency in various cells relative to that in the wild-type cells was calculated. Yeast colonies were separately analyzed by PCR to recover a 670 bp region of the replicated plasmid. The specificity of translesion synthesis was determined by sequencing the PCR-amplified DNA fragments.
To examine the role of the Polζ pathway in bypassing the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, we performed in vivo translesion synthesis assays in yeast rev3 (lacking Polζ) and rev1 deletion mutant cells. In rev1 mutant cells, the translesion synthesis of the (+)-trans-anti-BPDE-N2-dG adduct was reduced to 49% of the wild-type level (P = 0.005), while that of the (−)-trans-anti-BPDE-N2-dG adduct was reduced to 22% (Figure 1). In rev3 mutant cells, translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts were reduced to a greater extent between 16 and 6.5%, respectively, of the wild-type levels (Figure 1). In wild-type cells, translesion synthesis of the (−)-trans-anti-BPDE-N2-dG adduct occurred consistently at a slightly higher efficiency than that of the (+)-trans-anti-BPDE-N2-dG adduct by ~1.3-fold. These results show that the Polζ pathway is the major mechanism for translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells.
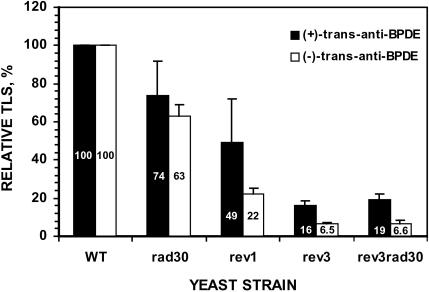
Relative frequencies of translesion synthesis (TLS) in various yeast strains. Using the plasmid pELUf1-BPDE containing a site-specific (+)- or (−)-trans-anti-N2-dG adduct, in vivo translesion synthesis assays were performed as described in Materials and Methods. Relative TLS was obtained by comparing translesion synthesis in various mutant strains to that in the wild-type cells. Slightly different transformation efficiencies as determined with the undamaged pELUf1 were taken into account in calculating the relative efficiencies. SDs are shown as error bars. WT, wild-type; rad30, lacking Polη; rev1, lacking Rev1; rev3, lacking Polζ; rev3rad30, lacking both Polζ and Polη.
Contribution of Polη to translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in vivo
To determine whether Polη affects bypass of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells, we performed in vivo translesion synthesis assays in the rad30 deletion mutant strain (lacking Polη). As shown in Figure 1, in the absence of Polη, translesion synthesis of the (+)-trans-anti-BPDE-N2-dG adduct was reduced to 74% (±18%) of the wild-type level (P = 0.004), while that of the (−)-trans-anti-BPDE-N2-dG adduct was reduced to 63% (±6%). To gain insights into the genetic relationship between Polη and Polζ in bypass of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, we examined translesion synthesis in rev3 rad30 double mutant cells that lacked both Polη and Polζ. As shown in Figure 1, translesion synthesis of either (+)- or (−)-trans-anti-BPDE-N2-dG in the double mutant cells was no more deficient than that in rev3 mutant strain. These results show that Polη plays a minor role in translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells, and suggest that Polη functions in the Polζ pathway in response to these lesions.
Mutagenic translesion synthesis in yeast cells of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts predominantly results from A and G insertions opposite the lesions
To determine translesion synthesis products at a nucleotide resolution, we amplified by PCR a 670 bp region surrounding the original lesion site from individual yeast colonies, and subsequently sequenced the amplified DNA fragments. In wild-type cells, the majority of translesion synthesis resulted from the correct C insertion opposite the (+)- (76%) and (−)-trans-anti-BPDE-N2-dG (89%) adducts (Tables 1 and and2).While2).While 10 and 7.3% of A and G, respectively, were inserted opposite (+)-trans-anti-BPDE-N2-dG (Table 1), only 4% each of A and G were inserted opposite (−)-trans-anti-BPDE-N2-dG (Table 2). Insertion of T, deletions, and tandem base substitutions constituted very minor translesion synthesis products (Tables 1 and and2).2). Other types of translesion synthesis, accounting for 3.6% and 0.9% for the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, respectively (Tables 1 and and2),2), resulted from the correct C insertion opposite the lesion, but contained mutations at adjacent sites (Figure 2). For simplicity, these are referred to as untargeted mutagenesis products (Figure 2). These results indicate that mutagenic translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells predominantly results from A and G insertions opposite the lesion, leading to G→T and G→C transversion mutations, respectively; and that the (+)-trans-anti-BPDE-N2-dG adduct is more mutagenic than the (−)-trans-anti-BPDE-N2-dG adduct.

