Abstract
Free full text

Development and application of a positive–negative selectable marker system for use in reverse genetics in Plasmodium
Abstract
A limitation of transfection of malaria parasites is the availability of only a low number of positive selectable markers for selection of transformed mutants. This is exacerbated for the rodent parasite Plasmodium berghei as selection of mutants is performed in vivo in laboratory rodents. We here report the development and application of a negative selection system based upon transgenic expression of a bifunctional protein (yFCU) combining yeast cytosine deaminase and uridyl phosphoribosyl transferase (UPRT) activity in P.berghei followed by in vivo selection with the prodrug 5-fluorocytosine (5-FC). The combination of yfcu and a positive selectable marker was used to first achieve positive selection of mutant parasites with a disrupted gene in a conventional manner. Thereafter through negative selection using 5-FC, mutants were selected where the disrupted gene had been restored to its original configuration as a result of the excision of the selectable markers from the genome through homologous recombination. This procedure was carried out for a Plasmodium gene (p48/45) encoding a protein involved in fertilization, the function of which had been previously implied through gene disruption alone. Such reversible recombination can therefore be employed for both the rapid analysis of the phenotype by targeted disruption of a gene and further associate phenotype and function by genotype restoration through the use of a single plasmid and a single positive selectable marker. Furthermore the negative selection system may also be adapted to facilitate other procedures such as ‘Hit and Run’ and ‘vector recycling’ which in principle will allow unlimited manipulation of a single parasite clone. This is the first demonstration of the general use of yFCU in combination with a positive selectable marker in reverse genetics approaches and it should be possible to adapt its use to many other biological systems.
INTRODUCTION
Despite the vast amount of information generated about the biology of Plasmodium since genetic transformation was first described, the development of the transfection technology has improved only slowly (1,2). This is mainly the result of low transfection efficiencies and the low number of selectable markers. Presently, it is possible to stably transform four species of Plasmodium, the human parasite Plasmodium falciparum, the primate parasite Plasmodium knowlesi and two rodent malaria parasites, Plasmodium berghei and Plasmodium yoelii [for a review see (1)]. Introduction and integration of foreign DNA into the genome of Plasmodium occurs mainly through homologous recombination and random integration is not frequently observed (1,3). The two rodent malaria parasites cannot be grown in long term in vitro cultures and selection of mutants is therefore carried out in vivo in laboratory rodents, which places constraints on the range of selectable markers that can be used. For example, the combination of puromycin and puromycin acetyl transferase that has been used for in vitro selection of mutants of P.falciparum and P.knowlesi (4,5) cannot be applied in the rodent parasites as the drug is lethal to laboratory animals. Currently only three drug-selectable markers are available for use with rodent malaria parasites. All are based upon dihydrofolate reductase (dhfr) of parasite (Toxoplasma gondii or P.berghei) and human origin, respectively. All confer resistance to the anti-malarial pyrimethamine whilst human dhfr (hdhfr) also confers resistance to a second drug, WR99210. These markers cannot be efficiently used in combination because of the cross resistance to pyrimethamine which hampers easy selection of such mutants (6). Therefore, relatively simple procedures that are routinely used in other organisms to analyse gene function by targeted gene disruption, such as complementation of function through restoration of the disrupted gene or by the introduction of transgenes that complement the loss of gene function are not common practice in Plasmodium (7,8).
The availability of a negative selectable marker, in combination with a small number of positive selectable markers, would facilitate a more flexible use of transfection technology in Plasmodium. The combination of a negative and a positive selectable marker in different vector configurations allows (i) restoration of disrupted genes (generated by positive selection pressure) through selection of reversion events where the selectable markers have been excised from the genome through homologous recombination (under negative selection pressure); (ii) improvement of selection for the integration of circular plasmids into the P.falciparum genome (9); (iii) selection of mutants that occur at such low frequency that an unacceptable background of drug-resistant parasites is observed presumably due to the accumulation of mutations that donate resistance in the transfected parasite population (9), a phenomenon that might be enhanced by electroporation (10); (iv) the development of ‘Hit and Run’ mutagenesis strategies for structure/function analyses; (v) recycling of both the positive and negative selectable markers leaving behind a modified locus, such as: a disrupted gene, an altered gene locus that expresses a modified product or expressing a novel transgene. Recycling would also permit sequential genetic manipulations in a single parasite line using a single positive selectable marker.
Here we report an investigation of the application of different negative selection procedures both in vitro and in vivo to the rodent malaria parasite P.berghei. The following markers that have been successfully used in other systems, some also in P.falciparum (9), were tested: thymidine kinase from Herpes simplex virus (hsvtk), the bacterial cytosine deaminase (bcd) (11) and a fusion gene comprising the open reading frames of the yeast cytosine deaminase and uridyl-phosphoribosyltransferase (yfcu) (12). The study culminated in the development and application of a negative selectable marker system based on yfcu for genetic transformation of P.berghei. The utility of a combination of a positive and negative selectable marker to sequentially disrupt and restore a gene to analyse gene function in a form of reversible recombination is demonstrated. This system of functional complementation provides an important tool, which may also enhance the investigation of gene function by targeted disruption in Plasmodium where transfection is problematic as a result of low transfection efficiency and low numbers of selectable markers.
MATERIALS AND METHODS
Construction of vectors
The hsvtk, the bcd and the yfcu were introduced in the expression vector pEFexpSSU(EV) (13). This plasmid contains the selectable marker cassette with dhfr-ts of T.gondii (tgdhfr-ts) under the control of the promoter of dhfr-ts of P.berghei (pbdhfr-ts), an expression cassette with the ef-1αa promoter upstream of a BamHI restriction site and a fragment of the d-ssu-rrna gene for targeted integration.
