Abstract
Free full text

Targeted delivery of ribavirin improves outcome of murine viral fulminant hepatitis via enhanced anti‐viral activity†
Abstract
Side effects of interferon–ribavirin combination therapy limit the sustained viral response achievable in hepatitis C virus (HCV) patients. Coupling ribavirin to macromolecular carriers that target the drug to the liver would reduce systemic complications. The aim of this study was to evaluate the efficacy of a hemoglobin–ribavirin conjugate (HRC 203) in murine hepatitis virus strain 3 (MHV‐3) induced viral hepatitis. HRC 203 had greater anti‐viral activity on both isolated hepatocytes and macrophages, whereas both ribavirin and HRC 203 inhibited production of the pro‐inflammatory cytokines interferon gamma (IFN‐γ) and tumor necrosis factor alpha (TNF‐α) by macrophages. In vivo, untreated MHV‐3–infected mice all developed clinical and biochemical signs of acute viral hepatitis and died by day 4 post infection. Livers recovered from untreated infected mice showed greater than 90% necrosis. In contrast, survival was enhanced in both ribavirin‐ and HRC 203–treated mice with a marked reduction in biochemical [ALTmax 964 ± 128 IU/L (ribavirin); 848 ± 212 IU/L (HRC 203)] and histological evidence of hepatic necrosis (<10% in ribavirin/HRC 203 vs. 90% in untreated controls). Clinically, HRC 203–treated mice behaved normally, in contrast to ribavirin‐treated mice, which developed lethargy and abnormal fur texture. In conclusion, targeted delivery of ribavirin to the liver alters the course of MHV‐3 infection as demonstrated by prolonged survival, improved behavior, and reduced signs of histologically evident disease, as well as inhibition of viral replication and production of inflammatory cytokines in vitro. (Hepatology 2006;43:581–591.)
Chronic hepatitis C virus (HCV) infection is a major public health problem affecting over 4 million people in the United States and more than 170 million individuals worldwide.1, 2 In addition, chronic HCV is a causative factor for approximately 50% of the cases of hepatocellular carcinoma in the United States, and is the single most common indication for orthotopic liver transplantation worldwide.3, 4 However, treatment of HCV infection remains problematic. The current standard of care is a combination of interferon alpha (IFN‐α) and ribavirin. This combination treatment is limited by severe side effects that often lead to premature cessation of therapy.5 The major toxicity associated with ribavirin is a dose‐dependent hemolytic anemia, which occurs in approximately 50% of treated individuals, resulting in a ribavirin dose reduction.6 The development of anemia usually starts after 4 weeks of therapy and can be precipitous.
Coupling of ribavirin to a carrier molecule offers the potential of a therapeutic with improved safety and efficacy by targeting drug delivery of ribavirin to key tissues infected by HCV while preventing the hemolytic anemia that is caused by exposure of red blood cells to free ribavirin. Targeting of drugs by attachment to carrier molecules for delivery to specific tissues via receptor‐mediated endocytosis is a recently established method for improving efficacy, decreasing toxic side effects, and providing an alternative to problematic systemic drug therapy.7, 8
Acellular hemoglobin (Hb) is rapidly bound to haptoglobin (Hp) in vivo and taken up by cells bearing receptors for the Hb‐Hp complex, principally by macrophages (Kupffer cells) and hepatocytes as a natural pathway for Hb clearance.9, 10, 11 The Hb‐Hp scavenger receptor, CD163, was identified as a marker for cells from the monocyte/macrophage lineage.9 The levels of this receptor are increased in the livers of patients with acute hepatitis.12 HRC 203 is a conjugate of human hemoglobin and ribavirin bound to haptoglobin (Hp‐Hb‐RV)13 designed to deliver ribavirin to the liver to avoid systemic toxicity associated with chronic ribavirin therapy. Selective uptake of the Hb‐Hp complex is mediated through binding of the complex to the receptor followed by entry into lysosomes. Receptor‐mediated binding and uptake of HRC 203 has been demonstrated in model cells expressing Hb‐Hp receptors.13 Acid phosphatase, a lysosomal enzyme, has been shown to release bioactive ribavirin from HRC 203 in vitro. 13 Similar release of active drug is expected after lysosomal uptake of the conjugate in target cells in vivo. Multiple ribavirin molecules are conjugated to each Hb molecule; therefore, HRC 203 has the potential to achieve higher intracellular doses of ribavirin in target tissues than are currently feasible. We estimated that comparable liver concentrations could be achieved or exceeded by administering endogenous Hp‐saturating doses of Hb‐ribavirin on the order of 1 to 2 g Hb.14 HRC 203 may be effective in maintaining optimal ribavirin levels in HCV patients who would otherwise require dose reduction or discontinuation of ribavirin therapy because of systemic consequences.
The aim of these studies was to evaluate the effect of HRC 203 in a mouse model of viral hepatitis induced by the coronavirus MHV‐3. MHV‐3 is the largest of the Coronavirus family of positive‐stranded RNA viruses with a genome size of 32 kb, a 5′ cap structure, and a 3′ poly(A) tail. MHV‐3 replicates in the liver‐derived macrophages (Kupffer cells), endothelial cells, and parenchymal cells (hepatocytes), with higher degrees of replication in the macrophage reservoir. Viral replication in macrophages appears to be a prerequisite for MHV‐3 to gain access to hepatocytes.15 Viral infection of macrophages leads to a marked inflammatory response associated with a Th2 cellular immune response and production of non‐neutralizing antibodies.16, 17 This response fails to control viral replication. Induction of pro‐inflammatory mediators tumor necrosis factor alpha (TNF‐α) and interferon‐γ (IFN‐γ) activate endothelial cells and increase their expression of adhesion molecules, resulting in the recruitment of inflammatory cells, vasoconstriction, intravascular thrombosis, fibrin deposition, and liver necrosis. Within 3 to 4 days post‐infection, fulminant hepatic failure results, leading to death in 80% to 90% of infected mice.18 The current studies were designed to examine the potential for HRC 203 to treat MHV‐3 infection through both anti‐viral and anti‐inflammatory pathways.
Materials and Methods
HRC 203.
