Abstract
Free full text

Adaptation and Acclimation of Photosynthetic Microorganisms to Permanently Cold Environments
Abstract
Persistently cold environments constitute one of our world's largest ecosystems, and microorganisms dominate the biomass and metabolic activity in these extreme environments. The stress of low temperatures on life is exacerbated in organisms that rely on photoautrophic production of organic carbon and energy sources. Phototrophic organisms must coordinate temperature-independent reactions of light absorption and photochemistry with temperature-dependent processes of electron transport and utilization of energy sources through growth and metabolism. Despite this conundrum, phototrophic microorganisms thrive in all cold ecosystems described and (together with chemoautrophs) provide the base of autotrophic production in low-temperature food webs. Psychrophilic (organisms with a requirement for low growth temperatures) and psychrotolerant (organisms tolerant of low growth temperatures) photoautotrophs rely on low-temperature acclimative and adaptive strategies that have been described for other low-temperature-adapted heterotrophic organisms, such as cold-active proteins and maintenance of membrane fluidity. In addition, photoautrophic organisms possess other strategies to balance the absorption of light and the transduction of light energy to stored chemical energy products (NADPH and ATP) with downstream consumption of photosynthetically derived energy products at low temperatures. Lastly, differential adaptive and acclimative mechanisms exist in phototrophic microorganisms residing in low-temperature environments that are exposed to constant low-light environments versus high-light- and high-UV-exposed phototrophic assemblages.
INTRODUCTION
Adaptation to Permanently Cold Environments
More than 70% of the earth exists as cold ecosystems that have a stable temperature below or close to the freezing point of water. Cold habitats include deep ocean, alpine, and polar environments. Metabolically active bacteria have even been isolated from supercooled, high-altitude cloud droplets (238). Cold environments are often dominated by microorganisms (including gram-negative and gram-positive bacteria, Archaea, yeasts, cyanobacteria, fungi, and protists), and represent the most abundant cold-adapted life-forms on earth at the level of species diversity and biomass (56).
In many cold ecosystems, the primary producers are the essential base of inorganic carbon fixation and autotrophic energy production, which allow food webs to develop by providing new organic carbon and nutrient sources (197, 212, 213, 270). Cold-adapted microorganisms include both psychrophilic (an organism with an optimal growth temperature at or below 15°C, and a maximum growth temperature below 20°C) (52, 170) and psychrotrophic (an organism exhibiting the ability to grow at temperatures below 15°C but exhibiting maximum growth rates at temperature optima above 18°C) organisms, and are often subjected to other extreme environmental parameters. Deep marine-dwelling species are exposed to extremely high pressures (baropsychrophiles) (280). Many microbial communities associated with the Antarctic and Arctic sea ice are subjected to salt concentrations several orders of magnitude higher than that of seawater (halopsychrophiles) (196, 266), and most of the terrestrial polar microorganisms are also exposed to osmotic stress and desiccation (53, 263). Microorganisms living in the Antarctic subglacial environment have been isolated from the atmosphere for more than 15 million years and exist at low-temperature, high-pressure, and low-nutrient levels (104, 221, 242). Phototrophic microorganisms growing on snow and ice surfaces in alpine or glacial environments are exposed to high light as well as UV radiation (72). In contrast, microalgal assemblages residing on the underside of the Arctic and Antarctic sea ice and planktonic algae growing beneath sea ice as well as under and in the permanent ice covers of the Antarctic dry valley lakes are photosynthetically active in a light environment less than 1% of incident photosynthetically active radiation (PAR) (119, 129). Importantly, all photoautotrophs residing in high-latitude polar environments must survive up to 5 months of total darkness between solar light cycles (196, 221).
To successfully colonize low-temperature environments, psychrophilic photoautotrophs have evolved a number of strategies that range from molecular to whole cell to ecosystem levels. The process of genetic change that accumulates over a time scale of many generations in response to an organism's specific environmental niche is termed adaptation. This is in contrast to acclimation, which refers to short-term physiological adjustments that occur during a lifetime in response to transitory changes in environmental conditions. Examples of adaptative mechanisms to low temperature include the evolution of cold shock and antifreeze proteins, the modulation of the kinetics of key enzymes, and the development of more fluid biological membranes through the accumulation of polyunsaturated fatty acyl chains. In contrast, acclimatory responses to transitory changes in the thermal environment are dependent upon sensor/signal pathways, and involve, for example, modulations in rates of transcription or translation of enzymes.
Photosynthetic microorganisms possess a myriad of mechanisms to acclimate to extremes in the light environment (from total darkness to extreme shade to photoinhibitory levels) to efficiently utilize photosynthetically available radiation while avoiding the detrimental effects of overexcitation of the photosynthetic apparatus. In addition, absorption of light energy (a temperature-independent process) must be tightly coordinated with the temperature-dependent processes of production of photochemically formed energy products (NADPH and ATP) and downstream catabolic consumption of these energy sources during organismal growth and metabolism. While comparative research on phototrophic organisms adapted to temperate versus low-temperature environments is in its infancy, laboratory-controlled research on pure-culture psychrophilic phototrophs (162, 163, 166) is beginning to reveal the unique adaptive mechanisms that these extremophiles employ to thrive in their environments, and how niche adaptation has impacted the capacity of low-temperature-adapted phototrophs to acclimate to environmental change (167-169).
Goals and Scope
The goals of this review are to identify adaptive and acclimative strategies exhibited by phototrophic microalgae to cold environments, to describe selected cold ecosystems on Earth that have been colonized by phototrophic microalgal communities, and to summarize the research on a well-characterized psychrophilic phototrophic eukaryotic microalga isolated from the liquid water column of a permanently ice-covered Antarctic lake and show how it can be a model of adaptation to a low-temperature ecosystem.
ADAPTATION AND ACCLIMATION TO LOW TEMPERATURE
Membrane Lipid Composition
A major adaptation of metabolic function influencing growth and photosynthesis at low temperatures is the maintenance of membrane fluidity (134, 162, 229, 252). The detrimental effects of low temperature on the rigidification of the membrane lipid bilayers such as loss of ion permeability have been clearly demonstrated (91, 134). Psychrophilic and psychrotrophic organisms utilize a combination of changes in fatty acid composition to regulate the fluidity of the membrane at low temperatures, such as incorporation of polyunsaturated, short-chain, branched, or cyclic fatty acids (272). In particular, the extent of unsaturation of the fatty acids in membrane lipids plays a major role in avoiding membrane rigidification at low temperatures.
Indeed, the role of unsaturation of lipids represents one of the most thoroughly investigated mediators of cold adaptation (30), and the production of polyunsaturated fatty acids has been used for chemotaxonomic classification of psychrophilic and psychrotolerant bacteria (81, 231) and microalgae (36), as well as a diagnostic indicator of thermal bleaching in corals (250). High polyunsaturated fatty acid concentrations have been detected in natural communities and isolated cultures of several low-temperature-adapted phototrophic microorganisms, including sea ice diatoms (85, 162, 163, 184), dinoflagellates (254), and green algae (167). In addition to temperature, the degree of unsaturation of membrane lipids in phototrophic organisms is also dependent upon factors such as high salinity (185), desiccation, light (253), and nutrient availability (227, 240).
In the paradigm for dissociative-type fatty acid biosynthesis, Escherichia coli, unsaturated fatty acids are synthesized de novo as part of the anaerobic fatty acid biosynthetic pathway: unsaturated fatty acids are produced via the isomerization of a trans-unsaturated fatty acid at the C10 level of the fatty acid biosynthetic pathway. Since this pathway is active during growth, modification of membrane fluidity via the introduction of unsaturated fatty acids can only occur in actively growing cultures.
In other bacteria and eukaryotes, double bonds are introduced postsynthetically into a fatty acid via an aerobic desaturation pathway that acts on membrane lipids (174). This reaction is catalyzed by a family of enzymes called desaturases, which introduce a double bond via an energy-dependent reaction which is both fatty acyl chain and bond position specific. In a variable thermal environment, microorganisms possessing the aerobic fatty acid desaturation pathway have an advantage over those relying on the anaerobic type of unsaturated fatty acid production in that fatty acyl chain modification can occur independently of cell growth.
Acclimation to low-temperature stress via an increase in expression of desaturases has been documented in poikilothermic organisms such as bacteria, algae, plants, and animals (2, 47, 84, 135, 139, 189, 255). Cyanobacteria have been particularly exploited as models of lipid unsaturation because several of the fatty acid desaturase genes have been genetically manipulated (234, 235). In the cyanobacterium genus Synechocystis, the expression of three desaturases (encoded by desA, desB, and desD) is inducible under cold temperatures. The sensor/signal pathway for regulation of transcription of the single desaturase found in Bacillus subtillus, encoded by des, has also been thoroughly investigated (35, 142).
In contrast, while the importance of unsaturated fatty acids in low-temperature adaptation of polar microorganisms is recognized, identification of the gene products involved in unsaturated fatty acid production in polar species has been largely unexplored. One exception is the identification of several putative enzymes involved in lipid biosynthesis and the production of unsaturated diether lipids in the Antarctic archaeon Methanococcoides burtonii (isolated from Ace Lake, Antarctica) (183). Low-temperature growth of M. burtonii cultures produced a high proportion of unsaturated lipids; however, the putative pathway to produce unsaturated fatty acids in the methanogen is distinct from either the anaerobic pathway in E. coli or the desaturase-mediated pathway in other bacteria and eukaryotes.
The fluidity of the membrane is closely connected with optimal photosynthetic function at low temperatures, which relies on the correct folding of complex multisubunit membrane-associated proteins which form the photosynthetic electron transport chain. The majority of protein components of the photosynthetic apparatus are anchored in the photosynthetic membranes via specific lipid species, the galactolipids (monogalactosyldiaclylglycerol [MGDG] and digalactosyldiacylglycerol [DGDG]), which are exclusively associated with the chloroplast. Membrane fluidity is also essential for electron transport between the photosynthetic complexes via mobile carriers such as plastoquinone (230), as well as the diffusivity of gases (226). Resistance to photoinhibitory damage, particularly at low temperatures, and the photosystem II repair cycle are also dependent upon the ability to desaturate fatty acids (70, 102).
While there is clearly a strong corelationship between the fatty acyl content of the photosynthetic membranes and optimal photosynthetic function at low temperatures, the role of lipids in low-temperature photosynthesis in polar microorganisms is largely unknown. Mock and Kroon (162, 163) recently reported on the interrelationship between membrane lipid composition and photosynthetic function during acclimation to low temperatures and either nitrogen limitation (162) or low irradiance (163) in mixed cultures of three sea ice diatom species. The sea ice microalgae exhibited large chloroplasts due to adaptation to the low-light conditions of the sea ice environment, producing cells whose cellular membranes were dominated by those in the thylakoid. Thus, major lipid classes under either N-replete or N-deplete conditions were the chloroplast-related classes MGDG and DGDG. Under nitrogen deplete conditions, diatom cultures exhibited a reduction in intracellular proteins and a concomitant rise in total lipids, particularly in the storage lipid triacylglycerol. The storage lipids act as a sinks for photosynthetic electron transport, which under nitrogen-replete conditions would be utilized for the reduction of nitrate. Furthermore, N limitation also resulted in a dramatic reduction in the MGDG-to-DGDG ratio.
MGDG is a non-bilayer-forming lipid class which can form a bilayer in thylakoid proteins due to the high proportion of proteins and pigments in the photosynthetic membranes. Presumably, the increase in overall lipids and in particular DGDG compensates for the loss of membrane-bound proteins and pigments and maintain membrane stability (162). Higher levels of unsaturated fatty acids in the photosynthetic membranes may also aid in assembly of D1 into photosystem II (see Fig. Fig.1),1), which has been shown to be dependent upon the degree of fatty acid unsaturation, particularly at low temperatures (174, 189). Despite these coordinated mechanism of adjusting lipid content and photosynthetic function to nitrogen stress, pools of the mobile electron acceptor plastoquinone were relatively reduced in N-limited cultures, indicative of an imbalance in absorbed light energy with utilization of stored photosynthetic energy in the form of reducing equivalents (162).

Oxygenic photosynthetic electron transport chain in the thylakoids of green algae and higher plants. Three major membrane-bound protein complexes functioning in series, photosystem II (PSII), cytochrome b6f complex (Cyt b6f), and photosystem I (PSI), are required to transport electrons from water to NADP+. Light energy is absorbed by the light-harvesting complexes, and excitation energy is transferred to the reaction centers, where it is used to drive charge separation of a chlorophyll pair (P680 and P700 for PSII and PSI, respectively). Electrons are transported from PSII to cytochrome b6f across the thylakoid membrane by the mobile transporter plastoquinone (PQ/PQH2), and from cytochrome b6f to PSI in the lumenal space by the small protein plastocyanin (PC). Electrons flow from NADPH to downstream metabolic reactions such as carbon and nutrient assimilation. QA, quinone A; QB, quinone B; FNR, ferredoxin-NADP oxidoreductase; A0, A1 FX, FA, and FB, intermediate electron acceptors of Photosystem I.
Priscu et al. (219) used the neutral lipid stain nile red to examine cell-specific neutral lipid levels in natural assemblages of sea ice microalgae in McMurdo Sound, Antarctica. They showed that neutral lipid:chlorophyll a, neutral lipid:particulate organic carbon, and neutral lipid-to-particulate organic nitrogen ratios were highest in assemblages dominated by the diatom algae Nitzschia spp. and Navicula glaciei. The lowest specific neutral lipid content was observed in the congelation ice samples dominated by the diatom Amphiphora spp. and in high-light surface assemblages dominated by the prymnesiophyte Phaeocystis ouchettii and the dinoflagellate Gymnodinium sp. Their data indicate that distinct differences in neutral lipid content and cellular C and N occur among natural assemblages of sea ice microalgal species and supports earlier work on lipid relationships in these microalgae by Nichols et al. (186, 187).
Cold-Adapted Enzymes
A critical adaptive feature of all cold-adapted microorganisms is the molecular adaptation of enzymes to compensate for the reduction in chemical reaction rates at low temperatures. The relationship between temperature and chemical reactions can be described by the Arrhenius equation (k = Ae−Ea/RT), where k is the rate constant, A is the constant for a particular reaction (e.g., frequency factor), Ea is the activation energy, R is the gas constant, and T is the absolute temperature in degrees Kelvin. Ea is related to Q10, the factor by which the rate changes by varying the temperature 10°C, according to the following relationship: ln Q10 = (Ea × 10)/(RT2T1), where T2 and T1 are the temperature limits for which Q10 is desired. In general a 10°C reduction in growth temperature causes biochemical reaction rates to decline two to three times (Q10 = 2 to 3). Therefore, the activity of a mesophilic enzyme can be reduced as much as 80-fold when the growth temperature is shifted from 37°C to 0°C. Despite the severe reduction of enzymatic activity, the doubling time of a psychrophilic bacteria at 4°C can be comparable to that of mesophilic bacteria grown at 37°C (57).