A BamHI fragment comprising the open reading frame of hsvtk (mutant sr39) was obtained from plasmid pET23d:HSVTK-SR39 [a kind gift from Dr M.E. Black (14)] and cloned into the BamHI site of the expression cassette of pEFexpSSU(EV) resulting in plasmid pEFTKSSU. For cloning of the bcd and the yfcu open reading frames in this expression cassette, we first filled in the BamHI site with dGTP and dATP using Klenow polymerase leaving a CT 3′ overhang. A XhoI digested bcd gene fragment was obtained from plasmid pCMVCODA [a kind gift from Dr C. Karreman (15)] and its cohesive ends were filled in with dTTP and dCTP using Klenow polymerase leaving an AG nucleotide 3′ protruding end. Ligation of this fragment in the right orientation into the pEFexpSSU(EV) vector resulted in plasmid pEFbCDSSU. The yfcu open reading frame was amplified from plasmid pCI-neoFCU1 [a kind gift from Dr P. Erbs (12)] using primers L1504 (5′-CTCGAGATGGTGACAGGGGGAATGG-3′) and L1505 (5′-CTCGAGTTAAACACAGTAGTATCTGTCAC-3′), cut with XhoI and the sites were filled in with Klenow as was done for the bcd gene fragment. Cloning of the fragment in its correct orientation into the pEFexpSSU(EV) vector resulted in construct pEFyFCUSSU. After linearization at the unique ApaI site, these three constructs can integrate into the ssu-rrna locus of the P.berghei genome by a single crossover event (13).
For use of the negative selection strategy for targeted gene disruption and subsequent restoration of the gene p48/45, the d-ssu-rrna fragment in pEFyFCUSSU was replaced with a 1.1 kb fragment of p48/45. To introduce a restriction site for linearization of this construct we cloned this p48/45 fragment in two steps. First, a fragment of 0.6 kb was PCR amplified from P.berghei genomic DNA using primers L2054 (5′-GGTACCAAGTGATATCTCATATCGATGGAAGAAGTGC-3′) and L2055 (5′-GAATTCGTACCAGGGCAACTAATACC-3′) and cloned into the Asp718 and EcoRI sites of pEFyFCUSSU, thereby removing the d-ssu-rrna fragment. Subsequently a second p48/45 fragment (0.5 kb) was amplified with primers L2056 (5′-GGTACCTCACTTTTTTGGGAACAGCC-3′) and L2057 (5′-GATATCACTTGTTCAGTATTATCACAAATG-3′) and cloned in the vector using the Asp718 and EcoRV sites. The resulting plasmid pEFyFCUp48/45 contains a fragment of 1050 nt of the open reading frame of p48/45 and was linearized with EcoRV before transfection.
Two constructs were made with fusion genes of the negative selectable marker yfcu and a positive selectable marker under control of the ef-1αa promoter. For the first construct we made a plasmid containing a fusion gene of tgdhfr-ts and yfcu by combining a PCR fragment of tgdhfr-ts [primers L2126 (5′-CTCGAGAAGAGAAGGAAGAC-3′) and L2127 (5′-GGTACCAAGCTTGCTAGCGACAGCCATCTCCATCTGG-3′)] and a PCR fragment of yfcu [primers L2128 (5′-GCTAGCGTGACAGGGGGAATGGC-3′) and 2129 (5′-GGTACCGAATTCTTTTCATCTAGACCTCTGTCG-3′)] in a vector using the NheI site for ligation of the two open reading frames. To introduce a [Gly4Ser]2 linker sequence between the two fragments of the tgdhfr-ts and the yfcu gene the primers 2373 (5′-CTAGCGGAGGAGGTGGATCTGGTGGAGGTGGAAGTG-3′) and 2374 (5′-CTAGCACTTCCACCTCCACCAGATCCACCTCCTCCG-3′) were annealed and the resulting synthetic DNA fragment was ligated in the right orientation into the NheI site of the fusion gene. The two selection cassettes containing the tgdhfr-ts and the yfcu in pEFyFCUp48/45 were then replaced with the single tgdhfr-ts-yfcu fusion gene using the HindIII and Asp718 sites resulting in plasmid pEFyFCUTgDHFRp48/45 that was linearized with EcoRV before transfection.
For the second construct a fragment was PCR amplified containing the ef-1αa promoter together with the hdhfr gene using primers 2198 (5′-ACTAGTCGTAATTATTTATATTTCTATG-3′) and 2199 (5′-GCTAGCATCATTCTTCTCATATACTTCAAATTTG-3′). A SpeI/NheI fragment of this fragment was subsequently cloned upstream of the linker sequence and yfcu of plasmid pEFyFCUTgDHFRp48/45 thereby replacing the tgdhfr-ts gene. The resulting construct pEFyFCUhDHFRp48/45 was linearized with EcoRV before transfection.
Transfection, positive selection of mutants and genotype analysis
Transfection of wild-type parasites of the reference line of P.berghei (cl15cy1 of the ANKA strain), selection and cloning of the mutant parasites were performed as described previously (3). The vectors described above were introduced separately into P.berghei and generated the following cloned lines of P.berghei: pEFTKSSU (239cl1), pEFbCDSSU (256cl2), pEFyFCUSSU (350cl1), pEFyFCUp48/45 (494cl1), pEFTgDHFRyFCU (635cl1) and pEFhDHFRyFCU (645cl1) (for overview of parasite lines see Table 1). All lines were selected by pyrimethamine treatment of mice as described (3) except for 645cl1 that had been selected by WR99210 treatment (6).