HRC 203 was prepared as described.13 Ribavirin (Inter‐Chemical, Shenzen, China) in phosphate‐buffered saline (PBS) was phosphorylated at its 5′‐hydroxyl group according to established methods19 and subsequently activated to a phosphorimidazolide according to the method of Fiume et al.20 Ribavirin‐phosphorimidazolide was reacted in a 150‐fold excess with human carbonmonoxyhemoglobin (Hemosol Corp., Mississauga, Ontario, Canada) in 0.1 mol/L sodium bicarbonate buffer, pH 9.5, resulting in phosphoramidate attachment to protein amino groups. The molar drug ratio of the hemoglobin–ribavirin (Hb–RV) conjugate was determined to be 6 to 8 ribavirin molecules per hemoglobin with little or no unmodified hemoglobin remaining after 96 hours of reaction. The Hb–RV conjugate was extensively dialyzed against PBS after completion of reaction. Purified rat haptoglobin was incubated with Hb‐RV in PBS to form the HRC 203 complex, which was subsequently purified by preparative, aseptic size‐exclusion chromatography to remove any unbound Hb‐RV. The final product was diafiltered against PBS and concentrated to 90 mg/mL total protein.
Virus.
MHV‐3 was obtained from the American Type Culture Collection (Rockville, MD) and was plaque purified on monolayers of DBTcells and grown to titers of 2 × 107 plaque‐forming units (PFU)/mL in 17CL1 cells. Virus was harvested by centrifugation at 4,500g for 1 hour at 4°C and was assayed on monolayers of L2 cells in a standard plaque assay.
Cell Isolation
Macrophages.
Peritoneal macrophages were harvested from Balb/cJ mice 4 days after an intraperitoneal injection of 1.5 mL 3% thioglycollate (Difco Laboratories, Detroit, MI) as described.21 Macrophages were re‐suspended in RPMI 1640 (ICN Biomedicals, Costa Mesa, CA) supplemented with 2 mmol/L glutamine (Sigma Chemical, St. Louis, MO) and 2% heat‐inactivated FBS (Flow Laboratories, Mississauga, Ontario, Canada). Macrophages were >95% pure as determined by morphology and non‐specific esterase stain, and viability exceeded 95% by trypan blue exclusion.
Hepatocytes.
Hepatocytes were isolated from the livers of Balb/cJ mice as previously described.22 Briefly, mice were anesthetized by intraperitoneal injection of pentobarbital (5 mg/100 g body weight). The abdomen was opened by midline incision, and the portal vein was exposed and cannulated with silastic tubing. The liver was perfused with oxygenated (95% O2 and 5% CO2) calcium‐ and magnesium‐free Hank's balanced salt solution (HBSS) at a flow rate of 10 mL/min at 37°C for 10 minutes. The perfusate was then changed to HBSS with calcium, magnesium, 0.02% collagenase B, 0.001% DNAse for a further 10 minutes. The liver was then removed and placed into a sterile Petri dish containing cold (4°C) HBSS with 10% FBS into which liver cells were dispersed. The cellular suspension was centrifuged at 50g at 4°C for 2 minutes twice. The cell pellet was re‐suspended in 10 mL HBSS and centrifuged at 400g at 4°C for 7 minutes. Cells were counted and re‐suspended at a concentration of 1 × 106/mL in complete RPMI 1640 fortified with 2% FBS. Viability of hepatocytes both pre‐ and post‐treatment was >95% as assessed by trypan blue staining. The purity of the hepatocytes was judged to be >98% by absence of immunofluorescence staining for CD11+ (macrophages/Kupffer cells) and CD31+ (endothelial cells) and immunofluorescence positivity for MHC Class II.
Viral Inhibition Studies.
Mouse peritoneal macrophages were isolated from Balb/cJ mice as outlined previously. The cells were pre‐incubated in the presence of HRC 203 (40 μmol/L conjugated ribavirin) or free ribavirin (800 μmol/L) 1 hour before infection with 1000 PFU MHV‐3 (MOI 0.3). Macrophages were harvested 8 hours after infection and analyzed for viral titers as described below. Hepatocytes isolated from Balb/cJ mice were pre‐incubated in the presence of increasing concentrations of either HRC 203 or ribavirin (1‐100 μmol/L) for 2 hours, after which the medium was replaced with fresh medium followed by infection with MHV‐3 (MOI 0.3) for a period of 72 hours. Cells were then harvested, and measurement of viral titers was carried out in a manner similar to that described for macrophages.
Cell‐Binding Studies.
Mouse peritoneal macrophages were isolated from Balb/cJ mice as outlined previously. 1 × 105 cells were placed in 5 mL polypropylene tubes. Complexes of Hp‐Hb were prepared fresh for each assay by combining fluorescein‐labeled haptoglobin (fHp) with Hb at a 1:1 molar ratio, and incubated for 1 hour at room temperature (RT) in the dark. Two hundred–microliter aliquots (diluted in RMPI 1640 + 0% FBS) were added to macrophages and incubated at 37°C for 1 hour. Cells were pelleted at 1500 rpm for 10 minutes, washed, and re‐suspended in PBS. Macrophages were pre‐stimulated with either MHV‐3 (MOI 2.0 in RMPI 1640 + 2% FBS) for 8 hours, dexamethasone (2.5 × 10−7M in RMPI + 10% FBS) for 24 hours, or control (RMPI 1640 + 10% FBS), all at 37°C. Cells were then washed and re‐suspended in RPMI 1640 + 10% FBS and placed at 4°C for 2 hours before incubation with fHp‐Hb compounds for 1 hour at 4°C. Binding of fluorescently labeled proteins to macrophages was detected using a Cytomics FC 500 MPL flow cytometer and analyzed with Cytometrics RXP Analysis software (Beckman Coulter, Fullerton, CA).
Cytokine Measurements
TNF‐α.