Clearly, the maintenance of appropriate reaction rates of enzyme-catalyzed reactions of essential metabolic processes must be one of the major challenges that cold-adapted microorganisms have overcome. One strategy to combat lowered reaction rates could be to increase enzyme concentrations; however, this would be energetically inefficient and there are only a few examples of this type of cold adaptation strategy (34, 185). While the underlying molecular mechanisms governing low-temperature adaptation in psychrophilic microorganisms are not well understood, there is a general consensus that a major adaptive strategy to compensate for reduced reaction rates is at the level of the catalytic efficiency (kcat/Km) of cold-adapted enzymes (67). This is accomplished either by higher turnover numbers (kcat) at the expense of Km (substrate concentration at half-maximum activity) or by optimizing both parameters (increasing kcat and decreasing Km). Several psychrophilic enzymes exhibit a temperature shift for maximal activity to lower temperatures and a concomitant unfolding at moderate temperatures (57, 103). These properties have been the result of amino acid substitutions that promote increased flexibility of the protein.
Recently, Napolitano and Shain (178, 179) have proposed an additional compensatory strategy for maintaining sufficient rates of biochemical reactions at low temperatures in a diverse collection of psychrophilic organisms. These authors found that in mesophilic and thermophilic organisms, levels of ATP and growth rates varied proportionally with respect to growth temperature, that is, at higher growth temperatures, an increase in energy demand (i.e., higher growth rates) coincided with an increase in energy supply (i.e., adenylate pools). Conversely, several psychrophilic organisms representing three kingdoms, Eubacteria, Fungi, and Protista (or two of the domains of life, Eucarya and Bacteria [277]) exhibited an inverse relationship between adenylate levels and growth temperature (178), despite the fact that growth rates (i.e., energy demand) varied proportionally with growth temperature in all the psychrophilic organisms. Thus, it appears that elevated ATP and total adenylate pools may represent an additional adaptive strategy to compensate for lower rates of biochemical reactions at low temperatures. It is likely that altered activity of a key enzyme(s) involved in adenylate metabolism, such as F1 ATPase or AMP phosphatase/deaminase, governs the differences in energy metabolism between the psychrophiles and either the mesophiles or thermophiles. However, biochemical and genetic evidence is currently unavailable to address this hypothesis (178).
While the research regarding enzymes from psychrophilic microorganisms has begun to increase in recent years, little attention has been paid to cold-adapted enzymes from phototrophic microorganisms. Loppes et al. (133) investigated temperature dependence and thermolability of nitrate reductase and argininosuccinate lyase from a psychrophilic Chloromonas sp. isolated from Petrel Island in Antarctica. Both psychrophilic enzymes exhibited a shift in maximal enzyme activity to lower temperatures. In particular, argininosuccinate lyase exhibited 25% of its maximum activity at 5°C, while the enzyme isolated from the mesophilic Chloromonas reinhardtii was completely inactive at this temperature. Lastly, both psychrophilic enzymes also exhibited a lower thermal stability than the mesophilic counterparts.
In contrast with argininosuccinate lyase, the temperature maximum for carboxylase activity of ribulose-1,5-bisphosphate carboxylase (Rubisco), one of the most critical enzymes for inorganic carbon fixation in phototrophs, was not altered in two isolates of the Antarctic Chloromonas sp., and the specific activity at low temperatures was actually lower in the psychrophilic compared with the mesophilic Rubisco (42). This was one of the few exceptions described so far of a psychrophilic enzyme not exhibiting higher catalytic activity at lower temperatures. In agreement with this report, Rubisco isolated from Antarctic hairgrass also exhibited lower activity at lower temperatures, and activity increased linearly up to an incubation temperature of 50°C (236). A similar trend was observed in winter-hardened-crops species (232). Conversely, the protein was thermally sensitive to inactivation in both Antarctic algae and plants (42, 236). While sequence analysis and modeling of the large subunit (rbsL) of Rubisco in the psychrophilic Chloromonas sp. indicated no changes in amino acid residues directly involved in catalysis, several substitutions may contribute to the stability of the enzymes and interactions with the small subunit (281). In contrast, heat inactivation of Rubisco in the Antarctic grass species was interpreted as an imbalance in the rates of Rubisco inactivation and reactivation by Rubisco activase (236).
A study comparing the temperature dependence of NO3− and NH4+ incorporation, and the activity of the assimilatory enzyme NO3− reductase with photosynthesis (CO2 incorporation) in natural assemblages of psychrophilic Antarctic sea ice microalgae (218) revealed that NO3− and NH4+ incorporation reached maximal rates between 0.5 and 2.0°C (Q10 = 10.1) and 2.0 and 3.0°C (Q10 = 15.7), respectively, which was close to that for CO2 incorporation (2.5 to 3.0°C; Q10 = 16.1).
These metabolic characteristics show the psychrophilic tendencies of the microalgal assemblage and, by virtue of the high Q10 values, further show that their metabolic activity can change rapidly given a small change in temperature. Conversely, NO3− reductase showed a distinctly higher temperature maximum (10.0 to 12.0°C) and a lower Q10 value (1.4) than either inorganic N or C incorporation. These results led the authors to conclude that, owing to differential temperature characteristics between N transport and N assimilation at the in situ growth temperature (−1.9°C), the incorporation of extracellular NO3− into cellular macromolecules may be limited by transport of NO3− into the cell rather than the intracellular reduction of NO3−. Such cases of differential temperature responses by selected biosynthetic pathways may play a role in the overall C/N acquisition ratios of organisms living in cold habitats, a contention that has been corroborated by other field studies on sea ice microalgae (216, 218).
Photosynthetic Electron Transport and Energy Balance
The photosynthetic electron transport chain in phototrophic organisms possesses a myriad of adaptive and acclimative mechanisms to perform transduction of light energy to chemical energy at low temperatures. In oxygenic phototrophic organisms (plants, green algae, and cyanobacteria), photosystem II (PSII) and photosystem I (PSI) are integral thylakoid membrane protein complexes (Fig. (Fig.1).1). The bulk of the chlorophyll and carotenoid present within the chloroplast thylakoid membrane is bound to the Lhcb (genes encoding light-harvesting complex II [LHCII] proteins) and Lhca (genes encoding LHCI proteins) families of light-harvesting polypeptides associated with PSII and PSI, respectively, the PSII core antenna polypeptides of PsbB (CP47) and PsbC (CP43), the PSII reaction center polypeptides PsbA (D1) and PsbD (D2), and the PSI reaction center polypeptides PsaA and PsaB (74).
A very prevalent group of oxygenic phototrophs found in low-temperature environments are the chromophytes, of which diatom algae in particular dominate marine and sea ice habitats (Table (Table1).1). The diatoms possess a typical oxygenic photochemical apparatus; however, chlorophyll b is replaced by chlorophyll c, and fucoxanthin is a major carotenoid (74). Green algae play various roles in low-temperature environments, which are often more likely to be dominated by prokaryotic photosynthetic microorganisms. Notable exceptions are found in two divergent low-temperature environments, the alpine snow ecosystem, which is dominated by psychrophillic Chlamydomonas and Chloromonas spp., and the permanent ice-covered lakes of the McMurdo Dry Valleys, which are vertically stratified layers of green algae (Table (Table1).1). Both ecosystems are discussed in detail later in this review.
TABLE 1.
Ecosystem properties and adaptive strategies of low-temperature photosynthetic microorganisms found in selected permanently low temperature environmentsa
| Ecosystem | Physical extremes | Photosynthetic members | Adaptive strategies |
|---|---|---|---|
| High alpine | High UV | Chlamydomonas spp. | UV and PAR screening |
| High PAR | Chloromonas spp. | MAA | |
| Variable dark periods | Filamentous Xanthophyceae | Flavenoids | |
| Spore formation | |||
| Mucilage production | |||
| Sea ice | Low light | Pennate diatoms dominate | PUFA |
| High brine salinity | Cryoprotectants | ||
| Rapid fluctuations in physical | Osmoregulators | ||
 environment environment | High light-harvesting capacity | ||
| Seasonal darkness | Heterotrophic carbon acquisition | ||
| Narrow spectral band | |||
| Ponds | High UV | Cyanobacteria (primarily Nostoc, | Mucopolysacchride maxtrix |
| High irradiance |  Phormidium, and Anabaena spp.) Phormidium, and Anabaena spp.) |  UV-screening pigments (e.g., mycosporine-type UV-screening pigments (e.g., mycosporine-type | |
| Prolonged desiccation | Eukaryotic algae |  amino acids) amino acids) | |
| Freeze-thaw cycles | |||
| Seasonal darkness | |||
| Ice-covered lakes | Low irradiance | Chlamydomonas spp. | Enhanced light-harvesting capacity |
| Narrow spectral light range | Ochromonas spp. | Blue light adapted | |
| P and N deficiency | Chroomonas spp. | PUFA | |
| Variable salinity | Pyraminomonas spp. | ||
| Vertical stratification | Purple nonsulfur bacteria | ||
| Seasonal darkness | Cyanobacteria |
In contrast to the intrinsic chlorophyll a and b light-harvesting pigment-protein complexes found in chloroplast thylakoid membranes of plants and green algae, the light-harvesting complex of cyanobacteria is an extrinsic pigment-protein complex called a phycobilisome which is bound to the outer, cytoplasmic surface of cyanobacterial thylakoids (69, 241). Phycobilisomes are rod-shaped chromoproteins called phycobiliproteins which may constitute up to 40% of total cellular protein. The phycobiliproteins usually associated with phycobilisomes include allophycocyanin, phycocyanin, and phycoerythrin. Cyanobacteria are distinct from chlorophytes because the redox carriers involved in respiratory as well as photosynthetic electron transport are located in the cyanobacterial thylakoid membranes, where they share a common plastoquinone (PQ) pool and a common cytochrome b6f complex (28, 29, 239). The metabolic diversity and structural conservation of prokaryotic microorganisms conferred a selective advantage (relative to eukaryotes) for survival in cold environments. Most low-temperature environments are dominated by bacteria, and photosynthetically active cyanobacterial mats are major providers of both organic carbon and nitrogen sources to these extreme environments, including Arctic and Antarctic ponds as well as numerous systems in the McMurdo Dry Valleys of Antarctica (215).
Cyanobacterial mats are particularly prevalent in shallow aquatic systems in both the Arctic and Antarctic. The cyanobacterial members of these systems are adapted and acclimated to a range of extremes in the thermal environment, freezing and thawing cycles, and photoprotection, as well as nutrient limitation (175). Numerous studies have shown that the microbial mats rely on adaptation of pigmentation to both maximize light-harvesting ability as well as protect against damaging light levels (16, 92, 171, 223, 262). Furthermore, Roos and Vincent (228) showed that the mat-forming cyanobacterium Phormidium murrayi isolated from the McMurdo Ice Shelf acclimated to laboratory-controlled changes in PAR, UV, and temperature by regulating ratios of light-harvesting and light-screening pigments.
Unlike the oxygenic photosynthetic process, the photochemical process of anoxygenic photosynthesis does not lead to the production of molecular oxygen. Two types of reaction centers are found in the anoxygenic phototrophic bacteria: type I, associated with green bacteria, and type II, associated with purple bacteria. Associated with the reaction center is a simple light-harvesting antenna, which can be partitioned into an inner antenna closely associated with the reaction center and a peripheral antenna. The pufM gene encodes the pigment-binding reaction center protein of all purple phototrophic bacteria, and pufM-specific primer sets have been utilized as a diagnostic tool to assess the abundance and diversity of purple sulfur and purple nonsulfur phototrophs in natural environments, including stratified lake ecosystems in the dry valleys of Antarctica (1, 106). The major light-harvesting pigments are bacteriochlorophyll and carotenoids. As in cyanobacteria, some of the photosynthetic electron transport chain components are shared with the respiratory chain.
Anoxygenic phototrophs form a diverse group that include green sulfur bacteria, green nonsulfur bacteria, heliobacteria, and purple bacteria and perform a primary role in carbon and sulfur cycling in aquatic ecosystems, particularly in anoxic zones in stratified lakes. Recently, Karr et al. (106) determined the distribution of the pufM gene to reveal the highly stratified nature of purple nonsulfur bacteria in the anoxic zone of Lake Fryxell, a perennially ice-covered lake located in the McMurdo Dry Valleys, Antarctica. While future pure-culture analyses of specific isolates are necessary to investigate adaptive and acclimative strategies specific to anaerobic photoautotrophs, the high diversity of the purple bacterial population implies successful exploitation of this extreme aquatic environment of low light intensities, near-freezing water column, and anoxic conditions by this group of anoxygenic photoautotrophs (106).
Balancing the energy flow through the process of photosynthesis is a challenge due to differential temperature sensitivities and differential rates between the photochemical reactions and the biochemical reactions. The balance of energy flow between the photophysical and photochemical processes that transform light and the metabolic sinks that consume the energy is called photostasis (93, 192). The following equation, derived by Falkowski and Chen (55), defines photostasis, σPSII × Ek = τ−1, where σPSII is the effective absorption cross section of PSII, Ek is the irradiance (I) at which the maximum photosynthetic quantum yield balances photosynthetic capacity estimated from a photosynthetic light response curve, and τ−1 is the rate at which photosynthetic electrons are consumed by a terminal electron acceptor such as CO2 under light-saturated conditions.
× Ek = τ−1, where σPSII is the effective absorption cross section of PSII, Ek is the irradiance (I) at which the maximum photosynthetic quantum yield balances photosynthetic capacity estimated from a photosynthetic light response curve, and τ−1 is the rate at which photosynthetic electrons are consumed by a terminal electron acceptor such as CO2 under light-saturated conditions.
An imbalance between energy absorbed versus energy utilized will occur whenever the rate at which the energy absorbed through PSII and the rate at which electrons are injected into photosynthetic electron transport exceed the metabolic electron sink capacity, that is, whenever σPSII × Ek > τ−1. Thus, photosynthetic microorganisms growing in low-temperature environments are potentially under a constant state of energy imbalance due to the decrease in τ−1. Excitation pressure (94, 147) or excessive excitation energy (105) is a relative measure of the reduction state of quinone A and reflects the redox state of the intersystem PQ pool (94). This can measured in vivo or in vitro by pulse amplitude-modulated fluorescence as either 1 − qP (in which qP is photochemical quenching) or as suggested more recently as 1 − qL ( in which qL is the fraction of open PSII centers) (113). Thus, excitation pressure is a measure of the imbalance in energy flow, that is, a measure of the extent to which I > Ek, and thus, σPSII × I > τ−1.
× I > τ−1.
The inequality illustrated above also provides insights into the possible mechanisms by which phototrophic organisms may respond to the imbalance in energy budget to attain photostasis. Figure Figure22 illustrates the possible fates of absorbed light energy through the photosynthetic apparatus. Energy balance is attained by either reducing σPSII by reducing light-harvesting antenna size and/or reducing the effective absorption cross-sectional area of PSII by dissipating energy nonphotochemically as heat (55, 89, 114). Roos and Vincent (228) reported that cultures of the Antarctic mat-forming cyanobacterium P. murrayi exhibited a similar acclimatory response to either low temperature or high PAR in a way similar to that of the mesophilic green alga Chlorella vulgaris (147), that is, to reduce the functional size of PSII by significantly increasing the carotenoid/chlorophyll a ratio under either low temperatures or high PAR or UV levels. This provides evidence that, like mesophilic organisms, low-temperature-adapted photoautotrophs sense and respond to excitation pressure (228).