Table 1
Overview of the negative selectable marker expressing P.berghei lines used in this study
| P.berghei line | Transfected construct | Negative selectable marker | Integration locus | Prodrug | In vivo selection possible |
|---|---|---|---|---|---|
| 239cl1 | pEFTKSSU | hsvTK | d-ssu-rrna | GCV | partial |
| 256cl2 | pEFbCDSSU | bCD | d-ssu-rrna | 5-FC | no |
| 350cl1 | pEFyFCUSSU | yFCU | d-ssu-rrna | 5-FC | yes |
| 494cl1 (dis1) | pEFyFCUp48/45 | yFCU | p48/45 | 5-FC | yes |
| 635cl1 | pEFTgDHFRyFCUp48/45 | TgDHFR-TS-yFCU fusion | p48/45 | 5-FC | no |
| 645cl1 (dis2) | pEFhDHFRyFCUp48/45 | hDHFR-yFCU fusion | p48/45 | 5-FC | yes |
The presence of the selectable marker tgdhfr-ts was demonstrated by PCR using primer sets L190/L191 for tgdhfr-ts (16). Integration at the ssu-rrna gene was detected by PCR amplification using primer pairs L739/L635 (13) and L1662 (5′-GATTCATAAATAGTTGGACTTG-3′)/L740 (13). For Southern analysis of ssu-rrna integration, genomic DNA was digested with HindIII and EcoRI and hybridized to the c/d-ets fragment [primers L260R and L372 (17)] or the tgdhfr-ts fragment (L190/L191). Integration in the p48/45 gene was detected by PCR using primer sets L1909 (5′-AGGTACCTTCATTCTACAATATGCGCATG-3′)/L635 (left) and L1662/L1821 (5′-CGGGGTACCGCGGAATCCAAATAAGTAATTTCCGAAG-3′) (right). The presence of the yfcu open reading frame was checked with primers L1504/L1505. For Southern analysis of p48/45 integration, genomic DNA was digested with XbaI and hybridized with a probe of the 5′ region of p48/45 [primers L1655 (5′-AGATATCATGGCAAATGCCAATGGGAA-3′)/L1656 (5′-TGGATCCAGAGAGAAAAGGGACACACA-3′)].
In vitro drug sensitivity assays
For testing the drug sensitivity of wild type (wt) and mutant parasites, synchronized blood stages were cultured in short-term in vitro cultures as described previously (18) in the presence of 10 nM to 1 mM GCV (ganciclovir; Cymevene, Roche) or 0.1 µM to 10 mM 5-fluorocytosine [(5-FC); Ancotil, ICN Pharmaceuticals]. Briefly, ring forms were cultured in 24 well-plates for a period for 24 h in drug containing medium. At the end of the culture period the development of the ring forms into mature schizonts was quantified in two wells per drug-concentration by counting parasites in Giemsa stained blood films. The developmental stage (ring, trophozoite, schizont) was determined from 100 to 200 parasites/slide (6).
Negative selection in vivo by treating infected mice with GCV and 5-FC
Mice infected with transgenic parasites expressing negative selectable markers were treated with GCV or 5-FC. Mice were infected by intraperitoneally (i.p.) injection of 105 parasites. Drug treatment was started at a parasitemia of 0.01–0.05% parasitemia. Mice infected with line 239cl1 (expressing hsvTK) were injected daily with a single dose of 10, 100 or 500 mg/kg bodyweight GCV (Roche, 20 mg/ml in 0.9% NaCl) either i.p. or subcutaneously (s.c.) for a period of 3 days. Mice infected with lines 256cl2, 350cl1, 635cl1, 645cl1 and 494cl1 (expressing bCD or yFCU) were injected i.p. once or twice a day with a fresh 5-FC solution (Sigma, 10–40 mg/ml in 0.9% NaCl, dissolved at 50°C) ranging from 125 mg to 1 g/kg bodyweight for a period of 3 days. Three to five days after the treatment period the surviving parasites were isolated by collecting 1 ml of infected heart blood with a parasitemia between 3–5% for further analysis of their genotype and phenotype.
Phenotype analysis of parasites after disruption and restoration of the p48/45 gene
Northern and western analysis of wild-type, mutants with a disrupted p48/45 and reverted parasites was performed according standard methods. Total RNA from blood stages enriched in gametocytes was hybridized to a p48/45 specific probe (primers L1384 5′-GCTCTAGATGAAAGAAGATCAGTAATATGTAG-3′/L1385 5′-CGCGGATCCACCAATTTTAATATTCATAAAACCAG-3′) and a p28 specific probe (primers L1271 5′-TCCCCGCGGCCATGGATGAATTTTAAATACAGTTTTATT-3′/L1276 5′-GCGAATTCTTACATTACTATCACGTAAATAAC-3′). Total protein isolated from blood stages enriched in gametocytes (19) was fractionated on 12% SDS–polyacrylamide gels. Immunopurified polyclonal antisera were used against P48/45 of P.berghei (20) and against the gametocyte specific protein P47 of P.berghei [M. van Dijk, unpublished data; (21)]. Antibody reactions were visualized by ECL detection (Amersham Biosciences). Fertilization and ookinete development were studied in in vitro fertilization assays as described (22,23).
RESULTS
Generation and positive selection of transgenic parasites expressing putative negative selectable markers
Several transgenic parasite lines of P.berghei were generated that express the following, putative negative selectable markers: hsvtk, bcd (11) and a fusion gene comprising the open reading frames of the yeast cytosine deaminase and yfcu (12). These markers were introduced into the genome using a DNA vector that contains a positive selectable marker (the pyrimethamine resistant dhfr-ts of T.gondii) and an expression cassette with the constitutive and strong promoter of the ef-1αa gene of P.berghei. In addition, this construct contains a fragment of the non-essential P.berghei d-rrna gene which allows targeted integration into the genome. The vectors, with the various negative selectable markers cloned into the expression cassette (Figure 1A), were introduced into P.berghei using standard protocols of transfection and positive selection (3). After cloning of the different transgenic parasites, correct integration of the vectors (Figure 1A–C) was demonstrated by PCR (Figure 1E) and Southern analysis of restricted genomic DNA (Figure 1F). The following transgenic lines were selected for further analysis: 239cl1 expressing hsvTK, 256cl2 expressing bCD and 350cl1 expressing yFCU (see Table 1).