Supernatants were collected from treated cells, and TNF‐α concentrations were assayed by ELISA. Monoclonal hamster anti‐murine TNF‐α Ab (Genzyme, Boston, MA) was coated to ELISA plates overnight at 4°C. After being washed with Tris buffer (pH 8.0), plates were blocked with 100 μL 6% bovine serum albumin in each well for 1 hour at RT. After washing, 100 μL standards or samples were added and incubated at RT for 3 hours. Subsequently, 100 μL polyclonal rabbit anti‐mouse TNF‐α Ab (Genzyme, IP‐400) was added to each well, and plates were incubated at 4°C overnight. Goat anti‐rabbit IgG alkaline phosphatase (100 μL) (Jackson ImmunoResearch Laboratories, West Grove, PA) was added, and plates were incubated at RT for 1 hour. After washing, 100 μL of diflusinal phosphate (1/10 in substrate buffer) was added, and plates were incubated for an additional 10 minutes at RT with shaking. Plates were read by Cyber Fluor (Beckman Instruments, Fullerton, CA). Units were assayed by comparison with a mouse TNF‐α standard (Genzyme).
IFN‐γ.
Supernatants were collected from treated cells, and IFN‐γ concentrations were assayed by the ability of the supernatants to inhibit or support the proliferation of Wehi 279 cells. Cultures were incubated for 40 hours in a humidified CO2 atmosphere at 37°C, pulsed with 1 μCi3 H thymidine (sp. Act. 2 Ci/mmol; Amersham, Arlington Heights, IL) and harvested 26 hours later onto glass fiber filters. Total cell‐associated radioactivity was measured in a Beckman scintillation counter, and bioassay data were expressed in nanograms/milliliter for IFN‐γ derived from a standard curve with mouse recombinant IFN‐γ (Genzyme).
Mice.
Female Balb/cJ mice, 6 to 8 weeks of age, were purchased from Charles River Laboratories (St. Constant, Quebec, Canada) and housed in micro‐isolator cages in the animal facilities of the Toronto General Hospital. Mice were fed with standard laboratory chow diet and water ad libitum. Animals were divided into 4 treatment groups (n = 10 mice per group). Group 1 were control, MHV‐3–infected (100 PFU/mouse); Group 2 received the HRC 203 via tail vein injection (6 mg conjugated ribavirin/kg/day) starting day −1 and daily thereafter + MHV‐3 (100 PFU/mouse); Group 3 received ribavirin by tail vein injection as described by Sidwell et al.23 (18 mg/kg/day) starting on day −1 and daily thereafter + MHV‐3 (100 PFU/mouse); and Group 4 control, uninfected mice. All control animals were given daily injections of 100 μL PBS via the tail vein. Mice were infected by an intraperitoneal injection with 100 plaque‐forming units (PFU) MHV‐3 in 100 μL PBS. Mice were killed on days 1, 2, 3, 4, and 5 and analyzed for evidence of hepatitis by liver biochemistry (alanine transaminase; ALT), hematologic disturbances (hemoglobin by hematocrit), white blood cell count, platelet count, and renal dysfunction (blood urea nitrogen).
Clinical Behavior.
The effect of treatment with ribavirin and HRC 203 on MHV‐3–induced liver disease was determined by monitoring of mice twice daily for subjective signs of clinical behavior, including: abnormalities of fur texture, decreased activity, increased respiration, and presence of tremor. The development of decreased activity, increased fur ruffling, difficulties in respiration, and increased shaking were signs of poor clinical behavior. Grading was on a scale of 1 to 5, in which 1 was indicative of marked presence of the abnormality and 5 was normal. A composite score for all parameters was calculated and was performed by 2 independent examiners who were blinded to the treatment regimens in an attempt to minimize bias.
Collection of Blood and Tissues.
On the day of sacrifice, mice were anesthetized using halothane followed by percutaneous cardiac puncture for collection of blood in an EDTA‐containing Microtainer tube (Becton‐Dickinson, Franklin Lakes, NJ) as previously described.21 Livers were removed at time of death as described in the following sections.
Viral Titers.
The effect of ribavirin and HRC 203 on viral replication for both in vivo and in vitro studies was determined in a standard plaque assay. For in vitro studies, monolayers of peritoneal macrophages or hepatocytes from Balb/cJ mice were pretreated with ribavirin, HRC 203, or medium (RPMI 1640) before the addition of virus at an MOI of 0.3. At 72 hours post infection, cells were harvested, subjected to one cycle of freeze thawing, and assayed for viral titers on monolayers of L2 cells. For in vivo studies, sections of liver tissue were weighed and snap frozen at −70°C until all tissues were collected for analysis. Subsequently tissues were thawed and homogenized in a dounce homogenizer in Dulbecco's modified essential medium supplemented with 2% FBS and 4 mmol/L glutamine as a 10% homogenate at 4°C, and assayed for virus using the plaque assay described previously. Hp‐Hb and free Hb were shown to have no hepatocyte or macrophage toxicity and had no viral inhibitory effects at the concentrations studied (data not shown).
Liver Histology.
At time of sacrifice, livers were removed, weighed, and fixed in 10% buffered formalin for a minimum of 4 hours. Tissues were processed and paraffin‐embedded by routine methodology, sectioned to a thickness of 5 μm, and stained with hematoxylin, eosin and Martius Scarlet Blue.24 In addition, livers were immunostained for fibrin using the Nexes immunostaining method.24 Sections from livers recovered from 4 mice per group at each time point were scored as to the extent of necrosis and fibrin deposition. The surface of the liver examined was equal in all instances and corresponded to 10 contiguous histological fields at low magnification.
Blood Analysis.
Blood was analyzed for hemoglobin and hematocrit, prothrombin time, and partial thromboplastin time, using the portable Hemochron Jr. II Microcoagulation System (International Technidyne Corporation, Edison, NJ). Sera from all animals were analyzed quantitatively for measures of liver cell necrosis (serum ALT) by the University Health Network Central Laboratory as previously described.23 Blood sugar, electrolytes, and blood urea nitrogen were analyzed in all samples using an Ektachem 700 analyzer in the Department of Biochemistry, University Health Network, as previously described.24 Lack of interference of the biochemistry tests was addressed by including samples of normal sera spiked with different concentrations of the Hp‐Hb‐RV conjugate complex. Unused sera were frozen and stored for additional tests as required.
Statistical Analysis.
Statistical analysis was conducted by Student t test, and a P value of .05 or less was considered statistically significant. Results were reported as the mean ± SD for at least 3 separate experiments, each performed in triplicate.