Schematic showing the function of the photosynthetic electron transport chain as a redox sensor. The process of photosynthesis integrates the fast temperature-independent photochemical reactions of light absorption and charge separation with the “slow” processes of electron transport and downstream utilization of electron sinks through growth and metabolism. Any environmental stress that perturbs the poise between energy absorbed and energy utilized is sensed by the photoautotrophic organism at the level of the redox state of intersystem electron transport pool (excitation pressure, redox) and/or the build-up of protons across the thylakoid membranes (proton motive force). Low temperatures (Low T) cause an imbalance between energy absorbed and energy utilized by reducing rates of energy consumption by downstream metabolic processes. The energy imbalance is corrected at the level of either light absorption via modulation in the absorptive cross-section of PSII (e.g., dissipation of excess energy as heat, NPQ) or at the level of energy utilization (e.g., modulations at the level of Calvin cycle enzymes). Ox, oxidized; red, reduced.
Photostasis can also be attained by increasing sink capacity (n × τ−1) (93). This may be accomplished by elevating the levels of Calvin cycle enzymes. In the Antarctic grass Deschampsia antarctica, cold acclimation under high light intensities involves maintenance of high rates of photochemical efficiency (199) in combination with high photosynthetic rates (279), rather than adjustments at the level of nonphotochemical quenching. Therefore, in the Antarctic grass species, adjusting the imbalance between light absorbed and energy utilization is at the level of sink capacity rather than the functional size of the photosynthetic unit.
Lomas and Gilbert (130) have proposed an alternate energy sink in diatoms under conditions when light absorption exceeds metabolic energy requirements. Under a growth environment of low-temperature-induced high excitation pressure, natural diatom-dominated populations exhibited relatively high rates of NO3− uptake. These authors suggested that the N uptake in excess of nutritional requirements was an adaptive mechanism to modulate the balance between photosynthetic energy production and to compensate for a reduced metabolic energy requirement at low temperatures. More recently, Parker and Armbrust (198) showed that laboratory-grown cultures of the diatom Thalassiosira pseudonana exposed to low-temperature-induced high excitation pressure balanced energy sink utilization between nitrogen metabolism and photorespiration in a complementary manner. When replete nitrate levels were available in the growth medium, excess energy was consumed via NO3− reduction; however, when NO3− levels were depleted, the photorespiration pathway became the major excess energy sink. These data indicate that diatoms exhibit an acclimatory ability to finely tune acclimatory responses to energy imbalances between photochemistry and cellular metabolism (198). This adaptation to low temperature may be an indicator of the dominance of diatoms in many permanently low-temperature marine environments, such as the polar sea ice.
PERMANENTLY COLD ECOSYSTEMS
Phototrophic prokaryotic and eukaryotic microorganisms are widely distributed across a variety of low-temperature environments. These environments can differ widely in their physical and chemical properties, which influence the survival strategies of the organisms living within each ecotype (see Table Table1).1). The various survival strategies often produce unique biogeochemical and consortial relationships among the organism within the habitats (194); therefore, knowledge of the physical and chemical properties of the environment is an integrative step to understanding of microbial photoadaption within each environment (61, 181, 211, 267).
High-Alpine Snowfields
The presence of “colored snow” in alpine snowfields which persists through the summer is a common occurrence worldwide at high altitudes (>2,500 m) and polar regions. At this altitude, the light level is extremely high and is exacerbated by PAR reflecting off the snow, so that PAR levels as high as 5,000 μmol m−2 s−1 reach algal cells (274). In addition, algal populations can be exposed to as much as 30% more UV compared with phototrophic organisms growing at sea level. This UV effect is more pronounced for wavelengths in the UV-B region (15).
UV-B and UV-A are both particularly damaging to phototrophic microorganisms, which typically exhibit suboptimal rates of growth and photosynthesis under UV stress. UV-B damages cells at the nucleotide and protein level, inhibits photosynthesis, and prolonged exposure can lead to cell death (268). UV-A exposure causes more indirect detrimental effects in the form of reactive oxygen species that attack nucleotides, proteins, and lipids (260). The potential for photooxidative stress under excessive high light is exacerbated by year-round low temperatures (around 0°C). Algal populations living below the snow surface are exposed to less damaging levels of PAR as well as UV radiation; however, during the summer months, snow melting occurs rapidly, so that cell transport in the meltwater changes the vertical profile of algal assemblages, resulting in a highly variable daily PAR exposure (75). On the other hand, snow cover in these environments can also effectively attenuate 100% of PAR, so that algal communities must also cope with variable periods of total darkness (11).
Despite this harsh environment of low temperatures and high and variable light, more than 100 species of chlorophytes (mainly Chlamydomonas and Chloromonas spp.) have been identified as the dominant organisms in the algal blooms contributing to red, yellow, gray, and green snow patches. Single cells isolated from these algal patches have been shown to be photosynthetically active (72, 274). Therefore, the algae inhabiting these environments must possess adaptive mechanisms to survive a temperature and light regimen that would cause severe photoinhibition and photooxidative damage to most plants.
One of the most-studied snow algae is Chlamydomonas nivalis, which is responsible for causing the more commonly observed red coloration of snow patches (271). The most prevalent photoinhibitory avoidance mechanism possessed by C. nivalis is the red coloration of the cells, caused by high levels of the secondary carotenoid astaxanthin. Astaxanthin accumulates around the single, centrally located chloroplast as lipid droplets. Badgered et al. (14) reported that astaxanthin was esterified to either a monounsaturated or a diunsaturated fatty acid in red vegetative cells and cysts, respectively, and the esterification of the auxiliary pigment to a fatty acid allows the chromophore to be concentrated within lipid globules to maximize photoprotection. Astaxanthin exhibits a maximal absorption wavelength of around 474 nm, and red vegetative or resting cells are almost entirely shaded from light in the shorter (blue) wavelengths. Recently, it was found that astaxanthin screens UV wavelengths in C. nivalis (72) and provides a photoprotective role by screening the chloroplast in other algae (80). C. nivalis is also known to accumulate phenolics in response to UV exposure (51) and, to a lesser extent, may also rely on mycosporine-like amino acids (strong UV-absorbing water-soluble molecules commonly synthesized by algae) (22, 72).
Since the majority of studies have focused on natural snow algal communities, evidence of acclimation to variations in environmental conditions is scant. One exception is a recent study by Baldisserotto et al. (11) regarding acclimation of thylakoid ultrastructure and photosynthetic apparatus in the filamentous snow alga Xanthonema sp. to prolonged exposure to complete darkness to mimic acclimation to the austral night. In response to prolonged darkness, Xanthonema cells exhibited a preferential disassembly of PSII while largely retaining the light-harvesting complexes of PSII for up to 35 days. Evidence of this acclimative response suggests that natural snow algal populations may preserve thylakoid ultrastructure organization via LHC-mediated stabilization of the photosynthetic membranes (11).
Polar Sea Ice
Polar sea ice covers 5% of the Northern Hemisphere and 8% of the Southern Hemisphere, but sea ice is also found in the Baltic, Caspian, and Knots seas, making it one of the major biomes in terms of surface area (up to 13% of the Earth's surface) (124). The most prevalent sea ice ecosystem is in the high latitudes of the Southern Hemisphere, where more than 40% of the Southern Ocean is covered during winter by sea ice that often exceeds 1 m in thickness (251). Sea ice supports rich single-celled microbial ecosystems, in addition to small metazoans (195). However, annual recession of the ice as well as breakup of the pack ice by physical forcing can make this a highly variable habitat from a seasonal down to a daily and even hourly level (60). Thus, physiological and metabolic adaptation of the sea ice biota must involve the ability to acclimate to rapid fluctuations in the physical environment due to the transient nature of the sea ice.
The food webs in the pack ice of the continent are supported primarily by photoautrophic production by phytoplankton, and many algal classes (Bacillariophyceae, Chrysophyceae, Chlorophyceae, Cryptophyceae, Dinophyceae, Prymnesiophyceae, Prasinophyceae, and Cyanobacteria) have been identified in the polar ice communities (111). The pennate diatoms (Fragilariopsis cylindrus and Fragilariopsis surta) are the most abundant organisms and are the major contributors to the brown coloration in the ice during summer algal blooms (124). During the transition from winter to spring in polar ice regions, large algal blooms are initiated and can attain very high biomass (up to 1,000 μg chlorophyll a/liter in the sea ice), much higher than typical of the diatom-dominated phytoplankton biomass of the surrounding waters of the Southern ocean (typically less than 5 μg chlorophyll a/liter). These algae are adapted to survive temperatures as low as −20°C as well as the dehydrating effects of high salinities.
As discussed above, adaptation of the lipid membranes via enrichment in polyunsaturated fatty acids is a common low-temperature adaptation mechanism in psychrophilic bacteria (157) isolated from sea ice as well as the sea ice diatoms isolated from the Antarctic (162, 163) and Arctic ice shelves (85). A higher degree of unsaturated fatty acids in the membrane lipids is also an adaptive advantage under high-salinity stress. Sea ice algae produce high levels of osmoregulators and cryoprotectants such as proline, polyols, betaines, and dimethylsulfonioproprionate (43, 46). Dimethylsulfonioproprionate production in the sea ice communities has received heightened attention, as it is a significant biological source of the volatile product dimethyl sulfide, which contributes to the sulfur load in the atmosphere (23, 45, 110).
The major environmental factor affecting the growth of sea ice communities is light. The light environment of sea ice is extremely low due to the reflection of the majority of PAR (85 to 99% surface irradiance) via the snow cover and sea ice and relatively high attenuation by the snow and ice itself (117, 149, 225). UV stress on the sea ice algae was initially assumed to be low owing to attenuation by the overlying ice; however, with the widening of the ozone hole over the Southern Hemisphere, sea ice communities are now being exposed to higher levels of UV-B radiation (258). A number of studies on natural and isolated cultures have indicated that the sea ice diatoms exhibit extreme shade adaptation (33, 96). Very low light compensation points (0.2 to 1 μmol m−2 s−1) (31) have been reported for natural populations residing on the underside of the ice sheet, with light saturation of growth reported for some ice algae to be below 20 μmol m−2 s−1 (73, 225, 273). Sea ice algae are adapted to low light levels by an augmented light-harvesting apparatus as well as by heterotrophic acquisition of carbon and energy sources, and can actively take up amino acids, sugars, and organic acids. However, heterotrophic growth appears to play a minor role in ice algal blooms. Palmisano et al. (196) estimated the heterotrophic capacity of ice algae to be about 0.3% relative to rates of photosynthesis.
Natural populations possess the ability to adjust pigmentation in response to changes in the light environment. In sea ice microalgal assemblages dominated by diatom algae, the ratio of the xanthophyll diatoxanthin relative to diadinoxanthin increased proportionally with an increase in irradiance levels from sunrise to afternoon (107). Variability in photosynthetic capability as well as pigmentation has also been observed at the level of changes in vertical positions in the sea ice (32, 108). Furthermore, ice algae were shown to possess a functional xanthophylls cycle, the diadinoxanthin cycle, when isolated cultures were exposed to irradiance levels that were four times higher than the natural light environment (116), and microalgae populations in Antarctic sea ice exhibit the ability to avoid short-term photoinhibitory conditions by dissipating excess light energy nonphotochemically (NPQ) (224). Thus, despite extreme shade adaptation, isolated diatom cultures have retained the xanthophyll cycle-mediated acclimatory mechanisms of dissipation of excess light energy via heat.
Photoacclimation of psychrophilic diatoms to variations in temperature and irradiance has also been reported in laboratory-controlled conditions (161, 164). Mock and Hock (161) reported that while initial exposure of laboratory-controlled cultures of the polar diatom Fragilariopsis cylindrus to a downshift in temperature induced a cold shock response at the level of both PSII photochemical efficiency and photosynthetic capacity, recovery from cold shock was observed after a few days of exposure; F. cylindrus possesses the ability to photoacclimate to changes in temperature environment. Furthermore, cultures acclimated to lower temperatures exhibited a higher capacity for NPQ in a manner that was comparable to high-light acclimation.
Mock and Valentin (164) recently constructed one of the first expressed sequence tag libraries in F. cylindrus. By monitoring gene expression during a downshift in temperature from 5°C to −1.8°C, these authors showed that when exposed to moderate irradiance levels, cells acclimated to lower temperatures by downregulating expression of PSII and carbon fixation genes while upregulating genes encoding chaperons and those involved in plastid protein synthesis and turnover. In contrast, cultures grown under low light did not respond to the temperature downshift by upregulating genes involved in chaperone or protein turnover function (164). These studies are some of the first to provide evidence that polar diatoms photoacclimate to low temperatures in a light-dependent manner and are probably sensing changes in excitation pressure.
Transitory Ponds
The ice shelves of the Arctic and Antarctic support shallow pond ecosystems that are created during the summer season when pockets of the ice melt to form bodies of liquid water of various sizes. While the formation of liquid water is seasonally transitory, the ponds often melt out in the same location every year, and the microorganisms, particularly the microbial mats, exhibit several decades of seasonal growth. The microorganisms that colonize these extreme habitats must be capable of surviving daily and annual freezing-thawing cycles, persistent low temperatures, continuously high exposure to solar radiation during the summer, and long periods of dormancy. Despite these constraints, there exist diverse and productive consortia of microorganisms in the form of microbial (cyanobacterial) phototrophic mats. These biota are representative modern-day examples of how life survived and evolved during global glaciation and extended periods of extreme cold (263-265).
According to the snowball Earth hypotheses, around 600 million years ago, the Earth was completely covered by ice exceeding 1 km in thickness. It has been suggested that photosynthetic cyanobacteria and bacteria may have survived global glaciation by residing in bacterial mats similar to present-day cyanobacterial mats found in Arctic and Antarctic ponds (87, 264, 265). The close association of microorganisms in these microrefugia would favor the development of symbiotic relationships, and even perhaps influence the development of the eukaryotic cell in a manner similar to the theory for eukaryotic cell development in thermal microbial mat ecosystems (263).
In contrast with the sea ice assemblages, which are dominated by diatom algae, the pond microbial mats are dominated by filamentous cyanobacteria. These organisms are tolerant to high UV, desiccation, and freezing-thawing cycles. They form mucilaginous mats which act as microrefugia, or small-scale refuges, for a plethora of other less-tolerant photosynthetic and heterotrophic forms of microbial life, including eukaryotic microalgae and microinvertebrates. Until recently, it was believed that ice shelf mat communities were restricted to Antarctica. However, Vincent and coworkers have documented widespread communities at the Ward Hunt Ice Shelf as well as the Markham Ice Shelf, both located on Northern Ellesmere Island, Nunavut, in the Canadian high Arctic (263, 266). The Arctic and Antarctic ice shelves are one of the environments most vulnerable to climate warming (88, 95, 122, 151, 261); increasing global temperatures has already had a significant negative impact on accelerating the fragmentation and loss of the Ward Hunt Ice Shelf and the draining of a 3,000-year-old Arctic lake that had been dammed behind it (172).