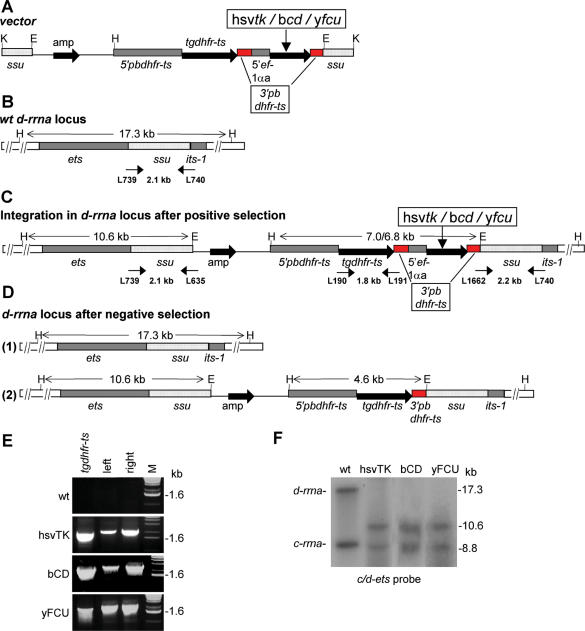
Generation of P.berghei parasites expressing negative selectable marker genes. (A) The constructs for expression of the hsvtk, bcd and yfcu negative selectable marker genes under control of the ef-1αa promoter. These constructs contain the tgdhfr-ts as a positive selectable marker and sequences of the small subunit (ssu) gene of the d-rrna locus for targeted integration into the genome. hsvtk, herpes simplex virus thymidine kinase; bcd, bacterial cytosine deaminase; yfcu, fusion gene of the yeast genes cytosine deaminase and UPRT; amp, ampicillin resistance gene; red boxes, 3′pbdhfr-ts repeat; K, KspI; H, HindIII; E, EcoRI. (B) Part of the genomic locus of the d-rrna gene that is used for integration of the expression constructs. ets, external transcribed spacer; its, internal transcribed spacer; ssu, small subunit. Sizes of PCR fragments and restriction fragments are indicated between short and long arrows, respectively. PCR primers are specified by their L-number. (C) The d-rrna locus after integration of the expression constructs in parasites that are selected by positive selection with pyrimethamine. (D) The d-rrna locus of parasites after negative selection with GCV or 5-FC of the mutant parasites as shown in (C). Parasites can be selected in which (1) the complete construct has been excised as a recombination event between the homologous d-ssu-rrna sequences or (2) the negative selectable marker cassette has been excised from the integrated construct by a recombination event between the two 3′pbdhfr-ts sequences. (E) Correct integration of the expression constructs in the parasite lines expressing hsvTK, bCD and yFCU after positive selection as shown by PCR. tgdhfr-ts, amplification of tgdhfr-ts (primers L190/L191); left, 5′ integration in the d-rrna unit (primers L739/L635); right, 3′ integration in the d-rrna unit (primers L1662/L740); M, marker. (F) Southern analysis of EcoRI and HindIII digested genomic DNA of wt and transformed parasites showing integration of the constructs in the d-rrna locus. DNA of wt and hsvTK, bCD and yFCU expressing parasites was hybridized to the c/d-ets probe.
Drug sensitivity of wt and transgenic parasites expressing putative negative selectable markers
Sensitivity of wt and transgenic parasites to the drugs GCV and 5-FC was first determined in standard short term in vitro cultures of the blood stages. Wt parasites were not sensitive to both drugs and only at the highest concentrations (GCV: 1 mM; 5-FC: 10 mM) a slight effect on the development of wt ring forms into mature schizonts was observed (Figure 2A). For transgenic parasites expressing hsvTK the sensitivity to GCV was strongly increased with an IC50 value of 0.5 µM (Figure 2A). The transgenic parasites expressing the bcd and those expressing the yeast fusion gene containing cytosine deaminase exhibited very different sensitivities to the prodrug 5-FC. Schizont development of parasites with the bacterial CD appeared to be unaffected by this drug, whereas parasites with the yeast fusion gene yfcu showed a strong increase in sensitivity to 5-FC with an IC50 value of 2 µM (Figure 2A).