Results
Effect of HRC 203 on the Growth of MHV‐3 in Macrophages
Previous reports have suggested that MHV‐3 infection is mediated through viral replication in macrophages before replication in hepatocytes.25 Therefore, experiments were performed to examine the efficacy of HRC 203 directly on viral replication in isolated macrophages. Cells were pre‐incubated with either PBS (control), free ribavirin (800 μmol/L), or HRC 203 (40 μmol/L conjugated ribavirin) for 1 hour before infection with the MHV‐3 virus. The concentration of ribavirin was chosen based on a previous report on the effect of ribavirin on viral replication in isolated macrophage cultures where a dose– response curve was conducted.25 Cells were harvested after 8 hours, and viral titers were measured as described previously. When added at a concentration of 800 μmol/L, free ribavirin had no effect on reducing viral titers. In contrast, a lower dose of ribavirin (40 μmol/L) conjugated to Hb did inhibit MHV‐3 replication (Fig. (Fig.1A).1A). The degree of inhibition was significant and represented an approximate 90% decrease in viral titers (1 log) compared with PBS‐ and ribavirin‐treated cells (Fig. (Fig.1B).1B). The inhibition in response to HRC 203 was not attributable to toxic effects on cells as demonstrated by trypan blue exclusion (data not shown).
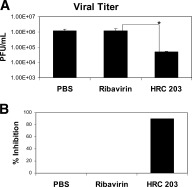
HRC 203 inhibits viral replication in macrophages after infection with MHV‐3 in vitro. Confluent layers of peritoneal macrophages isolated from Balb/cJ mice were pre treated with media (control), ribavirin (800 μmol/L), or HRC 203 (40 μmol/L conjugated ribavirin) for 1 hour before infection with 1000 PFU MHV‐3 virus. Viral titers were measured on monolayers of L2 cells in a standard plaque assay. Data represent mean ± SD of four independent experiments done in triplicate. (A) Viral titer; (B) % inhibition. *Comparison of HRC 203 to control and ribavirin by an unpaired t test (*two‐tailed P < .0001).
Binding of fHp‐Hb to Macrophages
The striking effect of HRC 203 on viral replication may be attributable to the targeted delivery of HRC 203 via the hemoglobin–haptoglobin receptor, CD163, located on the surface of cells from the monocyte/macrophage lineage. Previous studies have shown that CD163 expression is increased in response to glucocorticoid (dexamethasone) stimulation.26 We measured Hb‐binding to macrophages by culturing peritoneal macrophages (>95% CD11b+) with fluorescein‐labeled Hp‐Hb (fHp‐Hb). Binding of Hp‐Hb was compared between un‐stimulated, MHV‐3–infected, and dexamethasone‐stimulated macrophages (Fig. (Fig.2).2). Binding increased in a dose‐dependent fashion in all cases. However, Hp‐Hb binding tended to be greater in dexamethasone‐treated macrophages. These data are consistent with previous reports that dexamethasone induces the expression of CD163 on the surface of macrophages.26
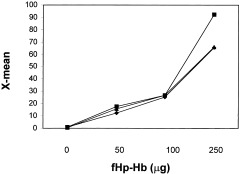
Binding of Alexa‐labeled haptoglobin–hemoglobin complex (fHp‐Hb) to macrophages. Macrophages were elicited with thioglycollate and divided into 3 groups: 1—untreated (diamonds); 2—MHV‐3–treated (triangles); or 3—dexamethasone‐treated (squares). The saturation curve compares fluorescent intensity (x‐mean values) between treatment groups, thereby measuring the amount of compound binding per macrophage.
Effect of HRC 203 on Production of TNF‐α and IFN‐γ in Macrophages
Experiments were performed to test the ability of HRC 203 to modulate the immune response of MHV‐3 macrophages. Ribavirin has been shown to alter the production of pro‐inflammatory cytokines by mouse peritoneal macrophages.25, 27 Supernatants from macrophages were analyzed for production of pro‐inflammatory cytokines TNF‐α and IFN‐γ. Macrophages produced significantly higher levels of TNF‐α and IFN‐γ in response to MHV‐3 infection in comparison with basal values (Fig. (Fig.3).3). Both ribavirin (800 μmol/L) and HRC 203 (40 μmol/L conjugated ribavirin) significantly inhibited the production of TNF‐α and IFN‐γ in MHV‐3–infected macrophages compared with controls. Both compounds prevented increases in cytokine levels above baseline; however, this effect was achieved using a much lower dose (1/20th) of ribavirin in the HRC 203 conjugated form than in the free ribavirin form.
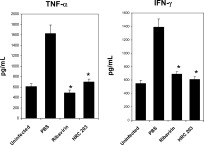
HRC 203 and ribavirin decrease pro‐inflammatory cytokine production by macrophages infected with MHV‐3 in vitro. One million (1 × 106) macrophages from Balb/cJ mice were infected with MHV‐3 at a multiplicity of infection of 2.5 in the presence or absence of phosphate‐buffered saline (PBS), ribavirin, or HRC 203. Levels of TNF‐α (A) or IFN‐γ (B) were measured in supernatants by ELISA after incubation for 8 hours. Data are presented as mean ± SD for 3 separate experiments done in triplicate. *P < .001 compared with PBS‐treated and MHV‐3–infected macrophages.
Effect of HRC 203 on Viral Replication in Isolated Hepatocytes
Experiments were performed to examine the efficacy of HRC 203 directly on viral replication in isolated hepatocytes. Hepatocytes isolated from Balb/cJ mice were pre‐incubated in the presence of increasing concentrations of either HRC 203 or ribavirin for 2 hours. After this incubation, the cells were then washed 3 times to remove unbound HRC 203 or ribavirin, then infected with MHV‐3, and kept in culture for 72 hours. The results from this experiment suggest that HRC 203 was more effective than free ribavirin in inhibiting viral replication (Fig. (Fig.4).4). Inhibition of viral replication by HRC 203 was approximately 75% at 1 μmol/L conjugated ribavirin, compared with no inhibition at the same concentration of free ribavirin. Inhibition by HRC 203 and free ribavirin were similar at 10 μmol/L of drug and higher.