Analysis of 16S rRNA genes from oscillatorians isolated from Antarctic and Arctic ice-shelf microbial mat communities indicates that filamentous cyanobacteria in both polar environments originated from temperate species (176). However, 16S rRNA analysis of psychrophilic filamentous cyanobacteria of the order Oscillatoria isolated from certain Antarctic algal mats shows they are more distantly related to temperate strains of the same genera, implying that these organisms may have been introduced to Antarctica as the continent cooled (~20 million years before the present) and evolved in their present-day habitat, compared to psychrotrophic strains that may have been introduced more recently (176). Tang et al. (249) found that many of the Arctic and Antarctic mat-forming cyanobacteria are not psychrophilic and exhibit growth temperature optima far above the temperatures found in their natural environments. Therefore it appears that adaptation to growth at low temperatures is not a requirement for successful colonization of these habitats, and other characteristics, such as UV screening and protection against photoinhibition, may have a greater selective advantage in the Arctic ice shelf environment (249).
The phototrophic microbial mats are dominated by the filamentous cyanobacteria of the order Oscillatoriales. Atmospheric nitrogen-fixing cyanobacteria, such as Nostoc and Anabaena spp., were found to be codominant, suggesting that bound inorganic nitrogen may be depleted in these environments and that N2 fixation may be an important source of nitrogen to these organisms as well as the entire mat community (223). An Antarctic algal mat from a pond near Ross Island, Antarctica, was also the sampling site for the isolation of the first psychrophilic anoxygenic phototrophic bacterium (Rhodoferax antarcticus sp. nov.) (140).
N2 fixation has also been shown to be important in the survival of filamentous cyanobacteria inhabiting the permanent ice covers of certain Antarctic lakes (194, 215). The UV-screening and N2-fixing cyanobacteria aid in the formation of a rich microhabitat that supports a highly concentrated assortment of other organisms, including bacteria, eukaryotic algae, ciliates, flagellates, nematodes, rotifers, and platyhelminthes. Their success in surviving and proliferating in these environments is likely due to a complexity of adaptive characteristics, such as maintenance of overwintering populations. One such strategy is the production of the mucopolysaccharide matrix, which not only traps sediment particles and aids in mat cohesion but also probably provides protection against freezing-thawing cycles and desiccation, thus allowing the mat communities to survive the winters and form seed populations for the resumption of growth in the summer (263).
In conjunction with habitat-specific stresses such as low temperatures and desiccation, the microbial mats during the summer are exposed to continuous high levels of UV radiation. Cyanobacteria possess a wide variety of avoidance and repair mechanisms to combat the negative effects of UV exposure, including the synthesis of intracellular (97) and extracellular (66) UV-screening compounds. Screening of UV and excessive PAR levels is a major adaptive strategy in the upper layers of the ice shelf mats, as was evident by the high carotenoid-to-chlorophyll a ratios.
Photoprotective carotenoids such as lutein, echinenone, and β-carotene have also been detected, but by far the major pigment present in both the upper and bottom layers of the mats is the UV-screening sheath pigment scytonemin as well as its degradation product, scytonemin-red (266). Scytonemin is a common UV-screening pigment found in many extreme environments and is effective at screening maximally in the UV-A and UV-C regions, as well as the UV-B region (222). De novo synthesis of this screening pigment occurs in response to exposure to UV-A, but can be induced by increases in temperature and photooxidative conditions in isolates from epilithic desert crust communities (44). The high levels of UV-screening pigments appear to screen out close to 100% of the short-wavelength radiation, so that the biota residing in the lower layers of the mats were exposed to wavelengths restricted to low intensity (<2% PAR) in the yellow-red waveband. Therefore, phototrophic microorganisms residing in the lower layers of the mat are likely adapted to shade conditions.
Cryoconites
Cryoconite habitats are transitory (with respect to liquid water) environments which form during polar summers when dark wind-blown particulate matter imbedded in glacial ice is heated by solar radiation and melts, forming a cylindrical basin of liquid water despite subfreezing air temperatures. Microorganisms associated with the wind-blown material serve as the seed populations of these unusual transitory microenvironments, which have been documented in both the Arctic and Antarctic as well as alpine glaciers (25, 71, 172a).
Photosynthetically active microalgal and cyanobacterial populations that fix inorganic carbon and nitrogen provide the nutrient foundation for a surprisingly complex microbial assemblage which includes bacteria, algae, and diatoms, as well as simple metazoans such as rotifers, tardigrades, and nematodes. During the polar winter, the holes refreeze and the organisms survive the cold, dark winter through dormancy. Each cryoconite hole potentially forms a unique ecosystem with relatively complex biogeochemical processes (256, 257).
Molecular analysis of the small-subunit rRNA gene of cryoconite holes that form in the McMurdo Dry Valley region have shown several sequences to be similar to rRNA gene species isolated from either microbial aggregates or microbial mats associated with the adjacent dry valley lake communities (25, 71). Cryoconite holes serve as a repository for terrestrial populations and may serve as glacial refuges for microorganisms in cold polar deserts. While there has been minimal work on adaptative mechanisms of organisms residing in cryoconite holes, there are presumably similarities with the ponds that form on the ice shelves.
Ice-Covered Lake Systems
The McMurdo Dry Valleys in southern Victoria Land, Antarctica (Fig. (Fig.3),3), is one of the most extreme deserts on our planet, with precipitation of <10 cm year−1 and an average annual air temperature near −20°C (range, −55°C to 5°C) (26, 49). These valleys, located adjacent to McMurdo Sound (latitude, 77 to 78.5oS; longitude, 160 to 164.5oE), form the largest ice-free region (~4,500 km2) on the Antarctic continent (59), and consist of a pristine mosaic of perennially ice-covered lakes, intermittent streams, arid soils, barren mountains, and surrounding glaciers (165, 269). There are no vascular plants or vertebrates and no established insects; microorganisms dominate life in the area. Based on these characteristics, the dry valleys have been considered the closest Earth analogues to conditions that exist on other icy worlds, such as Mars and and the moon Europa (48, 152, 210).
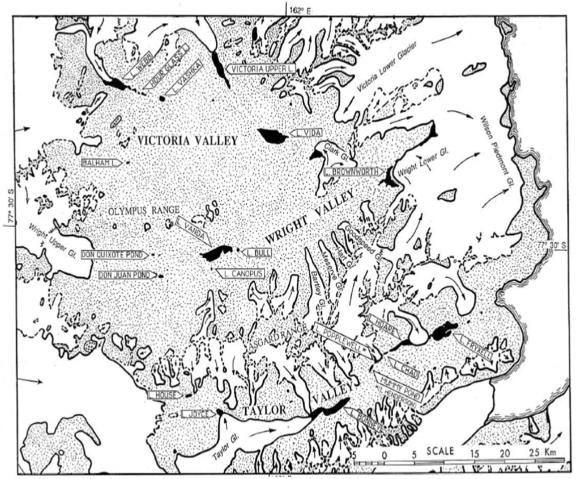
Map of the McMurdo Dry Valleys, Antarctica. Unusual meteorological conditions in the dry valleys produce the only permanently ice-covered lakes on Earth and the liquid water beneath the ice allows one of the few refugia for microbial communities in continental Antarctica. Each lake exhibits a unique biogeochemistry and therefore a unique microbial consortium. A Chlamydomonas sp. has been identified in many of the lakes. C. raudensis UWO 241 was isolated from the east lobe of Lake Bonney, Taylor Valley.
The perennially ice-covered lakes of the McMurdo Dry Valleys provide one of the few habitats where liquid water and associated life persist throughout the year on the Antarctic continent. It should be noted however that ice can cover lakes in other areas of the Antarctic continent as well as the high Arctic for extended periods of time (13). McKay et al. (153) developed a model of the physical processes that describes how a relatively thin (3 to 6 m) ice cover can persist over a liquid water column. These authors concluded that the existence of a perennial ice cover overlying liquid water was determined by the generation of glacial meltwater during the brief summer periods, when air temperatures are near freezing. It is this delicate meteorological balance that provides the only annual oasis for aquatic life in the McMurdo Dry Valleys in what would otherwise appear to be an inhospitable environment.
Photosynthetic production of organic carbon drives biogeochemical reactions (that is, the partitioning and cycling of chemical elements and/or compounds between living and nonliving parts of an ecosystem) and influences species abundance and diversity in all ecosystems. Polar lake systems have exceptional stresses imposed on photosynthesis by amplified seasonal patterns in sunlight and the permanent ice covers that greatly reduce the amount of light that reaches the water column. Despite the apparent lack of ecological complexity, we know now that these polar desert lakes harbor a complex assemblage of interacting autotrophic and heterotrophic microorganisms (123, 137, 210, 221) (Fig. (Fig.44).
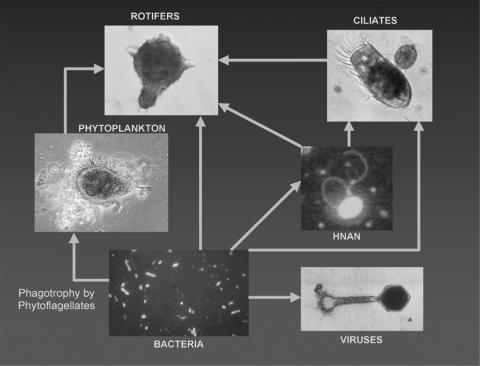
Pictorial representation of the major food web components and their linkages within Lake Bonney (Taylor Valley, Antarctica). The rotifer is a Philodina sp., the large ciliate is a Euplotes sp., and the phytoplankton is a Chlamydomonas sp. Note the bacteria attached to the surface of the Chlamydomonas cells which appear as threadlike structures on the upper portion of the cell; flagella are evident on the right apex of the cell. Note the absence of higher trophic levels. (Reprinted from reference 221 with permission of the publisher.)
The next section of this article focuses on the extensive work regarding the biogeochemistry of the lakes as well as the ecophysiology of the natural flora. We emphasize the biological and geological characteristics of Lake Bonney, located in the Taylor Valley, where a highly characterized psychrophilic phytoplankton was isolated by Priscu and coworkers (181).
McMURDO DRY VALLEY LAKE PHOTOTROPHIC COMMUNITIES
The McMurdo Dry Valleys are the site of numerous permanently covered lake systems. Lakes Bonney, Hoare, and Fryxell located within Taylor Valley (Fig. (Fig.3)3) have been the focus of the U.S. National Science Foundation's McMurdo Dry Valleys Long-Term Ecological Research (LTER) program, which has been studying these lakes systematically over the past 13 years (http://huey.colorado.edu/LTER/). Phytoplankton play an essential role in the functioning of the food web of the dry valley lake ecosystems (Fig. (Fig.4),4), and understanding the diversity and variability in the phytoplankton numbers is paramount to the overall goal of the McMurdo LTER.
Lake Bonney, which lies at the snout of the Taylor Glacier (Fig. (Fig.3),3), consists of two basins, the east lobe (3.5 km2) and the east lobe (1.3 km2) (207). A narrow sill separates the two lobes, isolating the saline, nutrient-rich deep waters of each basin while allowing the fresher surface waters to exchange (207). As discussed below, the presence of a year-round ice cover strongly influences the aquatic biology, chemistry, and physical properties of the lake. The ice cap itself is a habitat for microorganisms due to the high porosity of the lake ice (214).
Biogeochemistry
Several important biogeochemical implications arise from the presence of the permanent ice that covers the lakes. Gas exchange between the water column and atmosphere is severely restricted (213) and vertical mixing within the liquid water column of the lakes is predominantly through molecular diffusion owing to the lack of wind- and river-induced turbulence (244, 245). One of the manifestations of these biogeochemical conditions is a highly layered distribution of chemical species in the water column of the lakes.
Each lobe of Lake Bonney has a distinct geochemistry and associated biology related to climate evolution and input from subglacial outflows (58, 158). For example, the west lobe contains the highest levels of dimethyl sulfide ever sampled in a natural system, whereas the east lobe has the highest dimethyl sulfoxide levels encountered in natural waters (121). These biogenetic sulfur compounds are thought to be produced by cryptophyte algae, which occupy certain layers in the water column in Lake Bonney (120). The east lobe also contains nitrous oxide concentrations that exceed 700,000% of air saturation (209, 213). The sources and sinks of these compounds are not completely understood and are often not supported by the thermodynamics of the system (120). This thermodynamic paradox has led several authors to suggest that the gradients now observed in Lake Bonney may have formed many thousands of years ago (121, 209, 211).
The dissolved oxygen concentration from just beneath the ice to 15 m exceeds 1,000 μM, which is between 250 and 350% higher than what would occur if the water was saturated with air above the lake. Oxygen at depths below 20 m shows less than 10% air saturation. The dissolved oxygen levels beneath the chemocline in the west lobe of Lake Bonney support anaerobic processes such as denitrification (58, 209, 213), whereas no significant bulk anaerobic metabolism occurs beneath the chemocline in the east lobe. Vertical dissolved inorganic nitrogen and phosphorus profiles are similar to the salinity profiles, with relatively low values above 15 m followed by large increases below 15 m (Fig. (Fig.5,5, upper panels). The average molar ratio of dissolved inorganic nitrogen to soluble reactive phosphorus ranges from 64 to 616 between 5 and 17 m, reaches a maximum of 1,620 at 20 m, and then averages about 600 from 21 m to the bottom. These ratios are well above that required for balanced phytoplankton growth, indicating phosphorus limitation, a contention that has been supported by experimental work on both phototrophic and heterotrophic production (50, 58, 211).
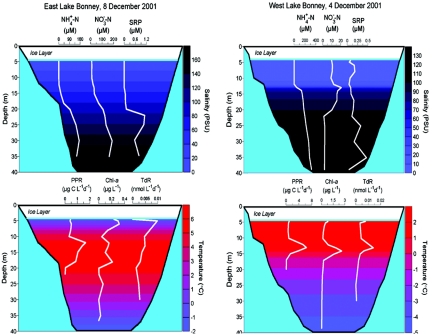
Physical, chemical, and biological parameters from the west and east lobes of Lake Bonney. SRP, soluble reactive phosphorus; PPR, phytoplankton primary productivity; Chl-a, chlorophyll a; TdR, bacterial productivity measured as [3H]thymidine incorporation into DNA; PSU, practical salinity units. (Reprinted from reference 221 with permission of the publisher.)
Temperature, Light Climate, and Phytoplankton Abundance
Temperatures in Lake Bonney range from near 0°C just beneath the ice cover to a maximum of 6.1°C at 14 m and to a minimum of −1°C in the deep saline water at 36 m (Fig. (Fig.5,5, lower panels). Despite the unusual temperature profile, the water column remains highly stable (i.e., it is not mixed by buoyant forces) because of the salt gradient (Fig. (Fig.5,5, upper panels). Spigel and Priscu (244, 245) have shown that the salinity profile is extraordinarily constant from year to year and that the curvature of the profiles is consistent with the effects of diffusion.
Lizotte and Priscu (129) conducted a detailed study of spectral irradiance in Lake Bonney and other dry valley lakes and showed that irradiance was always less than 50 μmol photons m−2 s−1 beneath the permanent ice covers. The wavelength of maximum transmission through the water column of Lake Bonney and other lakes is in the range from 480 to 520 nm (126), with longer wavelengths (>600 nm) being greatly diminished. Lizotte and Priscu (129) showed that the water itself was the dominant absorber of available light under the ice (38 to 75% of the total absorption coefficient), but that phytoplankton played a major role in attenuating shorter wavelengths (<520 nm) (11 to 47%).