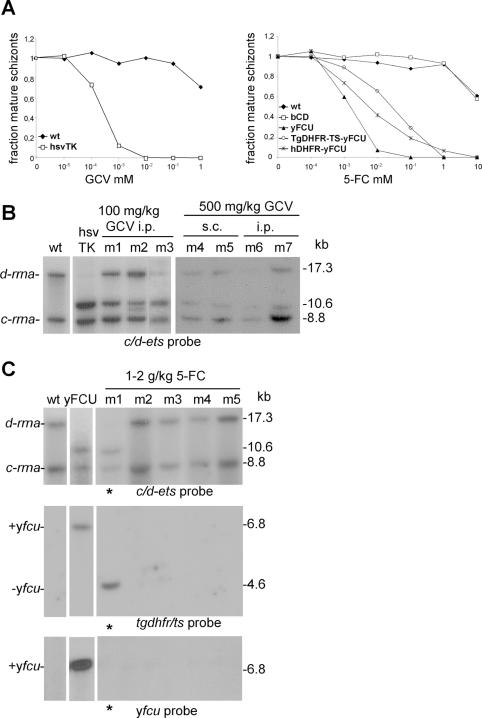
Drug sensitivity of mutant parasites expressing negative selectable markers and in vivo selection of parasites. (A) Left panel: In vitro sensitivity of wt parasites and hsvTK expressing parasites to GCV. Right panel: Sensitivity of wt parasites and parasites expressing bCD, yFCU, TgDHFR-TS-yFCU and hDHFR-yFCU to 5-FC. Sensitivity is defined as the fraction of ring forms that developed into mature schizonts during a 24 h culture period in the presence of different concentrations of the drugs relative to the no drugs control. In each experiment, parasite development was determined by counting 100–200 parasites in Giemsa stained slides and percentages shown are the mean of counting duplicate wells of each drug concentration. (B) Southern analysis of the d-rrna locus in parasites expressing hsvTK before and after in vivo selection in mice with 100 mg (i.p.; m1–m3) or 500 mg (s.c.; m4–m5, i.p.; m6–m7) GCV/kg/day. Selection resulted in parasite populations consisting of mixtures of parasites with a disrupted d-rrna locus (10.6 kb, see Figure 1C and D) and parasites with a restored wt locus (17.3 kb, see Figure 1D). EcoRI and HindIII digested genomic DNA of wt, hsvTK and m1–m7 parasites was hybridized to the c/d-ets probe. (C) Southern analysis of the d-rrna locus in parasites expressing yFCU before and after in vivo selection in mice with 5-FC (m1–m5), showing selection of parasites with a restored wt d-rrna locus (17.3 kb fragment, upper panel, see Figure 1D) or parasites (asterisked lanes) that still contain a disrupted locus (10.6 kb fragment, upper panel) but in which the yfcu gene is lost (4.6 kb, middle panel and no signal, lower panel). The reduced size (4.6 kb instead of 6.8 kb) of the fragment that hybridizes to the tgdhfr-ts probe indicates a recombination event between the two 3′pbdhfr-ts sequences resulting in the excision of the yfcu gene in these parasites (see Figure 1D). EcoRI and HindIII digested genomic DNA was hybridized to the c/d-ets probe (upper panel), tgdhfr-ts probe (middle panel) or yfcu probe (lower panel).
Negative selection of recombinant parasites in vivo
Since we observed a strong in vitro inhibition of development of hsvTK and yFCU expressing parasites by GCV and 5-FC respectively, we investigated whether drug selection in vivo could result in efficient killing of all parasites expressing the negative selectable marker gene and in this way select for parasites that have lost this gene by a recombination event. In two independent experiments, groups of three mice infected with hsvTK expressing parasites were treated with a single dose of GCV (10 or 100 mg/kg bodyweight/day) for a period of 3 or 4 days. Only with the higher dose of 100 mg we observed a significant effect on the parasite development and by comparing the growth rate of wt parasites and hsvTK expressing parasites we calculated that more then 99.9% of the transgenic parasites were killed by the treatment (data not shown). Southern analysis of the parasites that survived this treatment revealed that heterogeneous populations had arisen consisting of parasites either with or without hsvtk remaining in their genomes (Figure 2B). These results demonstrate that although in vivo GCV treatment was not sufficient to kill all hsvTK-expressing parasites, this prolonged treatment significantly enriched for parasites that had lost hsvtk. Based on the hybridization signal intensities of the Southern blots we estimated that 40–80% of the parasites in these drug-treated populations consisted of hsvtk-negative recombinants. Using standard procedures of cloning parasites by limiting dilution it is feasible to isolate hsvtk-negative parasite lines from this population. With higher concentrations of GCV (500 mg/kg/day, administered either i.p. or i.c.), mice were affected by toxic side effects and Southern analysis of the DNA of the surviving parasites revealed that the selection had not been more efficient (Figure 2B).
Next, we analysed whether in vivo 5-FC drug treatment of yFCU-expressing parasites was more efficient in selecting for parasites that have lost the negative selectable marker gene. In two independent experiments, groups of three mice were treated with a single dose of 5-FC (1 or 2 g/kg/day) for 3 to 4 days. By comparing the growth rate of wt parasites and yFCU-expressing parasites we calculated that more then 99.9% of the transgenic (yFCU-expressing) parasites were killed by the drug treatments (data not shown). Analysis of the parasites that survived the treatment showed that most of these parasite populations consisted of parasites that had lost yfcu by a reversion event restoring the wt rrna locus (Figure 2C). These results demonstrate that in vivo 5-FC treatment effectively kills yFCU-expressing parasites and selects for mutants that lost yfcu. In one mouse (m1, Figure 2C), the drug-selected parasites did not show the genotype of wt parasites. Interestingly, hybridization with a tgdhfr-ts probe revealed that the positive selectable marker was still integrated in the d-rrna locus of these parasites but the hybridizing fragment was 2.2 kb smaller than the 6.8 kb fragment found for the parental yFCU-expressing parasites (Figure 2C). This size difference can only be explained by a recombination event between the two 3′pbdhfr-ts regions that are present in the original integrated construct resulting in excision of a 2.2 kb fragment that contains the yfcu gene (Figure 1D). This was confirmed by the lack of hybridization of m1 genomic DNA with the yfcu probe (Figure 2C). The 3′pbdhfr-ts regions are only 452 bp in length, implicating that relatively short regions of homology are sufficient for recombination to occur.