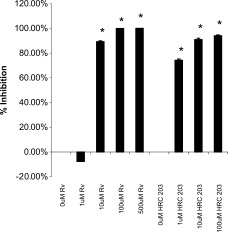
Percent inhibition of viral replication by HRC 203 and ribavirin (RV) in isolated hepatocytes. Bars represent the mean ± SD of 4 independent experiments done in duplicate compared with control (no drug) by an unpaired t test (*two‐tailed P < .001).
Another measure of improved efficacy was the ability of HRC 203 to inhibit cytopathic effects of the virus as indicated by the formation of hepatic cell fusion (syncytia) in response to viral infection (Fig. (Fig.5).5). When compared with normal uninfected hepatocytes (Fig. (Fig.5A),5A), MHV‐3–infected, untreated hepatocytes developed virus‐induced pathological conditions as evidenced by presence of syncytia within 24 hours of infection (Fig. (Fig.5B).5B). Pretreatment of MHV‐3–infected hepatocytes with HRC 203 (100 μg/mL) resulted in a marked reduction in virus‐induced pathological conditions (Fig. (Fig.5D).5D). In contrast, the same concentration (100 μmol/L) of free ribavirin had no effect on reducing the formation of syncytia by MHV‐3–infected macrophages (Fig. (Fig.55C).
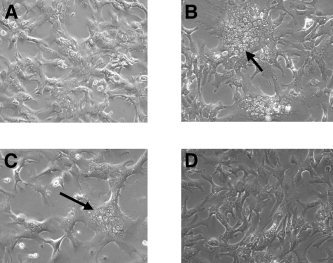
Effect of HRC 203 and ribavirin on syncytia formation in isolated cultures of hepatocytes. Hepatocytes were isolated from Balb/cJ mice. Normal uninfected hepatocytes are shown in (A). Viral cytopathic effect as indicated by syncytia formation is seen in MHV‐3–infected hepatocytes in (B) as well as in ribavirin‐treated (100 μmol/L) MHV‐3–infected hepatocytes (C) (arrows), whereas HRC 203–treated (100 μmol/L) MHV‐3–infected hepatocytes appear normal (D).
Survival and Clinical Outcome
Results from these in vitro studies demonstrate the improved efficacy of HRC 203 over free ribavirin. Therefore, a series of experiments were performed to evaluate the in vivo efficacy of HRC 203 on the course of fulminant hepatic failure induced by MHV‐3 in susceptible Balb/cJ mice. Balb/cJ mice were infected with the MHV‐3 virus and treated with PBS, free ribavirin, or HRC 203. After treatment, mice were examined daily for effects on survival and clinical behavior. One mouse from the untreated group and 2 mice from both the ribavirin and HRC 203–treated groups were sacrificed each day of the experiment for evaluation of viral titer, hematology, and biochemistry. Untreated MHV‐3–infected mice all developed clinical and biochemical signs (ALTmax 7986 ± 325 IU/L) of acute viral hepatitis, and died by day 4 post infection (Fig. (Fig.6,6, Table Table1).1). In contrast, survival was enhanced in both ribavirin‐ and HRC 203–treated MHV‐3–infected mice (Fig. (Fig.6A).6A). In association with increased survival was a reduction in liver necrosis as indicated by decreased levels of liver ALT (ALTmax 964 ± 128 IU/L ribavirin; 848 ± 212 IU/L HRC 203) (Table (Table1).1). Livers were assayed for determination of viral titers. By day 3, viral titers had risen to above the minimal detectable limit of the assay, although no significant difference between groups was observed (Fig. (Fig.6B).6B). By day 3, all PBS‐treated MHV‐3–infected mice died. Measurements of viral titers from livers of ribavirin‐ and HRC 203–treated mice on day 4 demonstrated a reduction in viral replication in HRC 203–treated animals compared with the ribavirin group (Fig. (Fig.66B).
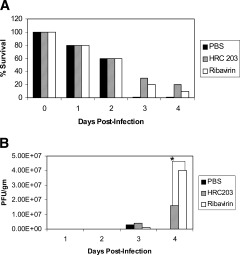
HRC 203 Therapy improves survival and reduces viral titers in MHV‐3–infected mice. Mortality in MHV‐3–infected mice is reduced in both HRC 203– and ribavirin‐treated mice compared with untreated controls (n = 10 per group). HRC 203 treatment is more effective than ribavirin in reducing liver viral titers in vivo. Data represent the mean ± SD of 3 experiments. (*P < .001)
Table 1
Effect of Treatment on Hematology and Biochemistry
| Days Post Infection | 5 | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Hematocrit (%) | ||||||
 Uninfected Uninfected | 0.41 ± 0.03 | 0.41 ± 0.02 | 0.40 ± 0.05 | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 0.41 ± 0.03 | 0.43 ± 0.03 | 0.42 ± 0.01 | — | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 0.42 ± 0.03 | 0.36 ± 0.04 | 0.33 ± 0.01 | 0.31 ± 0.02 | 0.30 ± 0.01 | 0.31 ± .01 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 0.41 ± 0.03 | 0.40 ± 0.01 | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.40 ± 0.02 | 0.42 ± .02 |
| Hemoglobin (g/L) | ||||||
 Uninfected Uninfected | 139 ± 12 | 140 ± 11 | 139 ± 11 | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 139 ± 11 | 139 ± 12 | 143 ± 7 | — | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 139 ± 11 | 122 ± 11* | 119 ± 4* | 112 ± 3* | 111 ± 4* | 114 ± 2* |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 140 ± 11 | 136 ± 4 | 136 ± 5 | 136 ± 4 | 134 ± 5 | 135 ± 2 |
| WBC (X10 9/L) | ||||||
 Uninfected Uninfected | 8.3 | 8.4 | ND | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 8.7 | 8.8 | 8.6 | — | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 9.0 | 8.6 | 9.1 | 9.0 | 8.9 | 8.6 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 8.4 | 8.7 | 9.1 | 9.1 | 9.1 | 9.0 |
| Sodium (mmol/L) | ||||||
 Uninfected Uninfected | 149 ± 1.2 | 148 ± 1.0 | 149 ± 1.2 | 147 ± 2.0 | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 145 ± 1.0 | 146 ± 1.7 | 147 ± 2.0 | 145 ± 1.3 | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 148 ± 1.2 | 151 ± 1.4 | 147 ± 1.3 | 151 ± 1.3 | 148 ± 1.4 | 152 ± 1.4 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 148 ± 1.4 | 152 ± 1.1 | 148 ± 1.0 | 149 ± 1.3 | 142 ± 2.0 | 143 ± 1.4 |