Products of untargeted mutagenesis. The damaged 33mer oligonucleotide contained in the plasmid pELUf1-BPDE is denoted as the template for translesion synthesis (TLS). Untargeted mutagenesis products resulting from TLS are shown below the damaged template. Strains from which the untargeted mutagenesis products were recovered are shown together with the number of such mutant plasmid clones. The correct C insertion opposite the lesion is indicated in blue, while mutations are illustrated in red. (A) Untargeted mutagenesis in the case of the (+)-trans-anti-N2-dG adduct. (B) Untargeted mutagenesis in the case of the (−)-trans-anti-N2-dG adduct. Δ, deletion; WT, wild-type; rad30, lacking Polη; rev3rad30, lacking both Polζ and Polη.
Table 1
Specificity of translesion synthesis opposite the (+)-trans-anti-BPDE-N2-dG adduct in various yeast strains
| Straina | Clones sequencedb | Base incorporation | Deletionc | Tandem substitutiond | Otherse | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | A | G | T | Total | |||||
| WT | 137 | 104 (76%) | 14 (10%) | 10 (7.3%) | 2 (1.5%) | 130 (95%) | 1 (0.7%) | 1 (0.7%) | 5 (3.6%) |
| rad30 | 74 | 58 (78%) | 2 (2.7%) | 13 (18%) | — | 73 (99%) | — | — | 1 (1.4%) |
| rev1 | 45 | 42 (93%) | 2 (4.4%) | — | — | 44 (98%) | 1 (2.2%) | — | — |
| rev3 | 54 | 51 (94%) | 2 (3.7%) | — | — | 54 (100%) | — | — | — |
| rev3 rad30 | 31 | 28 (90%) | — | — | — | 28 (90%) | — | — | 3 (10%) |
aWT, wild-type; rad30, lacking Polη; and rev3, lacking Polζ.
bNumber of independent clones sequenced following in vivo translesion synthesis assays using the damaged pELUf1-BPDE plasmids containing a site-specific (+)-trans-anti-BPDE-N2-dG adduct.
cDeletions were: 5′-CTCGATCGCTAACGCTACCATCCGAATTCGCCC-3′ in wild-type cells, and 5′-CTCGATCGCTAACGCTACCATCCGAATTCGCCC-3′ in rev1 mutant cells, where the damaged G is in boldface and the deleted sequence is underlined.
dThe tandem substitution product was: 5′-CTCGATCGCTAACTTTACCATCCGAATTCGCCC-3′, in which the damaged G and its 3′ C were replaced by TT (underlined).
eOther translesion synthesis was derived from the correct C insertion opposite the lesion plus mutations 3′ or 5′ of the lesion site, yielding untargeted mutagenesis products. The sequences of these products are shown in Figure 2.
Table 2
Specificity of translesion synthesis opposite the (−)-trans-anti-BPDE-N2-dG adduct in various yeast strains
| Straina | Clones sequencedb | Base incorporation | Deletion | Tandem substitutionc | Othersd | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | A | G | T | Total | |||||
| WT | 222 | 198 (89%) | 9 (4.1%) | 9 (4.1%) | 3 (1.4%) | 219 (99%) | — | 1 (0.5%) | 2 (0.9%) |
| rad30 | 88 | 84 (95%) | — | 2 (2.3%) | — | 86 (98%) | — | — | 2 (2.3%) |
| rev1 | 63 | 61 (97%) | 1 (1.6%) | 1 (1.6%) | — | 63 (100%) | — | — | — |
| rev3 | 41 | 40 (98%) | 1 (2.4%) | — | — | 41 (100%) | — | — | — |
| rev3 rad30 | 40 | 40 (100%) | — | — | — | 40 (100%) | — | — | — |
aWT, wild-type; rad30, lacking Polη; and rev3, lacking Polζ.