Unlike many pelagic systems, where spring growth of phytoplankton is triggered by a combination of decreasing mixed layer depth and increasing incident PAR (243), the initiation of spring phytoplankton growth in the nonturbulent waters of Lake Bonney is solely a function of the seasonal increase in incident PAR. The annual underwater light climate from 1999 through 2003 shows the large seasonal difference in underwater PAR both within and between seasons (Fig. (Fig.6).6). Measurable PAR is present only from late September through mid-March for most seasons.
Measurements made within each field season show that phytoplankton productivity and biomass increase when solar radiation first penetrates the ice cover of the lake, generally reaching their highest levels in January, when field studies typically end owing to logistic constraints (221). The depth of transmission also varies considerably with the 2000 to 2001 season, showing much deeper penetration than other seasons. This long-term variability in PAR reflects changes in environmental factors, such as irradiance (through cloud cover, changes in ice transparency, or turbidity associated with stream flow), UV radiation, and variation in stream-derived nutrients related to glacial melt over this period. In addition, temperatures were warmer during the late 1980s, resulting in higher ice cover porosity (62) and higher stream flow (27, 154), the latter yielding greater nutrient loading to the lake (see also reference 58). Lastly, Vincent et al. (267) also showed that sufficient UV radiation penetrates the ice cover of certain dry valley lakes to significantly inhibit algal growth. These facts, together with the region's sensitivity to ozone depletion (141) and climate warming and cooling (49, 150), make measurements of phytoplankton photosynthesis in these lakes an important gauge of environmental change.
Figure Figure77 shows the distinct vertical stratification of phytoplankton species, determined by inverted light microscopy of gravity-settled samples, in the east lobe of Lake Bonney at selected intervals between 1989 and 2000. The taxonomic scheme of Seaburg et al. was utilized (239a). The layer immediately beneath the ice is consistently dominated by the cryptomonad Chroomonas sp. over the entire period. Chlamydomonas intermedia also inhabits the upper part of the water column, but at much lower biomass levels than Chroomonas organisms. The chrysophyte Ochromonas sp. displays the greatest variation in vertical distribution but is often confined to the upper and middle depths of the water column. This species reaches some of the highest biomass levels within Lake Bonney. A Chlamydomonas sp. (later identified as C. raudensis UWO241 [205]; see below) is confined to the deep saline and low-irradiance portion of the photic zone (15 to 18 m). These vertical profiles are supported by chemotaxonomic pigment analysis (125, 128).
Phytoplankton Nutrient Status
Early reports on nutrient deficiency in the lakes of the McMurdo Dry Valleys were based primarily on indirect evidence such as nitrogen-to-phosphorus ratios in the water column (86), nutrient ratios in streams entering the lakes (20), photobiological responses of phytoplankton (126, 181), and direct measurement of nitrogen uptake using 15N-labeled compounds (210, 220). With the inception of studies focusing on photosynthesis (126, 128, 180, 181) and nitrogen transformations (120, 209, 213, 220), it became clear that detailed information on nutrient regulation of phytoplankton photosynthesis was necessary to understand the microbial dynamics of the lake ecosystems.
Priscu (211) conducted the first detailed long-term phytoplankton nutrient bioassays in these lakes and showed that phytoplankton photosynthesis in Lake Bonney just beneath the ice cover (5 m; Chroomonas sp. dominated) and at 13 m (Ochromonas spp. dominated), was extremely phosphorus deficient. However, photosynthesis in a sample from 18 m (dominated by a Chlamydomonas species later identified as C. raudensis UWO 241; see below) did not respond to either N or P addition. Priscu (211) showed that it was the upward diffusion of deep nutrient pools that were formed many thousands of years ago (138) that supports much of the phytoplankton photosynthesis now observed in Lake Bonney, particularly in the deep waters just above the chemocline (i.e., between 17 and 18 m). Phosphorus deficiency in both phytoplankton and bacterioplankton has been confirmed in Lake Bonney by other investigators (50, 58).
Photosynthetic Characteristics
Detailed photosynthesis-irradiance curves conducted on phytoplankton populations of the east lobe of Lake Bonney from 5 m (just beneath the ice; 0°C), 6 m (3°C), 10 m (5.5°C), and 17 m (6°C) by Lizotte and Priscu (127, 128) showed evidence of extreme shade adaptation, including low saturation points for photosynthesis (Ek = 15 to 45 μmol photons m−2 s−1), and extremely low maximal photosynthetic rates, PBm < 0.3 μg C (μg chlorophyll a)−1 h−1. Deeper phytoplankton (10 and 17 m) were shown to have PBm and photosynthetic efficiencies (α, slope of initial portion of P versus E curve) three to five times higher than those at the ice-water interface, despite Q10 values of only ~2 for PBm, implying that a simple temperature response cannot explain all of the differences between phytoplankton populations. Lizotte and Priscu (128) concluded that the deep chlorophyll layers in Lake Bonney may be caused by factors such as in situ growth of phytoplankton enhanced by higher nutrient availability at a nutricline, physiological adaptation to decreased irradiance, decrease in sinking rate of phytoplankton with depth, and/or behavioral aggregation of phytoplankton.
The extreme shade adaptation shown by Lizotte and Priscu (128) for the in situ phytoplankton populations indicates that these organisms are highly efficient at converting light to photosynthetic energy and may have a large number of chlorophyll pigments associated with each of the photosynthetic reaction centers. Neale and Priscu (180-182) used in vivo fluorescence yield to define the depth profile of relative changes in quantum yield of photosynthesis. The dark-adapted in vivo fluorescence per unit chlorophyll was higher and the photochemical yield, measured as the fluorescence parameter Fv/Fm (where Fv is variable chlorophyll a fluorescence and Fm is maximal chlorophyll a fluorescence) (19) were lower in the shallow populations dominated by the cryptophyte Chroomonas sp. compared to the deep populations dominated by Chlamydomonas sp. The Fv/Fm data in particular imply a low quantum yield in shallow populations, increasing quantum yield in the region of 10 to 15 m, and nealy maximal values in the Chlamydomonas sp.-dominated deep populations between 16 and 18 m (181, 182). This is in agreement with the trend of increasingly higher initial slopes of the photosynthesis-irradiance curves measured at 5, 10, and 17 m by Lizotte and Priscu (127, 128) and increasing quantum yields of photosynthesis and fluorescence with depth computed by Lizotte and Priscu (126).
Neale and Priscu (180) obtained further information on the structure and function of the photosynthetic apparatus in phytoplankton populations from several dry valley lakes through analysis of the slow (minute time scale) fluorescence transients. The steady-state fluorescence yield (Fs) in samples from Lake Bonney after 5 min of illuminations was lower (quenched) for irradiances greater than 10 μmol photons m−2 s−1, indicating induction of protective mechanisms to dissipate excess excitation energy (nonphotochemical quenching) at an unprecedented low irradiance. These results are consistent with adaptation to a constant-shade environment and maintenance of low excitation pressure.
Phototaxis
The low hydraulic kinetic energy in the McMurdo Dry Valley lakes imposes an ecological selection pressure for flagellated phytoplankton, which can outcompete nonmotile species (e.g., diatoms). Marshall and Laybourn-Parry (143) suggested that the flagellated phytoplankton utilize their motility to maintain their position in the stratified dry valley lake water column.
Priscu and Neale (217) observed that relatively high-density vertical sampling of the cryptomonad-dominated population occurred within the ice sample hole and just beneath the ice-water interface. Nutrient measurements indicated that no major gradients in inorganic N or P occurred in this region, suggesting that the phytoplankton within the lake maintain their depth as the result of positive phototaxis. Experiments designed to assess the phototactic capabilities of the major trophic zones of east lobe Bonney showed that the shallow (4 m) cryptophyte-dominated population exhibited a strong phototactic response. Conversely, no phototactic response was observed in either the Ochromonas- (12 m) or the Chlamydomonas (18 m)-dominated populations. Priscu and Neale (217) further tested the effect of increased light on the phototactic behavior of the 12- and 18-m populations by displacing them up in the water column to a region of higher light but similar temperature (within 1°C). These experiments showed that the 12-m population responded positively to the increase in light level, whereas no positive response was noted for the 18-m population.
What factor(s) governs the maintenance of position in the water column in the lower depth communities? Distinct increases in water column inorganic nitrogen and soluble reactive phosphorus occur near 13 and 19 m in the east lobe of Lake Bonney (58, 211). These nutrient gradients led Priscu and Neale (217) to suggest that chemotactic response to nutrients may override phototactic responses in the Ochromonas (12 m) and Chlamydomonas (18 m) populations. The distinct vertical layering of phytoplankton in the east lobe of Lake Bonney (and presumably other dry valley lakes) may be the result of complex physiological responses regarding trade-offs in nutrient and light utilization by each genus.
STUDY OF AN ANTARCTIC LAKE PHYTOPLANKTON
Identification and Phylogeny of the Lake Bonney Psychrophile
Koob and Leister identified a chlorophyte, Chlamydomonas subcaudata Wille, as a dominant species in the planktonic communities in the east lobe of Lake Bonney, Taylor Valley, Antarctica (112). Recently, a phylogenetic study was undertaken to identify the Lake Bonney Chlamydomonas sp. isolated by Neale and Priscu (181) from the deepest biotic zone of the east lobe of Lake Bonney (205). Sequence analysis of the internal transcribed spacer 1 (ITS 1) and ITS 2 rRNA genes was performed on the psychrophilic isolate studied by Morgan-Kiss et al. (166, 167, 169), strain CCMP-1619 (a psychrophilic Chlamydomonas sp. isolated by M. Lizotte and M. Lesser from Lake Bonney [http://ccmp.bigelow.org/]), and compared to the rRNA gene sequences of the type strains of C. raudensis (SAG 49.72) and C. subcaudata (SAG 12.87). It was determined that the rRNA gene sequences of the Lake Bonney isolated exhibited 100% identity with both CCMP-1619 and C. raudensis Ettl., but differed significantly from the authentic C. subcaudata (205). In a recent study (78, 191), sequence comparison of the petA-petD region of the chloroplast genome also confirmed that CCMP-1619 was the same species as the Lake Bonney psychrophile studied by Hüner and coworkers. As a consequence, the psychrophile previously named C. subcaudata by Koob and Lister (112) was renamed C. raudensis Ettl (UWO241) (205).
C. raudensis (UWO 241) is closely related to C. bilatus, a cold-tolerant Chlamydomonas species isolated from an alpine pool in the High Tetra Mountains of the Czech Republic (205). However, the type strain C. raudensis (SAG 49.72) is a mesophile that was isolated from a meadow lake in Czechoslovakia. The mode of deposition of C. raudensis (UWO241) in the east lobe of Lake Bonney is unknown. The permanent ice cover over Lake Bonney is a relatively recent event, about 200 years ago (138), and therefore there was a high potential for deposition of microorganisms from the surrounding environment (soil and atmosphere) to the lake ecosystem (144). Lastly, a Chlamydomonas species (identified as C. subcaudata by morphological inspection) has also been identified in the west lobe of Lake Bonney as well as several of the other dry valley lakes (112). Given the unique ice cover histories of these lakes, it would be interesting to confirm the identity of these Chlamydomonas populations and to verify whether they have the same origins as the east-lobe Lake Bonney C. raudensis.
Cell Morphology
A recent morphological study (205) showed that C. raudensis is a biflagellate single cell of approximately 10 to 15 μm in length (Fig. (Fig.8A).8A). C. raudensis also exists as nonmotile colonies of approximately 30 μm that contain 16 to 32 flagellated daughter cells (Fig. (Fig.8B).8B). A single cup-shaped chloroplast is present, as well as a basally located pyrenoid body which is surrounded by starch platelets (Fig. (Fig.8A).8A). The eye spots of C. raudensis were typically very small (<1.1 μm) and difficult to locate, in comparison with the very long eyespots of C. subcaudata and the multirowed eyespots of C. reinhardtii (205). Morphological investigation supports the conclusions from the phylogenetic analysis that the Lake Bonney psychrophile is distinct from its former namesake, C. subcaudata.

Electron micrographs of C. raudensis grown under laboratory-controlled conditions (8°C/20 μmol photons m−2 s−1) showing a single cell (A) and a membrane-bound colony (B). Note starch accumulation within the pyrenoids (P). C, chloroplast; N, nucleus; G, Golgi apparatus; M, mitochondrion; F, flagellum. Scale bars, 1.0 μm. (Reprinted and modified from reference 205 with permission of Blackwell Publishing.)
Growth and Photosynthetic Characteristics
Photosynthetic function and acclimation to environmental change in Chlamydomonas raudensis UWO241 have been studied for over a decade, primarily by Huner and coworkers (166-169, 205). Early studies characterized environmental limitations for growth of axenic cultures under laboratory conditions (Fig. (Fig.9).9). C. raudensis exhibited an optimal growth temperature below 10°C, although cultures exhibited exponential growth rates up to a temperature of 16°C. However, cultures failed to grow and cells were nonviable at growth temperatures above 18°C, which classifies C. raudensis as a true psychrophilic phytoplankton (Fig. (Fig.9A9A).
Dependence of rates of growth in C. raudensis on temperature (A) and irradiance (B). A. Cultures were grown under low irradiance (20 μmol m−2 s−1) and variable growth temperatures. B. Cultures were grown under optimal growth temperature (8°C) and variable growth irradiances.
Photosynthetic light response curves were performed under a range of measuring temperatures on C. raudensis cultures grown at 8°C and 20 μmol O2 m−2 s−1 (204). Rates of apparent O2 evolution (ΦO2) were temperature dependent in C. raudensis in a laboratory setting, exhibiting the highest ΦO2 at 8°C and then a steady decline at higher measuring temperatures. The ΦO2 was exceptionally high at 8°C in C. raudensis, indicating maximal efficiency in converting light to photosynthetic energy at low temperature.
A lower ΦO2 at higher temperatures can be explained in part by the temperature-dependent induction of the energy-dissipative xanthophyll cycle. The maximum photosynthetic capacity (Pmax) in C. raudensis measured under light-saturating conditions was highest at 8°C and, unlike ΦO2, was little affected by temperature up to temperatures of 25°C. Relative to the optimum temperature of 8°C, Pmax in C. raudensis was 82% lower at 35°C and 98% lower at 45°C. This indicates that the limitation for growth of C. raudensis at temperatures above 20°C is not immediately due to high-temperature damage to the photosynthetic apparatus or primary carbon metabolism.
Earlier ecophysiological studies by Priscu and coworkers (180, 181) of Lake Bonney phytoplankton communities indicated that photoprotective mechanisms have been sacrificed in favor of augmentation of light-harvesting capacity. However, isolated cultures of C. raudensis grown at the optimal growth temperature (8°C) and variable irradiance can tolerate growth irradiance levels that were significantly higher than the natural light environment (about 10-fold), and exhibited a linear increase in growth rate with respect to growth irradiance up to 150 μmol m−2 s−1 (Fig. (Fig.9B).9B). This ability to modulate growth rate at low temperatures under variable irradiance differs from that of low-temperature-grown cultures of mesophilic algae (such as the model organism Chlorella vulgaris), which are unable to adjust growth rate in response to variable growth irradiance (147, 275).