| Bicarbonate (mmol/L) | ||||||
 Uninfected Uninfected | 19.7 ± 2.3 | 20.1 ± 2.0 | 19.4 ± 2.0 | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 19.1 ± 2.1 | 18.0 ± 1.4 | 17.7 ± 2.5 | 17.0 ± 2.1 | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 18.9 ± 2.0 | 22.0 ± 2.0 | 18.0 ± 2.1 | 17.0 ± 1.1 | 21.0 ± 2.0 | 19 ± 2.2 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 20.0 ± 2.0 | 25.0 ± 2.2 | 18.0 ± 2.1 | 24.0 ± 2.1 | 21.0 ± 2.0 | 21 ± 1.8 |
| BUN (mmol/L) | ||||||
 Uninfected Uninfected | 8.0 | 9.5 | ND | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 8.0 | 11.2 | 19.2 | 14.3 | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 9.0 | 11.0 | 9.5 | 8.0 | 11.5 | 11.2 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 9.0 | 10.5 | 9.5 | 6.0 | 7.0 | 6.0 |
| ALT (IU/L) | ||||||
 Uninfected Uninfected | 30 ± 5 | 29 ± 6 | 31 ± 5 | ND | ND | ND |
 MHV‐3 PBS MHV‐3 PBS | 31 ± 7 | 65 ± 35 | 130 ± 110 | 7986 ± 325 | — | — |
 MHV‐3 Ribavirin MHV‐3 Ribavirin | 35 ± 5 | 45 ± 7 | 70 ± 10 | 205 ± 10 | 878 ± 238 | 964 ± 128 |
 MHV‐3 HRC 203 MHV‐3 HRC 203 | 40 ± 6 | 65 ± 5 | 45 ± 10 | 310 ± 20 | 587 ± 124 | 848 ± 212 |
NOTE. Data represents mean ± SD of 3 experiments.
Abbreviation: ND, not done; —, died before analysis.
The composite clinical score (Fig. (Fig.7A)7A) was obtained by combining the clinical score for all measured symptoms. MHV‐3–infected mice were observed for symptoms of abnormal fur texture (Fig. (Fig.7B),7B), respiration (Fig. (Fig.7C),7C), and tremor (Fig. (Fig.7D).7D). In all cases, PBS‐treated mice were observed to have the most severe symptoms. Ribavirin or HRC 203 therapy improved the clinical score of MHV‐3–infected mice. However, by day 4 HRC 203 therapy had improved clinical score to a greater degree than did ribavirin alone. Clinically, mice treated with ribavirin developed clinical symptoms compatible with drug toxicity or ongoing viral infection; in contrast, HRC 203–treated mice maintained improved scores in all behavioral categories to the end of the study (Fig. (Fig.77).

HRC 203 treatment improves clinical behavior. Mice were examined daily by 2 independent examiners for potential benefits of HRC 203 and ribavirin on clinical behavior after MHV‐3 infection, including; fur texture (B), respiration (C), and tremor (D), as well as a composite score for all 3 independent parameters (A). Each parameter was scored from 1 to 5, where 1 was markedly abnormal and 5 normal. Data represent the mean of 5 animals per group scored by each examiner for each parameter. A value of zero indicates no survivors.
Hematocrit and hemoglobin levels (Table (Table1)1) were measured to assess any effect of HRC 203 on the known hemolytic toxicity of ribavirin.28 Hemoglobin levels in HRC 203–treated mice were not greater than in the control, uninfected mice, suggesting that the dose of Hb in the form of HRC 203 did not contribute significantly to the total blood Hb concentration. HRC 203 administration provided an additional 4 mg Hb daily, providing only a modest 2.8% increase in total Hb (based on the total Hb concentration of 139 g/L for control untreated mice). Hb levels in the HRC 203–treated mice were statistically higher than those of ribavirin‐treated mice post‐infection (P < .01), and not statistically different from uninfected control mice or MHV‐3–infected and untreated mice from day 1 onward (Table (Table1),1), suggesting a decrease in blood total Hb and hematocrit in ribavirin‐treated mice over the course of the study. In addition, levels of blood urea nitrogen were higher and bicarbonate levels lower (not significantly lower) in ribavirin‐treated mice compared with HRC 203–treated mice, suggesting that ribavirin treatment resulted in disturbances in metabolic function in MHV‐3–infected mice. Collectively, these data suggest that conjugation of ribavirin to a carrier protein such as hemoglobin reduces the systemic toxicity associated with free ribavirin.
Effect of HRC 203 on Liver Histology
In addition to improving overall survival and clinical behavior, ribavirin and HRC 203 reduced the histological signs associated with MHV‐3 infection. Livers were removed and examined for histological evidence of necrosis and fibrin deposition (Fig. (Fig.8).8). The livers recovered from PBS‐treated MHV‐3–infected mice showed greater than 90% necrosis by day 3 post‐infection. In contrast, ribavirin or HRC 203–treated infected mice showed a marked reduction in histological evidence of hepatic necrosis with a maximum level of 10% hepatic necrosis (Fig. (Fig.8A).8A). The intensity of immunostaining for fibrin was significantly reduced in sections from the livers of ribavirin‐ and HRC 203–treated mice (Fig. (Fig.8A).8A). Histological grading of liver sections from the various treatment groups illustrates that the greatest intensity of fibrin staining was observed in PBS‐treated mice on days 3 and 4 of infection (Fig. (Fig.8B).8B). This intensity correlates with the intensity of observed clinical symptoms (Fig. (Fig.7).7). In contrast, the livers from ribavirin‐treated mice were negative for signs of necrosis and fibrosis. Although not completely free of disease, the livers from HRC‐203–treated mice showed a significant decrease in hepatic necrosis and fibrin deposition when compared with PBS‐treated mice (Fig. (Fig.88B).