bNumber of independent clones sequenced following in vivo translesion synthesis assays using the damaged pELUf1-BPDE plasmids containing a site-specific (−)-trans-anti-BPDE-N2-dG adduct.
cThe tandem substitution product was: 5′-CTCGATCGCTAACTTTACCATCCGAATTCGCCC-3′, in which the damaged G and its 3′ C were replaced by TT (underlined).
dOther translesion synthesis was derived from the correct C insertion opposite the lesion plus mutations 3′ or 5′ of the lesion site, yielding untargeted mutagenesis products. The sequences of these products are shown in Figure 2.
Contributions of Polζ, Rev1 and Polη to the mutagenic specificity of the (+)- and (−)-trans- anti-BPDE-N2-dG adducts
To gain insights into understanding the roles of Polζ, Rev1 and Polη in translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, the bypass products were recovered by PCR from the corresponding yeast mutant strains, and the amplified DNA fragments were sequenced. Specificity of translesion synthesis in the absence of these bypass polymerases was then compared with that of the wild-type cells. Without Polη, insertion of A was largely reduced opposite the (+)-trans-anti-BPDE-N2-dG adduct (Table 1), and was not detected opposite the (−)-trans-anti-BPDE-N2-dG (Table 2). Additionally, insertion of G opposite the (+)-trans-anti-BPDE-N2-dG adduct was significantly increased (Table 1). Translesion synthesis in rev1 and rev3 (lacking Polζ) mutant cells yielded similar products. In rev3 mutant cells, insertion of G opposite the lesion was not detected, and insertion of A was significantly reduced (Tables 1 and and2).2). Similarly, in rev1 mutant cells, insertion of A opposite the lesion was significantly reduced, and insertion of G was not detected opposite the (+)-trans-anti-BPDE-N2-dG or greatly reduced opposite the (−)-trans-anti-BPDE-N2-dG adduct (Tables 1 and and2).2). In the absence of both Polη and Polζ (rev3 rad30 double mutant), insertions of A and G opposite the lesions were abolished (Tables 1 and and22).
When the altered bypass in the mutant strains was directly compared with the bypass of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in wild-type cells, expressed as translesion synthesis relative to that in wild-type cells, a major contribution by Polη to A insertion and the general dependence of mutagenesis on Rev1 and Polζ were clearly seen (Tables 3 and and4).4). The effect of Polη on G incorporation, however, differed for (+)- versus (−)-trans-anti-BPDE-N2-dG. In the absence of Polη, G insertions were increased by ~2-fold opposite the former lesion, but decreased ~4-fold opposite the latter lesion (Tables 3 and and4).4). Apparently, the untargeted mutagenesis (Figure 2) induced by (+)- and (−)-trans-anti-BPDE-N2-dG adducts, which constituted only a very minor fraction of the overall translesion synthesis, required Polζ and Rev1, but was independent of the Polη function. As for error-free translesion synthesis, reflected by C insertion opposite the lesion, Polη did not have a major impact. In contrast, such error-free bypass significantly depended on Rev1 and mostly required the function of Polζ (Tables 3 and and44).