Reversion of a disrupted gene and restoration of the wt phenotype using negative selection
One of the advantages of the availability of an efficient negative selection system in P.berghei is its application in the analysis of phenotypes of mutant parasites that are generated by targeted disruption. Negative selection allows the selection of parasites with reversion of their disrupted genes back to wt, which will provide the possibility of verifying whether the observed mutant phenotype is the result of the disruption event. To demonstrate the selection of wt parasites through negative selection pressure on mutant parasites with a disrupted gene, we constructed a vector that contains both a positive and a negative selectable marker and used it to disrupt the gene p48/45. It has been shown before that disruption of this gene results in a distinct phenotype, reducing the fertility of male gametes (20). The disruption vector was based on the expression vector pEFyFCUSSU, containing yfcu as a selectable marker in which we replaced the d-ssu-rrna targeting sequence with a 1.1 kb fragment of the open reading frame of p48/45 (Figure 3A).
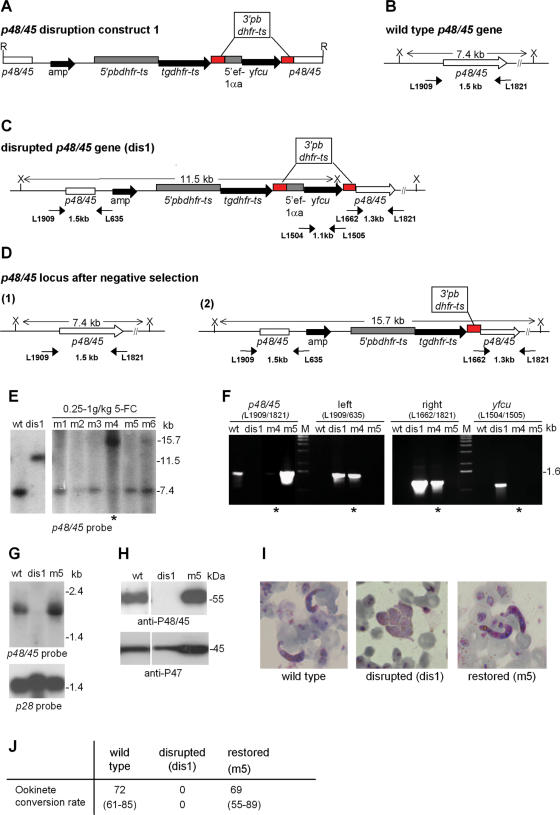
Generation and analysis of parasites with a disrupted and subsequently restored p48/45 locus. (A) Construct used to disrupt p48/45 containing both a positive (tgdhfr-ts) and a negative selectable marker (yfcu). R, EcoRV; amp, ampicillin resistance gene; red boxes, 3′pbdhfr-ts repeat. (B) The genomic p48/45 locus. Sizes of PCR fragments and restriction fragments are indicated between short and long arrows, respectively. PCR primers are specified by their L-number. X, XbaI. (C) The disrupted p48/45 locus (dis1) after integration of construct 1 in parasites that are selected by positive selection with pyrimethamine. (D) The p48/45 locus after negative selection with 5-FC of the mutant parasites as shown in (C). Parasites can be selected in which (1) the wt genotype is restored as a result of a recombinant event between the homologous p48/45 sequences or (2) the negative selectable marker cassette has been excised from the integrated construct by a recombination event between the two 3′pbdhfr-ts sequences. (E) Southern analysis of the p48/45 locus in dis1 parasites before and after (m1–m6) treatment of infected mice with 5-FC, showing selection of parasites with a restored p48/45 locus [7.4 kb band in m1, 2, 3, 5, 6)]. The 15.7 kb band in m4 indicates a recombination event between the two 3′pbdhfr-ts sequences (see Figure 3C and D) resulting in the excision of the yfcu gene in these parasites. Genomic DNA was digested with XbaI and hybridized to the 5′p48/45 probe. (F) PCR analysis of the p48/45 locus in dis1 parasites before and after negative selection, showing a disrupted p48/45 locus in parasite line dis1, a restored wt p48/45 genotype in mouse m5 and a disrupted p48/45 locus with the yfcu sequence removed in m4 (asterisked lanes). p48/45, amplification of p48/45 (primers L1909/L1821); left, 5′ integration in p48/45 (primers L1909/L635); right, 3′ integration in p48/45 (primers L1662/L1821); yfcu, amplification of yfcu (primers L1504/L1505); M, marker. (G) Transcription of p48/45 as analysed by northern blotting in wt parasites, in mutants with a disrupted p48/45 locus (dis1) and in parasites with a restored p48/45 locus (m5) after 5-FC selection. RNA of blood stages enriched in gametocytes was hybridized to a p48/45 probe (upper panel) or the gametocyte specific control probe p28 (lower panel). (H) The presence of P48/45 as analyzed by western blotting in wt parasites, in mutants with a disrupted p48/45 locus (dis1) and in parasites with a restored p48/45 locus (m5). Proteins extracted from blood stages enriched in gametocytes reacted with polyclonal antibodies raised against recombinant P48/45 (upper panel) or against recombinant protein of the gametocyte specific protein P47 (lower panel). (I) Gametes of mutant parasites with a disrupted p48/45 (dis1) fail to fertilize, resulting in the lack of ookinete formation and the presence of clusters of female unfertilized gametes in ookinete cultures. Parasites with a restored p48/45 locus (m5) produce normal ookinetes. Parasites were stained with Giemsa and photographed at 1000× magnification. (J) Ookinete production in vitro in wt parasites, mutants with a disrupted p48/45 locus (dis1) and parasites with a restored p48/45 gene (m5) after negative selection. Parasites with a restored p48/45 locus produce normal numbers of ookinetes as shown by the percentage of female gametes developing in mature ookinetes (ookinete conversion rate).