The psychrophilic C. raudensis also exhibits unusual growth response to light of specific qualities. While cultures of the Antarctic alga grew exponentially under either white light or blue light, cultures failed to grow under red light (although cells were still viable and resumed exponential growth when transferred back to white light) (169). Presumably, the inability to grow under red light is a consequence of natural populations of C. raudensis being exposed to virtually no light in the longer-wavelength spectrum. Therefore, they are adapted to a stable light environment of low intensity enriched in blue-green wavelengths.
Membrane Lipid and Fatty Acid Composition and Pigmentation
C. raudensis exhibits a typical distribution of lipid species that has been characterized in other Chlamydomonas sp., including the mesophile C. reinhardtii. The galactolipids (MGDG, DGDG, and sulfoquinovosyldiacyglycerol), lipids specifically associated with the chloroplast, made up more than 75% of the total lipid content, a common feature in organisms where more than 80% of the total membranes are associated with the single large plastid. In contrast, fatty acid content differed significantly between the mesophilic and the psychrophilic membranes. C. raudensis exhibited a significantly higher unsaturated fatty acyl bond index in comparison with C. reinhardtii (167). Most notably, the all chloroplast galactolipids (MGDG, DGDG, and sulfoquinovosyldiacyglycerol) of C. raudensis possessed high levels of polyunsaturated fatty acids (167), suggesting low-temperature adaptation specifically associated with the photosynthetic membranes.
In contrast, characteristic of Chlamydomonas sp., the majority of polyunsaturated fatty acids in photosynthetic membranes of the mesophile C. reinhardtii were restricted to the galactolipid MGDG, while the other major plastid lipids possessed saturated or monounsaturated fatty acyl species. Lastly, C. raudensis exhibited several novel polyunsaturated fatty acids with positional shifts in the unsaturated bond closer to the lipid head group. It is hypothesized that a shift in the double bond to positions closer to the head group increases the cross-sectional area of the fatty acyl tails, causing higher degrees of disruption in the lipid membrane and therefore higher degrees of fluidity. Since the desaturation of the fatty acyl chains is catalyzed by position-specific desaturases, presumably C. raudensis must also possess enzymes with novel positional specificity.
C. raudensis grown under a regimen of low temperature and low light exhibited a pigment complement typical of a Chlamydomonas species, with lutein and neoxanthin being the most abundant light-harvesting carotenoids (166, 205). One of the most striking characteristics of the pigment composition of C. raudensis was an unusually low chlorophyll a to b ratio (1.6 to 1.8) (166, 205), consistent with the notion that C. raudensis exhibits an enhanced light-harvesting capacity as a consequence of adaptation to a constant low-light environment in its natural habitat (181). Under controlled growth conditions, C. raudensis also exhibited significantly lower levels of both antheraxanthin and zeaxanthin (166), products of the xanthophyll cycle which are involved in energy dissipation during conditions of excessive light absorption (40).
Biochemistry and Physiology of the Photosynthetic Apparatus
Given the complex set of extreme natural environmental parameters to which the psychrophile is adapted, it was not surprising to find that the organization and composition of the photosynthetic apparatus of this enigmatic green alga exhibit a number of unusual features (166, 168, 181). At the level of the photosynthetic reaction center complexes, C. raudensis possesses comparable levels of D1, a major reaction center protein of photosystem II, but reduced levels of PSI reaction center proteins (PsaA/B) in comparison with the model species, C. reinhardtii (Fig. 10A). The low PSI-PSII reaction center stoichiometry was accompanied by the absence or reduction of all light-harvesting I proteins (for, e.g., LHCI proteins p18.1 and p22.1; Fig. 10A) determined by immunoblot analysis, and was confirmed by a low ratio of chlorophyll-bound LHCI to LHCII on nondenaturing polyacrylamide gel electrophoresis (166). Furthermore, C. raudensis possesses significantly higher levels of the oligomeric form of light-harvesting II complexes, the major chlorophyll b-binding multiprotein complexes of the photosynthetic apparatus (166, 168, 169). Therefore, the organization of the photosynthetic apparatus in the Antarctic alga favors photosystem II under growth conditions simulating its natural habitat, resulting in an unusually high PSII-to-PSI ratio. This strategy would be an adaptive advantage under constant exposure to blue light of low fluence levels, since the light-harvesting apparatus of PSII utilizes chlorophyll b and short-wavelength-absorbing chlorophyll a to absorb light predominantly in the blue region (155).

A. Abundance of major thylakoid proteins of photosystems I and II in the mesophilic species C. reinhardtii (M) and the psychrophillic C. raudensis (P) grown at the optimal growth temperature (29 and 8°C, respectively) and low irradiance levels. PsaA/B, photosystem I core reaction center proteins; D1, major photosystem II reaction protein; p18.1 and p22.1, representative light-harvesting I polypeptides. B. Low-temperature 77 K fluorescence emission spectra measured in vivo in C. reinhardtii (dotted line) and C. raudensis (solid line) cultures grown under same conditions as in A. (Modified from reference 166 with kind permission of Springer Science and Business Media.)
C. raudensis exhibits an apparent molecular mass of cytochrome f, a major polypeptide of the cytochrome b6f complex, which is about 7 kDa smaller than the 41-kDa cytochrome f from C. reinhardtii (168). Despite this mass difference, cytochrome f from either Chlamydomonas species exhibited the ability to bind the heme cofactor, and no differences in the apparent molecular mass of either cytochrome b6 or the Rieske Fe-S centers was observed. The petA gene, encoding the cytochrome f protein from UWO 241, was isolated and sequenced (AY039799) (79). The amino acid sequence of cytochrome f from C. raudensis was 79% identical to that of C reinhardtii (Fig. (Fig.1111).
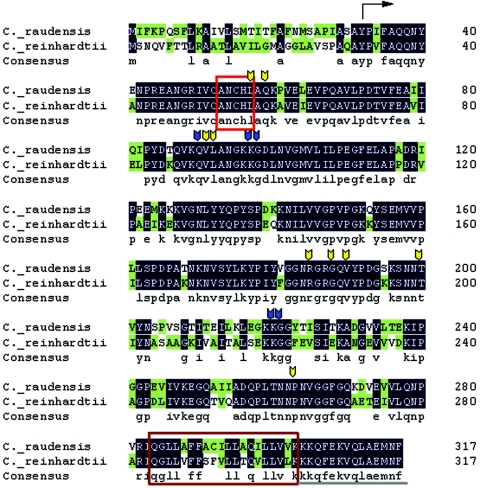
Sequence alignment of cytochrome f proteins from C. raudensis and C. reinhardtii. The protein sequence was predicted from the DNA sequence and aligned using the DNAman program. Black boxes indicate identical amino acids. Green shading, similar amino acids. Black arrow, N terminus of mature cytochrome f protein; red box, heme binding motif; brown box, transmembrane helix; gray underline, C terminus localized in stromal side of thylakoid membrane; blue arrows, lysine residues involved in plastocyanin docking; yellow arrows, amino acid residues involved in water chain formation.
The major difference in sequence was the presence of three cysteine residues (C21, C24, and C261) in the psychrophile rather than the two Cys residues (C21 and C24) typically found in other cyanobacterial, algal, and plant species and involved in covalent heme binding. The C261 residue is present in the transmembrane helix of cytochrome f of C. raudensis, is not involved in heme binding, and does not appear to be redox sensitive (78). However, the heme in cytochrome f of C. raudensis is significantly less stable to high temperature than that of C. reinhardtii, which has been interpreted to reflect an adaptation to a psychrophilic environment at the protein sequence level. This interpretation is consistent with the observation that components of the thylakoid membrane of C. raudensis denature at a temperature that is about 10°C lower than that of the mesophile C. reinhardtii (78, 167).
An additional structural alteration in the stoichiometry of the thylakoid protein complexes was observed at the level chloroplast ATP synthase. C. raudensis exhibited higher levels of both the α and β subunits of CF1 relative to the mesophilic control, C. reinhardtii (166). This may be an adaptive strategy to maintain sufficient levels of plastid-derived energy and avoid low-temperature-induced reduction in ATPase reaction rates. Alternatively, the higher levels of plastid ATP synthase may indicate greater pool sizes of ATP in the psychrophile. As discussed above, one adaptive explanation for higher adenylate pools in the psychrophiles may be to compensate for reduced rates of molecular diffusion at low temperatures. Thus, higher levels of adenylates could represent an adaptive mechanism to provide adequate energy for ATP-dependent biochemical reactions at low temperatures (178, 179).
The unusual structural characteristics of the thylakoid apparatus of the psychrophilic alga had unique consequences on the functional photochemistry at the level of low-temperature (77 K) fluorescence emission. The model species C. reinhardtii possesses a classic 77 K fluorescence emission spectra, with a major emission maxima at 685 and 717 nm (Fig. 10B) (166), corresponding to chlorophyll a fluorescence originating from LHCII and LHCI-PSI complexes, respectively (114). In striking contrast, cells of C. raudensis possessed virtually no long-wavelength (>700 nm) fluorescence emission, indicating that the psychrophile possessed little to no fluorescence emission originating from PSI-LHCI complexes (166-169).
The absence of the PSI-associated 77 K fluorescence in the psychrophile is clearly associated with the reduced levels of PSI reaction center and, in particular, LHCI polypeptides (Fig. 10A). This phenomenon has been reported in a number of mutants harboring deficiencies in PSI content (38, 136, 278), as well as under specific stress conditions that target PSI function, such as iron deficiency in cyanobacteria (98), hyperosmotic stress, and low temperature (233). However, C. raudensis represents the first naturally occurring organism to exhibit this characteristic under controlled laboratory growth conditions.
Functional differences in photosynthetic electron transport between the psychrophile and the model mesophilic species C. reinhardtii have revealed striking differences in intersystem electron transport and the redox status of the intersystem pool between the two algal species. In one study, PSI activity was monitored in vivo by measuring the change in absorbance at 820 nm as an estimate of the oxidation-reduction of P700+ (168). The mesophilic species exhibited a 1.5-fold higher steady-state change in A820 (ΔA820/A820) in comparison with the psychrophilic alga, in support of earlier findings that C. raudensis possesses a structurally and functionally down-regulated PSI. However C. raudensis exhibited 1.5- to 2-fold higher levels of dark reduction of P700 (168), indicative of higher rates of PSI-driven cyclic electron transport (9).
Neale and Priscu (181, 182) studied the light-harvesting characteristics of C. raudensis isolated from the east lobe of Lake Bonney through measurement of the PS I reaction center (P700) content and spectral variation in PSII fluorescence kinetics. Based on their experiments, Neale and Priscu (181) concluded that the stable light environment experienced by Lake Bonney phytoplankton enables a high degree of acclimation to low-intensity blue-green light. This acclimation is accomplished partially by augmentation of the antenna size of chloroplast PSII with light-harvesting carotenoids and reducing the content of photoprotective carotenoids.
Short-Term Photoacclimation
The ability of the organism to adjust quickly and efficiently to maximize photosynthetic efficiency while avoiding the damaging effects of overexcitation of the photochemical apparatus involves a variety of regulatory processes. These photoacclimatory mechanisms can be broadly separated into two distinct groups, based on differential time scales: short-term acclimation allows the organism to adjust within minutes, while long-term acclimation involves response mechanisms that respond on time scales that vary from hours to days to months.
Antenna quenching.
Antenna quenching refers to the reversible, light-dependent down-regulation of PSII photochemistry leading to a decreased efficiency in energy transfer from the light-harvesting antenna to PSII reaction centers. This down-regulation of PSII protects PSII reaction centers from overexcitation through the nonphotochemical dissipation of excess energy as heat through xanthophyll cycling. The cycling of xanthophylls represents an important general mechanism underlying the regulation of NPQ. The most-studied xanthophyll cycle involved in photoprotection is the reversible conversion of violaxanthin to zeaxanthin (39-41, 68, 90, 193). Under conditions of excess light, the trans-thylakoid ΔpH activates violaxanthin deepoxidase, which converts the light-harvesting carotenoid violaxanthin to the energy-quenching carotenoids antheraxanthin and zeaxanthin. This photoprotective mechanism is induced on a time scale of minutes when plants and green algae are shifted from low-light to high-light conditions and its activity can be monitored by assessing the epoxidation state of the xanthophyll cycle pigments (39-41, 68, 90, 193).
The major xanthophyll cycle involved in photoprotection in C raudensis is the conversion of violaxanthin to zeaxanthin (166). This, in part, accounts for the observation that C raudensis is not an obligate shade-adapted psychrophile since this green alga can grow at irradiances that are 10 times that to which it is typically exposed in its natural environment. A detailed examination of all xanthophylls failed to uncover alternative xanthophyll cycles in this green alga. Thus, short-term photoprotection through antenna quenching has been retained in this Antarctic psychrophile even though it is rarely exposed to irradiances greater than 100 μmol m−2 s−1.
State transitions.
In terrestrial plants as well as green algae, light absorbed preferentially by PSII relative to PSI (state 2) leads to an overreduction of the PQ pool, whereas preferential excitation of PSI relative to PSII (state 1) results in oxidation of the PQ pool. The redox state of the PQ pool regulates a thylakoid protein kinase which controls the phosphorylation state of the peripheral Lhcb antenna polypeptides and energy transfer between PSII and PSI (4). The regulation of energy transfer by the redox state of the thylakoid PQ pool reflects a short-term photoprotective mechanism to counteract the potential for uneven absorption of light by PSI and PSII and to maintain maximum photosynthetic efficiency. Thus, the state transition is a dynamic mechanism that enables photoautotrophs as varied as cyanobacteria, green algae, and terrestrial plants to respond rapidly to changes in illumination.
By monitoring the increase in the 77 K chlorophyll a fluorescence emission at 720 nm associated with PSI relative to the decrease in PSII chlorophyll a fluorescence emission at 688 nm, Morgan-Kiss et al. (168) detected state I to state II transition in C. reinhardtii which was sensitive to the redox state of the PQ pool and was associated with the phosphorylation of the major LHCII polypeptides. Furthermore, the state I-state II transition in C. reinhardtii was inhibited by the protein kinase inhibitor staurosporine. In contrast, state I-state II transitions could not be detected in the psychrophilic C. raudensis UWO 241 (Fig. 12A), although reversible phosphorylation of one major LHCII polypeptide was observed (168) (Fig. 12B). It was concluded that the psychrophile is locked in state 1 and as a consequence is considered a “natural state transition mutant” (168). Thus, adaptation of C. raudensis to the unique environment of Lake Bonney has resulted in loss of the fundamental short-term photoprotective mechanism of state transitions.