Table 3
Changes in translesion synthesis specificity opposite the (+)-trans-anti-BPDE-N2-dG adduct in mutant cells relative to that in wild-type cellsa
| Strainb | Base incorporation | Deletion | Tandem substitution | Othersc | Total | |||
|---|---|---|---|---|---|---|---|---|
| C | A | G | T | |||||
| WT | 0.76 | 0.10 | 0.07 | 0.02 | 0.01 | 0.01 | 0.01 | 1 |
| rad30 | 0.58 | 0.02 | 0.13 | — | — | — | 0.01 | 0.74 |
| rev1 | 0.46 | 0.02 | — | — | 0.01 | — | — | 0.49 |
| rev3 | 0.15 | 0.01 | — | — | — | — | — | 0.16 |
| rev3 rad30 | 0.17 | — | — | — | — | — | 0.02 | 0.19 |
aTranslesion synthesis in various mutant strains is expressed relative to that in the wild-type strain. Calculations were based on Figure 1 and Table 1.
bWT, wild-type; rad30, lacking Polη; and rev3, lacking Polζ.
cOther translesion synthesis yielded untargeted mutagenesis products, whose sequences are shown in Figure 2.
Table 4
Changes in translesion synthesis specificity opposite the (−)-trans-anti-BPDE-N2-dG adduct in mutant cells relative to that in wild-type cellsa
| Strainb | Base incorporation | Deletion | Tandem substitution | Othersc | Total | |||
|---|---|---|---|---|---|---|---|---|
| C | A | G | T | |||||
| WT | 0.89 | 0.04 | 0.04 | 0.01 | — | 0.01 | 0.01 | 1 |
| rad30 | 0.60 | — | 0.014 | — | — | — | 0.014 | 0.63 |
| rev1 | 0.213 | 0.004 | — | — | — | — | — | 0.22 |
| rev3 | 0.0637 | 0.0016 | — | — | — | — | — | 0.065 |
| rev3 rad30 | 0.066 | — | — | — | — | — | — | 0.066 |
aTranslesion synthesis in various mutant strains is expressed relative to that in the wild-type strain. Calculations were based on Figure 1 and Table 2.
bWT, wild-type; rad30, lacking Polη; and rev3, lacking Polζ.
cOther translesion synthesis yielded untargeted mutagenesis products, whose sequences are shown in Figure 2.
Taken together, these results show that (i) Polη, Polζ and Rev1 combined are required for G→T mutagenesis induced by the (+)- and (−)-trans-anti-BPDE-N2-dG adducts; and (ii) G→C mutagenesis induced by these two lesions is generated through the Polζ pathway, independent of Polη opposite (+)-trans-anti-BPDE-N2-dG, but involving the Polη function opposite (−)-trans-anti-BPDE-N2-dG. Additionally, these results suggest that the Polζ pathway is the major mechanism, while Polη plays a minor role, for error-free translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts.
DISCUSSION
Polη was originally discovered as an error-free translesion synthesis polymerase in response to ultraviolet (UV)-induced TT dimers (39,40). However, the first clue suggesting that this polymerase may be involved in error-prone translesion synthesis of benzo[a]pyrene DNA adducts came from biochemical studies using templates containing a site-specific (+)-trans-anti-BPDE-N2-dG adduct (27). Purified Polη is able to recognize this bulky DNA lesion, and predominantly inserts an A opposite the lesion (27). Similar studies with the (−)-trans-anti-BPDE-N2-dG adduct showed that this lesion is less efficiently bypassed by Polη than the (+)-trans-anti-BPDE-N2-dG adduct (23,28,29). In this study, we tested our hypothesis that Polη participates in error-prone translesion synthesis by inserting A opposite the (+)- and (−)-trans-anti-BPDE-N2-dG adducts. Using an in vivo genetic assay that is based on the replication of site-specifically damaged plasmids in yeast cells, we showed that Polη indeed plays a major role in the insertion of A opposite these two stereoisomeric lesions, resulting in G→T transversion mutations. Therefore, in contrast to its anti-mutagenic role in response to UV radiation, Polη functions to promote mutagenesis induced by the (+)- and (−)-trans-anti-BPDE-N2-dG DNA adducts.