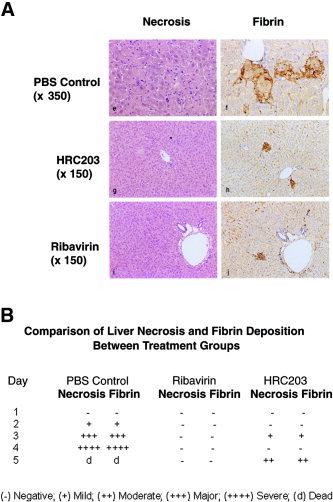
Effect of HRC 203 and ribavirin on hepatic histopathology after MHV‐3 infection. All liver tissues were stained with hematoxylin‐eosin and by a robotic immunoperoxidase method using antibody to fibrin/fibrinogen (DAKO). (A) At day 3 post infection, upper panel shows that PBS‐treated and MHV‐3–infected livers have marked hepatic necrosis (>50%) and deposits of fibrin (×350). In middle and lower panels, HRC 203– and ribavirin‐treated and MHV‐3–infected liver tissues show only focal areas of hepatic necrosis (×150) and minimal fibrin deposition (×150). The differences in magnification were used to illustrate details of the liver cell necrosis in the control animals. (B) Comparison of liver necrosis and fibrin deposition between untreated, ribavirin‐treated, and HRC 203–treated groups on all days of study. Liver tissues were harvested from animals on days indicated and examined for liver necrosis and fibrin deposition. Data represent mean of examination of 3 animals per group scored in a blinded fashion.
Discussion
Ribavirin is used in combination with interferon for the treatment of acute and chronic hepatitis C; however, its effectiveness is limited by hemolytic toxicity that requires dose reduction or discontinuation in a portion of the treatment population.28 Because of renal clearance and the need to maintain the sustained virological response (SVR), large daily doses of ribavirin are necessary to sustain a beneficial response.29 We have investigated the efficacy of a targeted form of ribavirin, HRC 203, against murine hepatitis virus strain 3 (MHV‐3), to assess the benefit of hemoglobin‐conjugated ribavirin compared with free ribavirin. MHV‐3 causes a spectrum of liver disease in mice including fulminant hepatitis, liver failure, and death.30 The in vitro and in vivo experiments reported here show that HRC 203 is more effective against MHV‐3 infection than higher doses of free ribavirin.
The results from in vitro experiments on MHV‐3–infected macrophages support the use of hemoglobin targeted drug therapy in vivo. Viral replication in macrophages is a prerequisite for MHV‐3 to gain access to the liver parenchymal cells. MHV‐3 replication was reduced in macrophages treated with HRC 203, in contrast to macrophages treated with an approximately 20‐fold higher dose of free ribavirin. This effect was not due to drug toxicity; macrophages incubated with either HRC 203 or ribavirin were viable as shown by exclusion of trypan blue. Free ribavirin produced no improvement in viral titers, compared with a 90% reduction in viral titers produced by HRC 203. Viral infection of macrophages also activates an immune cascade mediated by cytokine production.25 The production of pro‐inflammatory cytokines including TNF‐α and IFN‐γ is increased in MHV‐3–infected macrophages compared with uninfected macrophages.25 The delivery of HRC 203 to macrophages via the hemoglobin–haptoglobin receptor, CD163, allows for direct targeting of anti‐viral therapeutics to an important member of the innate immune system. Recent studies have demonstrated the increased expression of CD163 in the liver of patients with acute hepatitis compared with those with chronic hepatitis or non‐infected individuals.12 The increased presence of CD163 was attributed to the increased numbers of activated macrophages in the livers of affected individuals.12 The benefit of targeting macrophages is supported by the demonstration of a significantly greater reduction in viral titers and a comparable reduction in the production of pro‐inflammatory cytokines using a lower dose of drug in the conjugated form compared with that of free drug.
The results presented in this study also demonstrated an enhanced antiviral activity of HRC 203 in isolated mouse hepatocytes infected with the MHV‐3 virus. Free ribavirin was ineffective at reducing viral replication at a dose of 1 μmol/L. In contrast, when hepatocytes were incubated with the same concentration of drug in the form of HRC 203, a significant reduction in viral replication (78%) was observed. The dose of HRC 203 required to inhibit viral replication was 10 micromolar (μmol/L) in isolated hepatocytes, whereas for similar viral inhibition in cultures of macrophages, a 40 μmol/L concentration of ribavirin was required. Even at a concentration of 1 μmol/L significant inhibition of viral growth was seen in isolated hepatocytes, whereas in macrophages at this concentration no inhibition was seen.25 We do not have an explanation for this finding, but differences in binding of drug, intracellular processing, or rate of degradation of compound could account for these differences. The release of ribavirin monophosphate might explain the enhanced anti‐viral activity in hepatocytes; however, the ribavirin released from HRC 203 has been shown to be free ribavirin and not the monophosphorylated form.13 HRC 203 also inhibited the viral cytopathic effect (syncytia formation) of MHV‐3, whereas free ribavirin did not. The effect was specific for HRC 203, because neither the Hb‐Hp complex alone nor Hb‐RV conjugate alone were inhibitory (data not shown). Taken together, these findings suggest a receptor‐mediated process underlying the improved activity of HRC 203 compared with free drug. Although CD163 is not expressed on hepatocytes, hemoglobin and hemoglobin–haptoglobin have been shown to bind to hepatocytes in a receptor‐mediated fashion, although the hepatocyte receptor has not been identified.10, 31, 32
The effect of HRC 203 was also compared with free ribavirin in an in vivo model of viral hepatitis. MHV‐3–infected control mice all died within 4 days of infection of massive liver necrosis. HRC 203 and free ribavirin improved survival compared with untreated control, and HRC 203 treated mice exhibited improved clinical behavior whereas mice receiving free ribavirin showed symptoms consistent with drug toxicity or ongoing viral infection despite better histology in the free ribavirin group. In addition, HRC 203 maintained hematocrit values near baseline compared with a decreasing hematocrit observed in the free ribavirin group. Because the hemolytic effects of ribavirin normally occur only after 4 weeks or more of daily dosing in humans,33 whether the apparent avoidance of this effect in the MHV‐3–infected mice is directly related to this known toxicity of ribavirin is unknown. Nevertheless, although the exact mechanism for the beneficial effects of HRC 203 is not known, the data presented suggest that the coupling of ribavirin to hemoglobin improves the safety and efficacy of ribavirin by targeting the delivery of ribavirin to infected cells.