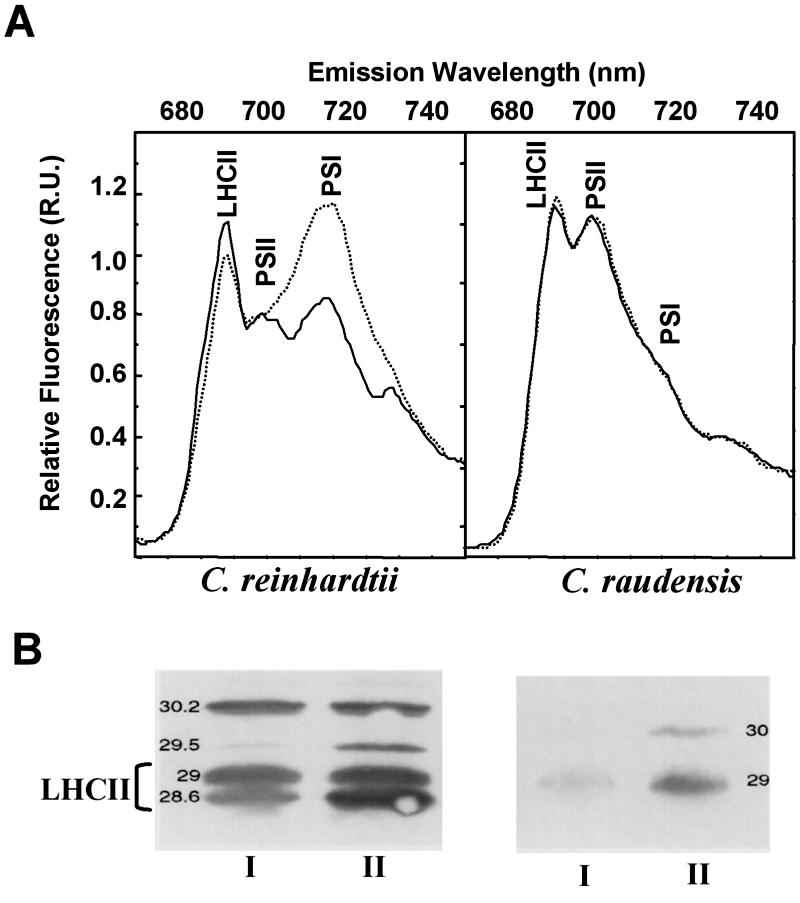
State transitions in the mesophilic C reinhardtii (left panel) versus the psychrophilic C. raudensis (right panel). A. The 77 K fluorescence emission spectra of whole cells exposed to state I (solid line) or state II (dotted line) conditions. B. Immunoblot of isolated thylakoid membranes with antiphosphothreonine antibodies to detect phosphorylation patterns in proteins of thylakoids exposed to state I (I) or state II (II) conditions. A state I response was induced by exposure of freshly harvested cells from an exponentially growing culture to far-red light for 15 min. A state II response was induced by incubation of samples in the dark under anaerobic conditions. (Modified from reference 168 with kind permission of Springer Science and Business Media.)
The cytochrome b6f complex has been shown to play a critical role in the regulation of LHCII phosphorylation, and mutants of this complex are unable to undergo state transitions (5, 282). To test whether the altered structure and stability of cytochrome f of C. raudensis is a determining factor in the absence of state transitions in this psychrophile, a petA deletion mutant of C. reinhardtii WT.11 (FIBE ΔpetA) was complemented with the petA gene from C. raudensis (78). The C. reinhardtii mutant expressed a cytochrome f that exhibited an apparent molecular mass that was 7 kDa smaller rather than the molecular mass expected for the WT.11 cytochrome f. Despite this complementation, the transformant was still able to undergo state transitions comparable to that observed for WT.11.
Clearly, the alteration in the structure and stability of cytochrome f from C. raudensis is not a limiting factor regulating state transitions (79). As an alternate hypothesis, Morgan-Kiss et al. (168) proposed that adaptation of C. raudensis to the unique conditions of Lake Bonney has altered the structure and function of its photosynthetic apparatus such that the PSII and stromal flux of electrons through the intersystem electron transport chain are lowered relative to the rate of cyclic PSI electron transport compared to that of C. reinhardtii, which predisposes C. raudensis to remain in state 1.
Photoinhibition and recovery.
Imbalances between the light energy absorbed through photochemistry and energy utilization through photosynthetic electron transport can ultimately lead to the inhibition of photosynthesis (7, 109, 115, 131, 208). Although PSI has been shown to be sensitive to excess light, PSII is considered the primary target of photoinhibition. It has been shown in some chilling-sensitive plant species and green algae that tolerance to photoinhibition may be accounted for, in part, by a higher rate of repair relative to the rate of photodamage to D1 (109, 156, 188). Recovery from photoinhibition in most plants and green algae involves a PSII repair cycle in which photodamaged D1 is degraded and newly synthesized D1 is reinserted into the thylakoid membrane to form a photochemically functional PSII reaction center (7, 8, 156).
The response of the Antarctic psychrophile C. raudensis to photoinhibition was investigated by Pocock et al. (206) and found to be unique in four specific ways. First, unlike any other eukaryotic or prokaryotic organism examined to date, C. raudensis was unable to modulate its sensitivity to photoinhibition as a function of exposure temperature. Second, although C. raudensis is adapted to low temperature, it is more sensitive to photoinhibition than the mesophile at both high and low photoinhibitory temperatures (206). This inability to acquire resistance to photoinhibition is in contrast with the common feature of green algae as well as cyanobacteria to develop resistance to photoinhibition when grown under cold acclimation conditions (93). Third, the psychrophile exhibited an exceptional capacity to recover from photoinhibition at low temperature. From 70 to 80% of maximal PSII photochemical efficiency is recovered after only 20 min at 8°C, which is matched by comparable recovery kinetics of O2 evolution. Last, the recovery kinetics of C. raudensis were minimally sensitive to the chloroplastic translation inhibitor lincomycin, whereas maximum recovery from photoinhibition in C reinhardtii was inhibited by at least 50 to 60% by lincomycin (206). This indicates that, unlike the model mesophilic species, recovery from photoinhibition in the Antarctic psychrophile appears to occur independently of the D1 repair cycle.
This is the first example of a cold-tolerant organism that has lost its ability to develop any resistance to photoinhibition but has retained an exceptional capacity for recovery that is independent of chloroplastic translation. In addition, the xanthophyll cycle was only minimally induced during low-temperature photoinhibition in C. raudensis and therefore this photoprotective mechanism does not play a significant role in recovery from high-light stress. It is clear that the mechanisms involved in the recovery of C. raudensis from photoinhibition are novel and as yet unknown.
Phototactic response.
Some green algae possess the ability to modulate the amount of exposure to light through a process termed the phototactic response. Previous studies have shown that cells will respond to exposure to light by swimming in directed paths towards the light source and that the wavelength preference for this response is in the blue region (190). The motile phototactic Chlamydomonas reinhardtii possess light-sensing eyespots that contain a complex sandwich between the plasma membrane and the thylakoid membranes of the chloroplast and consist of two to four layers of carotenoid-filled lipid globules (177) as well as the photoreceptor chlamyrhopsin (37). Light absorption by chlamyrhopsin molecules triggers transient opening of calcium channels over the plasma membrane, which in turn induce calcium-sensitive voltage gate calcium channels in the flagella and changes in intracellular calcium concentrations affect flagellar activity differentially, causing swimming (101).
C. raudensis cells isolated from laboratory-controlled cultures exhibited a strong, positive phototactic response observed at the nonpermissive growth temperature (29°C), although no response was observed at the permissive growth temperature of 7°C. This could explain why Priscu and Neale (211) were unable to elicit a phototactic response from 18-m Lake Bonney phytoplankton populations (dominated by C. raudensis) and supports the conclusion that some other factor, perhaps chemotaxis, is responsible for keeping C. raudensis (UWO 241) cells in their vertical position within the stratified water column (112, 181).
Thermosensitivity of the photosynthetic apparatus.
The photosynthetic process is one of the most thermosensitive functions in higher plants (83) and green algae (65, 247). The thermolability of PSII, one of the most sensitive pigment-protein complexes, was monitored in the psychrophilic alga C. raudensis via the heat-induced increase in the room temperature fluorescence parameter F0. The heat-induced rise in F0 has been attributed to a physical disassociation of LHCII from PSII cores due to the conversion of trimeric to monomeric LHCII (6, 18), as well as an accumulation of closed PSII centers (21). The temperature of maximum F0 yields in the psychrophilic alga was 10°C lower (40°C) in comparison with the mesophilic species C. reinhardtii (50°C) (167), suggesting a higher thermal liability of the photosynthetic apparatus in the psychrophile. However, the heat-induced rise in F0 was not accompanied by a functional dissociation of LHCII from PSII in C. raudensis. Moreover, the heat-stressed thylakoids of the C. raudensis exhibited 2.3-fold higher levels of oligomeric LHCII than those of the mesophile, indicating that the conversion of trimeric to monomeric LHCII was less heat labile in the psychrophile. Thus, it appears that the oligomeric form of LHCII is relatively heat stable in the psychrophilic alga. This enhanced stability of LHCII is unlikely to be an adaptive response to elevated temperatures per se, but may be an artifact of an adaptive strategy to enhance maximum light absorption and energy transfer from LHCII to PSI under the natural light environment of extreme shade (129).
The degree of fluidity of the photosynthetic membranes has been associated with resistance of the photosynthetic apparatus to heat stress. The photosynthetic apparatus of plants acclimated to low temperatures, and thus exhibiting higher levels of unsaturated fatty acids in their membranes, is more thermally sensitive than that of plants acclimated to higher temperatures. Conversely, Arabidopsis thaliana mutants possessing lesions in desaturases possess lower levels of unsaturated fatty acids and exhibited higher levels of thermal tolerance compared with wild-type plants (229). While the fluidity of C. raudensis membranes has not been measured directly, the correlation between degree of unsaturated fatty acyl in the membrane lipids and membrane fluidity is well established (174). C. raudensis possesses relatively high levels of unsaturated fatty acids associated with specific plastid lipids. Therefore, it is likely that higher photosynthetic membrane fluidity in the psychrophilic alga contributed to higher thermosensitivity of PSII.
Heat treatment of thylakoid membranes has also been shown to redistribute light energy from PSII to PSI in conjunction with a stimulation of PSI-associated photochemistry due to heat-induced alterations in thylakoid membranes in favor of a disorganized membrane arrangement (99, 237). While heat-stressed mesophilic cultures exhibited both a rise in PSI 77 K fluorescence emission and a stimulation of photoxidation of P700, psychrophilic cultures under heat-stressed conditions exhibited no major functional adjustments at the level of PSI (167). It is thought that the stimulation of PSI activity in the mesophilic species may be a short-term acclimatory strategy to protect photodestruction of PSI when PSII is preferentially inhibited under elevated temperatures. It is likely that the relatively low levels of PSI and LHCI polypeptides exhibited by C. raudensis limit the ability of the psychrophile to adjust PSI activity during heat stress and may represent the loss of this short-term acclimatory mechanism.
Long-Term Photoacclimation
Composition and structure of light-harvesting complexes.
A major long-term photoacclimatory mechanism in green algae (55, 118, 155, 246) and the phycobilisomes of cyanobacteria (55, 77) involves the modulation of the physical size and composition of LHCII. Cyanobacteria have proven to be an excellent model system to elucidate the molecular mechanism regulating light-harvesting in response to nutrient limitations. Seminal research by Grossman and coworkers (76, 77) provided important insights into the global regulation of phycobilisome turnover in response to N and S limitations in Synechococcus spp. In addition, Huner and coworkers (159, 160) have observed in the filamentous cyanobacterium Plectonema boyanum that there is an inverse relationship between growth irradiance and light-harvesting antenna size regardless of growth temperature. Exposure to increased irradiance in cultures grown under either low (15°C) or moderate (29°C) temperatures results in a reduction in the rod lengths of the phycobilisomes. In addition, in response to higher irradiance levels, decreases in chlorophyll a content are associated with concomitant increases in the major carotenoid myxoxanthophyll.
Similar trends have been shown for photoacclimation in model mesophilic green algae such as Chlorella vulgaris (146, 275), Dunaliella tertiolecta (54, 246), and Dunaliella salina (82, 145, 248). Recently, it was shown that both the redox state of the PQ pool and the transthylakoid ΔpH act as signals in the retrograde regulation of nuclear gene expression of the Lhcb gene family and the subsequent polypeptide and pigment composition of LHCII in green algae (24, 54, 147, 148, 275).
The regulation of LHCII size in green algae and phycobilisomes in cyanobacteria is not a simple response to light intensity, since low growth temperature (5°C) mimics the effects of high light on the composition and function of LHCII (146, 148, 275, 276) and phycobilisomes (159, 160). Thus, photoacclimation reflects a response to excitation pressure rather than a response to either temperature or light per se. Thus, photostasis is attained upon exposure of green algae and cyanobacteria to high excitation pressure generated either by high light or low temperature through a decrease in the efficiency of light harvesting.
In contrast to the green algae C. vulgaris and D. salina and the cyanobacterium P. boryanum, C raudensis exhibits minimal changes in pigmentation LHCII abundance when grown at either low temperature or increasing irradiance (see Fig. 14A to C). Furthermore, unlike the mesophilic species Chlorella vulgaris, C. raudensis exhibited the ability to modulate growth rate in response to the higher growth irradiance (Fig. (Fig.9B,9B, Fig. 13D). This was a remarkable characteristic of the psychrophile, considering the light conditions were more than 10-fold higher than the natural light environment to which C. raudensis is adapted. This ability to increase growth rates correlates with an increase in photosynthetic energy utilization, and accounts for the lower excitation pressure exhibited in cultures of C. raudensis in comparison with cultures of the mesophilic species. Thus, on the basis of pigment composition and structural alteration of LHCII, C. raudensis may to respond to increased excitation pressure in a manner similar to wheat, rye, and Arabidopsis thaliana as well as Antarctic vascular plants (17, 279).

Effect of a low-temperature growth regimen on pigmentation (A), abundance of thylakoid polypeptides (B), LHCII abundance (C), and growth and chlorophyll fluorescence parameters (D) in cultures of mesophilic (Chlorella vulgaris) and psychrophilic (Chlamydomonas raudensis UWO 241) green algae. A. Mid-log-phase cultures grown under regimens indicated above each culture(°C/μmol m−2 s−1) B. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of thylakoid polypeptides isolated from cultures grown under the above regimens C. Immunoblots of major light-harvesting polypeptides and Lhcb. D. Growth and fluorescence parameters of cultures. Growth rate is expressed as h−1. Room-temperature chlorophyll a fluorescence parameter 1 − qP measured as growth temperature/light regimen.

Putative model illustrating the functional changes in the organization of PSII as a survival strategy in populations of C. raudensis during the transition between winter and short Antarctic summer. Left panel, microbial populations exhibit cessation in growth during the transition from summer to winter. Cells exhibit minimal photosynthesis and high respiration rates. PSII is functionally down-regulated by the disconnection of major LHCII from the PSII core, and light is dissipated nonphotochemically as heat via an energy-dependent fluorescence quenching (qE)-type mechanism. The PQ pool is largely reduced due to metabolically derived (e.g., starch breakdown) electron donors. In summer, excysting populations provide the seed cultures for active growth. Reassociation of oligomeric LHCII antenna with the PSII core allows rapid transition from energy-dissipative processes to efficient light energy capture and utilization during the short growing season. Respiration is low and starch reserves are replenished for the upcoming winter season.