The vector was introduced into P.berghei using standard protocols of transfection and positive selection (3). After cloning of the transgenic parasites, correct integration of the vector and disruption of the p48/45 locus (Figure 3A–C) was demonstrated by Southern analysis of restricted genomic DNA (Figure 3D) and PCR (Figure 3E). Parasite clone dis1 was analysed further by northern and western analysis, showing the absence of the wt 2 kb p48/45 transcript (Figure 3G) and the P48/45 protein, respectively (Figure 3H).
In order to restore the interrupted p48/45 gene, we treated six mice infected with parasites of clone dis1 with 5-FC (250 mg to 1 g/kg/day) for 3 days. Southern analysis of the surviving parasites showed that in five out of the six mice (m1, 2, 3, 5 and 6) the p48/45 gene of the parasites had reverted to wt (Figure 3D and E). Northern and western analysis of one of the selected parasite populations (m5), showed the recurrence of the wt 2 kb p48/45 transcript (Figure 3G) and a 55 kDa protein that reacted with an antibody to P.berghei P48/45 (Figure 3H). These parasites showed normal rates of zygote (ookinete) formation, demonstrating that the reversion of the p48/45 gene had resulted in complete restoration of the phenotype (Figure 3I and J).
Restoration of the disrupted p48/45 gene in the parasites selected in five out of the six mice is the result of a recombination event between the two homologous p48/45 sequences that are introduced through integration of the construct, resulting in excision of the complete integrated construct. In one experiment (m4) we selected a population of parasites that did not show the wt genotype (Figure 3E and F). In this case a 15.7 kb fragment hybridized to the p48/45 probe and this fragment corresponds with a recombination event between the two 3′pbdhfr-ts sequences that are present in the integrated construct, thereby excising a 2.2 kb fragment containing the yfcu gene (Figure 3D and E). PCR analysis confirmed that the p48/45 gene is still disrupted in the m4 parasite line, but that the yfcu sequence has been removed (Figure 3F).
Improvement of negative selection vectors by generation of gene fusions encoding positive and negative selectable markers
For routine application of a positive/negative selection system in future reverse genetics experiments and for selectable marker recycling, the vector used to introduce DNA requires an optimal configuration with regard to the size of the selectable marker cassettes and to the desired recombination events. We therefore constructed vectors containing fusions of the positive selectable markers tgdhfr-ts and hdhfr with the negative selectable marker yfcu in order to reduce the size of the selectable marker cassettes (2.7 and 4.0 kb smaller, respectively). Since these vectors contain a single fusion gene as a selectable marker, only one 3′pbdhfr-ts sequence is needed, thereby avoiding the unfavourable recombination events that occurred in the other constructs that contain two of these sequences (Figures 1D and and3C).3C). The two vectors, containing the tgdhfr-ts-yfcu (data not shown) or hdhfr-yfcu fusion gene in addition to p48/45 target sequence (Figure 4A) were introduced into P.berghei using standard protocols resulting in the generation of parasites with a disrupted p48/45 gene (Figure 4B–D). The positive selection of these parasites demonstrated that the fusion proteins displayed sufficient DHFR activity to allow in vivo selection of mutants. To investigate whether the yFCU fusions affected the activity of the yFCU moiety, we determined the in vitro sensitivity of the mutant parasites to the drug 5-FC. Indeed, parasites containing either fusion gene showed a decreased sensitivity to 5-FC compared to mutant parasites containing an independent yfcu gene (Figure 2A). We next applied negative selection by treating infected mice with 5-FC (1 g/kg) for 4 days. In two experiments (three mice per experiment) using mutants containing the fusion gene tgdhfr-ts-yfcu we were not able to select parasites with a restored p48/45 gene (data not shown). This failure of negative selection confirms that the TgDHFR-TS-yFCU fusion protein has a decreased and insufficient yFCU activity. On the other hand, using the mutants containing the fusion gene hdhfr-yfcu (dis2), we obtained populations of parasites that were enriched in parasites with a restored p48/45 gene (m1–m3, Figure 4D). After optimizing the selection protocol either by injection of 5-FC (500 mg/kg) twice a day (m4–m6, Figure 4D) or by a second negative selection round (m7–m9, Figure 4D) we were able to select for parasite populations in which the restoration of the p48/45 was complete. The results demonstrate that the hdhfr-yfcu fusion can be applied for gene disruption and restoration experiments by using positive and negative selection.
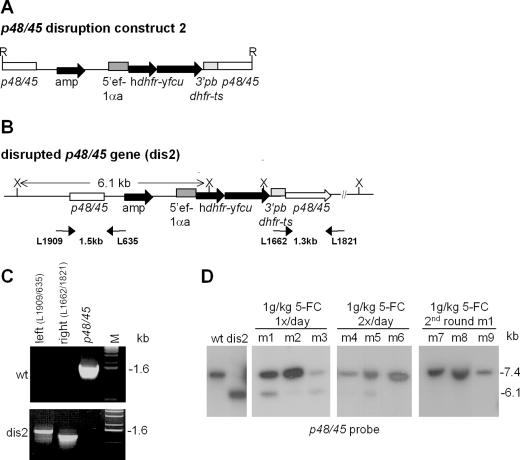
Generation of parasites with a disrupted and restored p48/45 locus using a fusion gene of the positive and negative selectable marker. (A) Construct used to disrupt p48/45 containing a fusion gene of the positive (hdhfr) and negative selectable marker (yfcu). R, EcoRV; amp, ampicillin resistance gene. (B) The disrupted p48/45 locus after integration of construct 2 in parasites that are selected by positive selection with WR99210. Sizes of PCR fragments and restriction fragments are indicated between short and long arrows, respectively. PCR primers are specified by their L-number. X, XbaI. (C) Correct integration of construct 2 in the p48/45 locus in parasite line dis2 as shown by PCR. Left, 5′ integration in p48/45 (primers L1909/L635); right, 3′ integration in p48/45 (primers L1662/L1821); p48/45, amplification of p48/45 (primers L1909/L1821); M, marker. (D) Southern analysis of the p48/45 locus in mutant parasites before (dis2) and after (m1–m9) treatment of infected mice with 5-FC showing that selection with 1 g 5-FC/kg/day (m1–m3) results in mixtures of parasites with a disrupted p48/45 locus and parasites with a restored wt locus. Increasing the selective pressure by 5-FC treatment twice a day (m4–m6) or by a second round of 5-FC-selection of parasite population m1 (m7–m9) leads to a further enrichment for parasites with a restored p48/45 genotype.