After 4 days of infection, both ribavirin‐treated and HRC 203–treated mice were alive. However, livers harvested from MHV‐3–infected mice treated with HRC 203 had significantly lower viral titers than livers from mice treated with approximately 3‐fold more free ribavirin. Data from our in vitro experiments suggest that the overall decrease in liver viral titers was most likely attributable to a decrease in viral titers in both macrophages and hepatocytes in response to HRC 203. Hemoglobin thus serves as a natural drug carrier for targeted therapy of such receptor‐bearing cells.
The production of the pro‐inflammatory cytokines TNF‐α and IFN‐γ was markedly reduced by both ribavirin and HRC 203 in MHV‐3–infected macrophages in vitro. Fibrin deposition in the liver is a response to the activation of the immune coagulation system, which involves these mediators. TNF‐α has been shown to be involved in the immune response to HCV and fibrosis progression.34, 35 In murine models, overexpression of IL‐12 and IFN‐γ can induce severe liver damage.36 Livers harvested from MHV‐3–infected mice treated with ribavirin or HRC 203 demonstrated a significantly lower level of fibrin deposition and necrosis compared with PBS‐treated mice. Therefore, a reduction of pro‐inflammatory cytokine production likely contributed to the overall improvement in liver histology.
Overall, the effects of HRC 203 on MHV‐3 infection in vivo demonstrated that conjugation and targeted delivery of ribavirin in the form of HRC 203 produced an enhanced anti‐viral activity, with significant improvement in all aspects of response to infection compared with untreated, infected mice, and provided a response comparable or better than a 3‐fold higher dose of free drug. In combination with the in vitro findings, the results presented here provide important proof‐of‐concept for the effectiveness of HRC 203 and the targeting of drug agents using hemoglobin. Collectively, these results suggest that targeting of ribavirin may allow for enhanced clinical and virologic benefit and set the stage for clinical trials designed to treat patients infected with hepatitis C. Although the results presented here do not suggest the identification of a new form of therapy for chronic hepatitis C, our data suggest that targeted delivery of ribavirin to macrophages and hepatocytes reduces many of the systemic side effects produced by free ribavirin. Therefore, sustained HRC 203 therapy could potentially alleviate systemic side effects such as hemolytic anemia that normally result in the premature cessation of ribavirin therapy.
We undertook this study to examine the potential benefit of a hemoglobin‐conjugated ribavirin compound (HRC 203) to prevent or ameliorate liver injury caused by murine hepatitis virus strain 3 (MHV‐3). We have shown that HRC 203 is effective against MHV‐3 infection, which causes a spectrum of liver diseases in mice, including fulminant hepatitis, liver failure, and death.30 We also found a marked anti‐viral and anti‐inflammatory effect of HRC 203 on macrophages, presumably mediated by CD163 cell‐specific uptake. This effect appears to be related to, or to directly ameliorate, liver injury in infected mice. The experiments performed provide evidence that that HRC 203 compound is biochemically and histologically efficacious in ameliorating the liver injury caused by MHV‐3.
Notes
†Potential conflict of interest: Dr. Levy is a consultant for Novartis, Astellas and Roche.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/hep.21072
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/hep.21072
Citations & impact
Impact metrics
Article citations
Inhibition of tick-borne encephalitis virus in cell cultures by ribavirin.
Front Microbiol, 14:1182798, 12 Jun 2023
Cited by: 4 articles | PMID: 37378295 | PMCID: PMC10291047
Antiviral Drug Delivery System for Enhanced Bioactivity, Better Metabolism and Pharmacokinetic Characteristics.
Int J Nanomedicine, 16:4959-4984, 22 Jul 2021
Cited by: 12 articles | PMID: 34326637 | PMCID: PMC8315226
Review Free full text in Europe PMC
Harnessing molecular recognition for localized drug delivery.
Adv Drug Deliv Rev, 170:238-260, 20 Jan 2021
Cited by: 7 articles | PMID: 33484737 | PMCID: PMC8274479
Review Free full text in Europe PMC
Advanced Prodrug Strategies in Nucleoside and Non-Nucleoside Antiviral Agents: A Review of the Recent Five Years.
Molecules, 22(10):E1736, 16 Oct 2017
Cited by: 27 articles | PMID: 29035325 | PMCID: PMC6151663
Review Free full text in Europe PMC
Equilibrative nucleoside transporter 1 expression in primary human hepatocytes is highly variable and determines uptake of ribavirin.
Antivir Chem Chemother, 25(1):2-10, 01 Jan 2017
Cited by: 5 articles | PMID: 28417642 | PMCID: PMC5890492
Go to all (23) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Synergistic antiviral effect of a combination of mouse interferon-alpha and interferon-gamma on mouse hepatitis virus.
J Med Virol, 69(2):188-194, 01 Feb 2003
Cited by: 20 articles | PMID: 12683406 | PMCID: PMC7166598
γδ T Cells Contribute to the Outcome of Murine Fulminant Viral Hepatitis via Effector Cytokines TNF-α and IFN-γ.
Curr Med Sci, 38(4):648-655, 20 Aug 2018
Cited by: 6 articles | PMID: 30128874
Telbivudine preserves T-helper 1 cytokine production and downregulates programmed death ligand 1 in a mouse model of viral hepatitis.
J Viral Hepat, 17 Suppl 1:24-33, 01 Mar 2010
Cited by: 10 articles | PMID: 20586931 | PMCID: PMC7166602
[Side-effects of pegylated interferon plus ribavirin therapy with or without protease inhibitor direct acting antiviral agents during treatment of chronic hepatitis C virus infection].
Orv Hetil, 152(50):1997-2009, 01 Dec 2011
Cited by: 3 articles | PMID: 22112373
Review
 1
,
‡
1
,
‡