Insertions of A opposite the (+)- and (−)-trans-anti-BPDE-N2-dG adducts are mostly abolished when either Polη, Polζ or Rev1 is absent. Thus, the induced G→T transversion mutations depend on the functions of all these three proteins. Our in vivo results are best explained by the two-polymerase two-step mechanism of translesion synthesis (18,41). In such a bypass pathway, A is inserted by Polη opposite the lesion followed by extension synthesis catalyzed by Polζ. Supporting this conclusion, A is effectively inserted opposite (+)- and (−)-trans-anti-BPDE-N2-dG adducts by Polη in vitro (15,23,27–29), but not by Rev1, or just barely by Polζ (15,42). However, purified Polη is ineffective in extension synthesis from opposite these lesions (15,23,27,28). The bypass intermediate is thus most likely extended by Polζ. According to this mechanism, the insertion step would not occur without Polη and the extension step would not occur without Polζ, thus explaining the observed dependence on both Polη and Polζ of the G→T transversion mutations induced by the (+)- and (−)-trans-anti-BPDE-N2-dG adducts.
A very weak activity is detectable for Polη to extend from opposite the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in vitro (15,23,27,28). This marginal activity was most likely responsible for the residual (5–10% of the wild-type level) G→T transversion mutations in the absence of Polζ (Tables 3 and and4).4). In the absence of Polη, a residual A insertion activity was also observed opposite the (+)-trans-anti-BPDE-N2-dG adduct (Table 3), which probably resulted from the very weak activity of A insertion by Polζ (15). Supporting this interpretation, the residual A insertion activity was eliminated in cells lacking both Polη and Polζ (Tables 3 and and44).
With respect to G→T transversion mutagenesis induced by the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, the phenotype of the rev1 mutant is similar to that of rev3 that lacks Polζ. Since Rev1 is unable to insert an A opposite these adducts (42), this protein must play a non-catalytic function for the induced G→T mutagenesis, which is similar to its role in UV-induced mutagenesis (42,43). So far, an in vivo catalytic activity (dCMP transferase) of Rev1 has been suggested only for the bypass of AP sites (37,43). Thus, the non-catalytic function of Rev1 may be responsible for its ubiquitous role in translesion synthesis in cells. The precise nature of this non-catalytic function, however, remains unknown.
In addition to A insertion, another major mode of mutagenic translesion synthesis is G insertion opposite the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, leading to G→C transversion mutations, which requires the Polζ pathway. Missing either Polζ or Rev1 abolishes this mode of translesion synthesis. Opposite the (+)-trans-anti-BPDE-N2-dG adduct, Polζ is capable of translesion synthesis and preferentially inserts G in vitro (15), whereas purified Polη is inefficient in G insertion (15,23,27,28). Thus, we conclude that the observed G→C transversion mutations are generated mainly by Polζ-catalyzed G insertion followed by Polζ-catalyzed extension through the Polζ pathway, i.e. a one-polymerase two-step mechanism. Supporting this conclusion, inactivating Polη led to an increase in G→C mutagenesis, probably due to the elimination of the competitive binding of Polη to the template and Polη-catalyzed A insertion. In the case of the (−)-trans-anti-BPDE-N2-dG adduct, Polζ is also capable of translesion synthesis and preferentially inserts G in vitro, but its activity is lower than in the case of the stereoisomeric (+)-trans-anti-BPDE-N2-dG lesion (15). On the other hand, G insertion opposite (−)-trans-anti-BPDE-N2-dG catalyzed by yeast Polη is more active than opposite (+)-trans-anti-BPDE-N2-dG in vitro (15). Consistent with these biochemical results, we observed that G→C mutagenesis induced by (−)-trans-anti-BPDE-N2-dG was significantly reduced in yeast cells in the absence of Polη (Table 4), suggesting a major role for Polη in catalyzing G insertions opposite this lesion. Thus, the precise mechanism of G→C mutagenesis induced by the (+)-trans-anti-BPDE-N2-dG adduct is somewhat different from that induced by the (−)-trans-anti-BPDE-N2-dG adduct, due to the different catalytic selectivity of Polη.