Despite the ability of the psychrophile to maintain lower excitation pressure under lower temperature and higher irradiance conditions by increasing utilization of photosynthetic energy products (by increasing growth rates), functional changes in the light energy distribution between LHCII and PSII were observed at the level of 77 K fluorescence emission. Cultures grown under high light exhibited a rise in LHCII-associated fluorescence in comparison with cultures grown at the low-irradiance regimen (R. Morgan-Kiss, unpublished data). It is likely that the increase in LHCII fluorescence is related to a functional switch in LHCII from efficient energy transfer to PSII to nonradiative dissipation of excessive absorbed light energy.
Photosystem stoichiometry.
When redistribution of LHCII via state transitions is insufficient to maintain the poise in light energy distribution between PSII and PSI, long-term adjustments at the level of PSII/PSI stoichiometry occur. This modulation in the ratio of the photosystems appears to occur primarily at the level of PSI reaction polypeptides (3, 64, 173, 202, 259). It was first proposed by Fujita et al. (63) that modulation of photosystem stoichiometry is a response to changes in the redox state of the intersystem electron transport chain to ensure equal rates of electron flow through both PSI and PSII (4). Subsequently, Pfannschmidt et al. (201) showed that the transcription of the chloroplast-encoded psbA (which encodes the PSII reaction center protein D1) and psaAB genes (which encode the PSI reaction center polypeptides) are controlled by the redox state of the PQ pool. Overreduction of the PQ pool by the preferential excitation of PSII not only favors energy transfer from PSII to PSI through phosphorylation of LHCII also favors the activation of psaAB transcription and the concomitant repression of psbA. Conversely, oxidation of the PQ pool by preferential excitation of PSI favors not only dephosphorylation of LHCII but also the activation of transcription of psbA and the repression of psaAB (4, 201). Thus, PQ, the redox sensor that controls state transitions, as well Lhcb expression appear to be the sensors that regulate chloroplast photosystem stoichiometry (200, 203).
Changes in the spectral quality of the light environment lead to preferential excitation of either PSII or PSI. The light-harvesting apparatus of PSI is composed of mostly long-wavelength-absorbing chlorophyll a, whereas LHCII utilizes chlorophyll b and short-wavelength-absorbing chlorophyll a (155). Under an environment enriched in red light, such as the habitat of plants growing under a leaf canopy, PSI is preferentially excited, and the pool of intersystem electron transporters is largely oxidized. Conversely, in a predominantly blue light environment, such as most aquatic ecosystems, PSII preferentially absorbs the majority of the available light and the intersystem electron pool is largely present in a reduced state.
In a study regarding acclimation of the photochemical apparatus to long-term changes in the light quality, Morgan-Kiss et al. (169) showed that C. raudensis has retained the ability to adjust photosystem stoichiometry, and the kinetics for changes in the PSI reaction center proteins, PsaA/PsaB, were comparable to that of the mesophilic alga C. reinhardtii exposed to a similar light regimen. Furthermore, overexcitation of PSII under blue light was reflected as a transient rise in excitation pressure in both Chlamydomonas species followed by a decline in 1 − qP to initial values. The transitory phenomenon of excitation pressure indicates that both species sensed the overexcitation of PSII under blue light and compensated for this imbalance by adjusting the PSII-to-PSI ratio in favor of PSI excitation. However, unlike the mesophilic species, the adjustments in photosystem stoichiometry in blue-light-treated C. raudensis cells were not accompanied by changes in the light energy distribution in favor of PSI at the level of 77 K fluorescence emission. Instead, on a functional level, the Antarctic alga responded to blue light by upregulating PSII photochemistry at the level of 77 K fluorescence, maximum PSII photochemical efficiency and yield of PSII electron transport. This novel acclimative response exhibited by the psychrophilic C. raudensis is likely to be an adaptive strategy to maximize light absorption and energy transduction under a natural light environment of extremely low intensity blue-green wavelengths that would be preferentially absorbed by PSII.
While both Chlamydomonas species responded to the PQ-oxidized environment of red light exposure in a predictable way by decreasing PSI reaction center proteins, the redox state of the PQ pool varied drastically between the two species. As expected, 1 − qP levels remained low throughout the red-light treatment in cultures of C. reinhardtii, indicative of an oxidized intersystem electron pool. However exposure of C. raudensis cultures to red light caused a fourfold increase in excitation pressure (169). This accumulation of a reduced PQ pool was also accompanied by functional downregulation of PSII (169). Furthermore, red-light-exposed cells of C. raudensis exhibited a rise in LHCII 77 K fluorescence accompanied by a blue-shifted fluorescence maximum, a response which mimicked high-light-grown cultures.
It is believed that the severely reduced levels of the PSI reaction center proteins PsaA and PsaB in red-light-treated cultures of C. raudensis limited the rate of oxidation of the PQ pool and were a major contributor to the build-up of reduced PQ and therefore the rise in 1 − qP. This inherent characteristic of a down-regulated PSI exhibited by the Antarctic alga limits this organism's capacity to maintain growth and photosynthesis under a light environment enriched in red light, where PSI is preferentially excited.
We propose two alternative hypotheses to account for the apparent paradox of a reduced PQ pool under preferentially oxidizing conditions in the red-light-treated cultures of C. raudensis. First, it is clear from our previous studies that the population of PSI in C. raudensis appears to be functionally and structurally down-regulated under any experimental conditions or growth regimen we have tested (166, 167). Therefore, under red light, it is possible that further loss of PSI via the decrease in abundance of PsaA/PsaB reaction center proteins resulted in a photochemical apparatus that possessed minimal functional PSI. Under these conditions, PSI-mediated oxidation of the intersystem electron pool could be very slow relative to the rate of its reduction by PSII and lead to a build-up in the redox status of the PQ pool. While the down-regulation of PSI may have contributed in part to the reduced PQ pool, we believe that other factors probably play a role. Higher rates of PSI-mediated cyclic electron transport and/or contribution via external stromal electron sources could also contribute to a relatively reduced PQ pool (168, 169).
Antarctic microbial communities exhibit a myriad of strategies to survive the long winter of continuous darkness and grow and reproduce during the short growing season of the Antarctic summer. Phytoplankton populations employ a number of survival mechanisms for winter survival, such as mixotrophy, cyst formation, and breakdown of metabolic reserves, such as starch (12). One of the keys to survival of the microorganism populations in the dry valley Antarctic lakes is to enter the short summer season with an actively growing population (119). This shift would also necessitate the requirement of adaptive and acclimative mechanisms to allow efficient photosynthetic rates soon after photosynthetically active light becomes available after the transition from winter to summer.
Due to logistical problems, few data are available regarding adjustments at the functional or structural level of the photosynthetic apparatus in natural Lake Bonney populations of C. raudensis. Based on our studies involving photoacclimation of laboratory-controlled cultures to light quality, we present a model for the rapid upregulation of the photosynthetic apparatus in C. raudensis during the transition from continuous dark conditions in the winter to the availability of light in the summer (Fig. (Fig.14).14). In the nongrowing season, it is probable that C. raudensis employs several adaptative strategies for winter survival, such as encysting or breakdown of starch reserves. However, while photosynthesis is probably down-regulated, we believe that cells maintain relatively high levels of the photochemical apparatus in a functionally down-regulated form, in a manner similar to overwintering evergreens (192) and dark adaptation of snow algae (11).
Retaining the photochemical apparatus would provide an adaptive advantage where the growing season is short, and upon onset of active photosynthetic light availability, synthesis and assembly of the photosynthetic apparatus could be severely delayed in such a low-temperature, nutrient-poor environment. Our model proposes that during the transition from the total winter darkness to the austral summer, there is a functional shift in the light-harvesting antenna from mainly a role of dissipating energy to one of efficient light energy transfer (Fig. (Fig.14).14). This transition from winter to summer was mimicked under our laboratory conditions by a transfer to blue light. Blue light is a key signal for a number of metabolic processes such as nitrate uptake and metabolism and chlorophyll biosynthesis (10, 100, 132), and it is not surprising that it would be a primary signal for a transition to efficient photosynthesis, since blue-green wavelengths dominate the natural light environment of this phytoplankton (181). Conversely, when cultures were shifted to red light, we believe that the cessation of growth, the down-regulation of PSII, and the accumulation of reduced intersystem electron transport (169) indicated that the red-light-transferred cultures responded in a similar manner to preparation of the cell population for overwintering. Thus, the red-light-induced responses observed in C. raudensis cultures may not be a consequence of red light per se, but of the absence of wavelengths in the blue-green region.
Model of the Photochemical Apparatus of a Eukaryotic Psychrophile
Based on the available functional and structural evidence, we have developed a functional model of the organization of the photochemical apparatus of the psychrophilic C. raudensis (Fig. (Fig.15).15). Prolonged isolation in an unusual light environment, that is, exposure to year-round extreme shade of an unusually narrow spectral quality (blue-green), has had a profound effect on the structural organization and function of the light-harvesting and photosynthetic electron transport chain of this extremophilic phototroph. Photosynthetic adaptation of the photosynthetic apparatus has involved functional and structural augmentation of LHCII-PSII, at the level of a high PSII-to-PSI ratio as well as tight coupling of energy transfer from LHCII to PSII. Concomitantly, PSI and associated LHCI are both structurally and functionally down-regulated.

Model for organization of thylakoid pigment-protein complexes of the electron transport chain in the psychrophilic Chlamydomonas raudensis UWO 241. In the natural, extremely stable light environment of extreme shade and predominantly blue-green wavelengths (blue lines), the majority of available light would be preferentially absorbed by PSII. Adaptation in C. raudensis to this light environment has led to an unusually high PSII/PSI stoichiometry and highly efficient energy transfer from LHCII to PSII. Conversely, PSI and associated light-harvesting complexes are both structurally and functionally downregulated. Given the severe reduction in light-harvesting capacity of PSI, it is proposed that PSI centers are largely excited via a spillover energy transfer mechanism from PSII (dotted line). Photosynthetic membranes may be arranged as loose stacks rather than distinct granal and stromal regions to promote energy spillover between the photosystems.
We propose that C. raudensis has adapted photosynthetically to rely entirely on LHCII for light-harvesting ability, and excitation of PSI occurs mainly via an energy spillover mechanism from LHCII-PSII centers. It is also probable that the organization of the photosynthetic thylakoid membranes favors loose stacks rather than distinct grana and stroma to promote light energy transfer between the photosystems. Furthermore, the extreme disunity between the ratio of PSII and PSI indicates that linear electron transport may be a minor cycle of photosynthetic electron transport in this enigmatic alga. While alternative electron transport cycles are not well described in psychrophillic organisms, C. raudensis exhibits high rates of PSI-mediated cyclic electron transport in conjunction with relatively high levels of chloroplast ATPase (166, 168), suggesting that a requirement for higher ATP may also play a role in the unusual functional organization of the photosynthetic electron transport chain. It is also likely that these dramatic adjustments have had an impact on the redox poise and maintenance of photostasis in this organism, which is evident in the loss of highly conserved short- and long-term photoacclimatory mechanisms.
CONCLUSIONS AND FUTURE DIRECTIONS
Permanently cold ecosystems make up one of the largest biospheres on the Earth. Paradoxically, the microorganisms that not only survive but thrive in these extreme habitats are still poorly understood. These two factors make low-temperature environments one of the last unexplored frontiers in our world. Research regarding the physiology and biochemistry of the primary producers, the microorganisms relying on photoautotrophic metabolism, of many of the food webs has been scant. For example, at least 10 genomes from heterotrophic extremophilic bacteria have been or are in the process of being sequenced, two of which are from psychrophilic Archaea. However, to date, no genomic sequences are available for a representative psychrophilic phototroph.
While genome sequencing is a first step in understanding the adaptation of microorganisms to life in one subset of extreme environments, a more powerful tool will be the integration of genomics with metabolic function through physiological and biochemical investigations. Therefore, our sequencing efforts should focus on polar phytoplankton genomes where we possess the most knowledge regarding the natural ecology, physiology, and biochemistry of the organism. Chlamydomonas raudensis UWO 241 would make a prime candidate for the first phototropic psychrophile genome to sequence, since it is one of the most researched psychrophilic phytoplankton to date. In addition, more detailed environmental genomic analyses of all low-temperature environments are needed to advance our knowledge of the dominant photosynthetic life forms in low-temperature food webs as well as aid in the identification of new photosynthetic models of low-temperature adaptation.
Acknowledgments
We thank Alexander Ivanov and Marianna Król for their contributions to the research described as well as valuable discussion over the years.
This work was supported by the National Science and Engineering Research Council of Canada (NSERC) and the National Science Foundation (NSF).
REFERENCES
Articles from Microbiology and Molecular Biology Reviews : MMBR are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/mmbr.70.1.222-252.2006
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1393254?pdf=render
Citations & impact
Impact metrics
Article citations
Permanent Stress Adaptation and Unexpected High Light Tolerance in the Shade-Adapted Chlamydomonas priscui.
Plants (Basel), 13(16):2254, 14 Aug 2024
Cited by: 0 articles | PMID: 39204690 | PMCID: PMC11359158
Regulation of Microalgal Photosynthetic Electron Transfer.
Plants (Basel), 13(15):2103, 29 Jul 2024
Cited by: 0 articles | PMID: 39124221 | PMCID: PMC11314055
Review Free full text in Europe PMC
Microbial assemblages and associated biogeochemical processes in Lake Bonney, a permanently ice-covered lake in the McMurdo Dry Valleys, Antarctica.
Environ Microbiome, 19(1):60, 20 Aug 2024
Cited by: 0 articles | PMID: 39160591 | PMCID: PMC11334312
Amoebozoan testate amoebae illuminate the diversity of heterotrophs and the complexity of ecosystems throughout geological time.
Proc Natl Acad Sci U S A, 121(30):e2319628121, 16 Jul 2024
Cited by: 1 article | PMID: 39012821
Accumulation Characteristics of Natural Ophiocordyceps sinensis Metabolites Driven by Environmental Factors.
Metabolites, 14(8):414, 27 Jul 2024
Cited by: 0 articles | PMID: 39195510 | PMCID: PMC11355974
Go to all (168) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences
- (1 citation) ENA - AY039799
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Photosynthetic adaptation to polar life: Energy balance, photoprotection and genetic redundancy.
J Plant Physiol, 268:153557, 13 Nov 2021
Cited by: 8 articles | PMID: 34922115
Review
The Antarctic Chlamydomonas raudensis: an emerging model for cold adaptation of photosynthesis.
Extremophiles, 17(5):711-722, 01 Aug 2013
Cited by: 29 articles | PMID: 23903324
Review
Identity and physiology of a new psychrophilic eukaryotic green alga, Chlorella sp., strain BI, isolated from a transitory pond near Bratina Island, Antarctica.
Extremophiles, 12(5):701-711, 26 Jul 2008
Cited by: 19 articles | PMID: 18661097
Comparison of photoacclimation in twelve freshwater photoautotrophs (chlorophyte, bacillaryophyte, cryptophyte and cyanophyte) isolated from a natural community.
PLoS One, 8(3):e57139, 19 Mar 2013
Cited by: 9 articles | PMID: 23526934 | PMCID: PMC3602455