Translesion synthesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast cells results mainly in base incorporation opposite the lesions. Furthermore, most bypass products result from the correct C insertion opposite the lesion, i.e. error-free translesion synthesis. In vitro, Polη, Polζ and Rev1 all have significant C insertion activities opposite these two lesions (15,23,27,28,42). In this study, we found that C insertions opposite the lesions in vivo were only slightly reduced in the absence of Polη, but were more significantly reduced in the absence of Rev1, and mostly abolished in the absence of Polζ (Tables 3 and and4).4). Therefore, error-free translesion synthesis of these lesions is mostly performed by the Polζ pathway. The C insertion step is likely catalyzed by both Rev1 and Polζ. Since Rev1 cannot perform extension synthesis following C insertion (42), the extension step must be catalyzed by Polζ. Consistent with this interpretation, error-free bypass is most deficient in yeast cells lacking Polζ. Polη only makes a minor contribution (~30%) to error-free bypass of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, for which the most likely mechanism is C insertion by Polη and subsequent extension by Polζ. A mechanistic model for translesion synthesis and mutagenesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts is summarized in Figure 3.
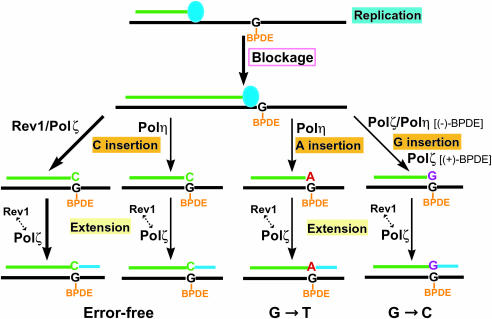
Mechanistic models for translesion synthesis of the (+)- and (−)-trans-anti-N2-dG DNA adducts in yeast cells. The (+)-and (−)-trans-anti-BPDE-N2-dG adducts strongly block the replication complex (represented by the filled blue oval). Translesion synthesis is mediated predominantly by C insertion opposite the lesion catalyzed by the Rev1 dCMP transferase and by Polζ. Extension synthesis by Polζ completes the lesion bypass. Polη makes a minor contribution (~30%) to error-free bypass, for which the most likely mechanism is C insertion by Polη and subsequent extension by Polζ. Less frequently, A is inserted opposite the lesion by Polη, which is subsequently extended by Polζ, leading to G→T transversion mutations. Insertion of G opposite the lesions occurred at a low frequency, which is catalyzed by Polζ in the case of the (+)-trans-anti-BPDE-N2-dG adduct and by Polζ and Polη in the case of the (−)-trans-anti-BPDE-N2-dG adduct. Subsequent extension is catalyzed by Polζ, leading to G→C transversion mutations. Rev1 likely facilitates Polζ-catalyzed extension. In this role, Rev1 may play a non-catalytic function independent of its dCMP transferase.
The results with the site-specific (+)- and (−)-trans-anti-BPDE-N2-dG adducts in this study are in general agreement with our earlier report on mutagenesis induced by racemic (±)-anti-BPDE using randomly damaged plasmids (15). However, two differences are noted: quantitatively more G→C transversion mutations and deletions and insertions of 1–3 nt in the randomly damaged system (15). Two factors may be responsible for these differences. First, treatment of DNA with (±)-anti-BPDE yields multiple types of lesions. In addition to the (+)- and (−)-trans-anti-BPDE-N2-dG adducts, the (+)- and (−)-cis-anti-BPDE-N2-dG adducts and other minor BPDE adducts are also formed (2,4). These additional forms of DNA lesions likely contribute to the mutation spectrum. Secondly, deletions and insertions induced by (±)-anti-BPDE occur more frequently within single nucleotide repeats (15). The (+)- and (−)-trans-anti-BPDE-N2-dG adducts in this study, however, were not positioned within such repeat sequences. Consequently, it is expected that frameshift mutagenesis is less likely to occur in the sequence context examined in this study.
Our studies further suggest that the (+)-trans-anti-BPDE-N2-dG adduct is more mutagenic than the (−)-trans-anti-BPDE-N2-dG adduct. Since the two lesions did not differ greatly in translesion synthesis efficiency, the less mutagenic property of the (−)-trans-anti-BPDE-N2-dG adduct is likely a direct result of its more frequent error-free bypass in cells. A greater mutagenic potential of the (+)-trans-anti-BPDE-N2-dG adduct was also reported in cultured simian kidney cells in different sequence contexts (12). Hence, the mutagenecity of the BPDE adduct is influenced by its stereochemistry. Moreover, the specificity of nucleotide incorporation opposite the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in these mammalian cells was similar to that reported here in yeast cells. Recently, it was reported that (±)-anti-BPDE-induced mutagenesis in cultured human cells is largely abolished when Polζ and REV1 expressions are suppressed (32,33). These similarities suggest that the fundamental mechanism of translesion synthesis and mutagenesis of the (+)- and (−)-trans-anti-BPDE-N2-dG adducts is conserved from yeast to mammals. By comparing results using the same experimental system, it is possible for us to evaluate the intrinsic mutagenic potential of various DNA lesions. Thus, it is apparent that the p-benzoquinone DNA adducts derived from benzene are intrinsically much more potent in mutagenesis than the (+)- and (−)-trans-anti-BPDE-N2-dG adducts in yeast (44).
Acknowledgments
We thank Zhongwen Xie for technical assistance. This work was supported by NIH grants CA92528 (to Z.W.), and CA20851 (to N.E.G.). Funding to pay the Open Access publication charges for this article was provided by the NIH grant CA92528.
Conflict of interest statement. None declared.
REFERENCES
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/gkj446
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/nar/article-pdf/34/2/417/7128839/gkj446.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/nar/gkj446
Article citations
Repair of programmed DNA lesions in antibody class switch recombination: common and unique features.
Genome Instab Dis, 2(2):115-125, 26 Mar 2021
Cited by: 4 articles | PMID: 33817557 | PMCID: PMC7996122
Review Free full text in Europe PMC
dUTPase inhibition confers susceptibility to a thymidylate synthase inhibitor in DNA-repair-defective human cancer cells.
Cancer Sci, 112(1):422-432, 20 Nov 2020
Cited by: 15 articles | PMID: 33140501 | PMCID: PMC7780055
Exposure of Human Lung Cells to Tobacco Smoke Condensate Inhibits the Nucleotide Excision Repair Pathway.
PLoS One, 11(7):e0158858, 08 Jul 2016
Cited by: 7 articles | PMID: 27391141 | PMCID: PMC4938567
DNA Polymerases η and ζ Combine to Bypass O(2)-[4-(3-Pyridyl)-4-oxobutyl]thymine, a DNA Adduct Formed from Tobacco Carcinogens.
Chem Res Toxicol, 29(3):303-316, 22 Feb 2016
Cited by: 8 articles | PMID: 26868090 | PMCID: PMC5081176
Roles of mutagenic translesion synthesis in mammalian genome stability, health and disease.
DNA Repair (Amst), 29:56-64, 21 Jan 2015
Cited by: 28 articles | PMID: 25655219
Review
Go to all (23) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Two-step error-prone bypass of the (+)- and (-)-trans-anti-BPDE-N2-dG adducts by human DNA polymerases eta and kappa.
Mutat Res, 510(1-2):23-35, 01 Dec 2002
Cited by: 37 articles | PMID: 12459440
Translesion synthesis of acetylaminofluorene-dG adducts by DNA polymerase zeta is stimulated by yeast Rev1 protein.
Nucleic Acids Res, 32(3):1122-1130, 11 Feb 2004
Cited by: 18 articles | PMID: 14960722 | PMCID: PMC373411
Role of DNA polymerase eta in the bypass of abasic sites in yeast cells.
Nucleic Acids Res, 32(13):3984-3994, 29 Jul 2004
Cited by: 57 articles | PMID: 15284331 | PMCID: PMC506798
Covalent binding of benzo[a]pyrene 7,8-dihydrodiol 9,10-epoxides to DNA: molecular structures, induced mutations and biological consequences.
Biophys Chem, 49(3):185-199, 01 Apr 1994
Cited by: 35 articles | PMID: 8018817
Review
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: CA20851
Grant ID: CA92528
Grant ID: R01 CA092528
Grant ID: R01 CA020851