DISCUSSION
Here we report the construction of a rapid gene knock-out/restoration system for mutant parasites of P.berghei following development of effective negative selection protocols. The availability of negative selectable markers markedly improves the flexibility of the application of reverse genetic technologies in studies on the biology of Plasmodium (see Introduction) and this report is the first that describes a method for in vivo negative selection of mutant rodent malaria parasites. The application of in vitro negative selection has been reported for P.falciparum to select for mutants with disrupted genes after transfection with circular plasmids (9). The protocol for P.falciparum is a relatively complicated procedure that requires: (i) the introduction and positive selection of plasmids containing a positive selectable marker in addition to hsvtk into the in vitro cultured blood stages, followed by (ii) simultaneous positive and negative selection. The procedure resulted in the isolation of mutants that contain disrupted genes through double crossover recombination events. For both P.falciparum and P.knowlesi it has been demonstrated that mutants expressing hsvTK are effectively killed in vitro by the drug GCV (5,9). The use of bcd in P.falciparum to select for double crossover integration events was assessed and discarded because for unclear reasons selection resulted in parasites that were resistant to 5-FC and had not undergone double crossover integration (9).
In this study we tested three candidate negative selectable markers in combination with two drugs for the development of in vivo negative selection of P.berghei. We found that hsvTK expression makes P.berghei highly sensitive to GCV with in vitro IC50 values that are comparable to that for P.falciparum (9). In vivo selection of transgenic hsvTK-expressing parasites with GCV to select for parasites that had lost the hsvtk resulted in a substantial enrichment for parasites that had lost this negative selectable marker. With doses of 100 and 500 mg/kg GCV the selected parasite populations always consisted of both parasites with and without hsvtk, showing that the in vivo treatment did not effectively kill all hsvTK-expressing parasites. Since haemotoxic side effects are observed in mice treated with doses of GCV higher than 100 mg/kg/day [this study, (24,25)] a more efficient selection cannot be achieved by the use of GCV. Although we were not able to completely eliminate parasites containing the hsvtk by GCV treatment, the enrichment of parasites without hsvtk indicates that the described hsvTK/GCV selection strategy might well be of value for negative selection applications if it is combined with parasite cloning.
We have also investigated the sensitivity of hsvTK-expressing parasites to acyclovir (ACV), a drug that is related to GCV and is better tolerated by mice, but this drug had no effect on the development of the parasites either in vitro nor in vivo on the development of the parasites (data not shown). The different effects of the two prodrugs on hsvTK-expressing parasites can be explained by the fact that the hsvtk mutant (sr39) we used in our experiments has a lower sensitivity [6.5×, see (14)] to ACV compared with GCV. Another possibility is that the Plasmodium nucleoside/base transporters that are responsible for prodrug transport across the membrane have different affinities for the two prodrugs, which has been observed for the mammalian transporters (26–28).
CD of Escherichia coli has been widely used as a negative selectable marker in mammalian cells (11,29). CD deaminates cytosine to uracil in bacteria and fungi and is not found in eukaryotes including Plasmodium. CD can convert the prodrug 5-FC into the highly toxic compound 5-fluorouracil (5-FU) and has been used in the related apicomplexan parasite, T.gondii (30). However in P.berghei we did not detect a significant difference in sensitivity to 5-FC between wt and bCD-expressing parasites as determined by inhibition of in vitro schizont development. On the other hand, the infectivity of such drug-treated schizonts as determined by injection into mice appeared to be significantly reduced compared to non-treated schizonts (data not shown). This observation indicates that bCD-expressing P.berghei parasites are affected by 5-FC in a manner similar to bCD-expressing P.falciparum (9) but the inhibitory effect appears to be too low to be able to select in vivo for P.berghei parasites that have lost bcd.
In order to improve the CD/5-FC strategy for negative selection in Plasmodium, we investigated the potency of a fusion gene encoding two yeast enzymes, cytosine deaminase and uracil phosphoribosyl transferase (UPRT), an enzyme involved in downstream metabolism of 5-FU. The yeast CD is more efficient in 5-FC conversion than the CD of bacteria (31,32) and it has been reported that adenoviral expression of the fusion gene of this enzyme with UPRT (forming yFCU) establishes an extra 1000-fold increase in sensitivity to 5-FC (12). Introduction of yfcu in P.berghei indeed strongly increased the sensitivity to 5-FC compared to bcd. Moreover, in vivo selection with 5-FC resulted in efficient killing of yFCU-expressing parasites, enabling for the first time in vivo selection of pure parasites populations that had lost the negative selectable marker. The efficiency of selection with 5-FC of parasites that have lost yfcu makes this system a practical tool to exploit negative selection in P.berghei. In this study we showed its applicability to the investigation of gene function by using an approach in which targeted disruption selected by positive pressure is followed by selection under negative pressure for reversion of the original disruptive integration restoring both phenotype and genotype. Such reversible recombination events offer a relatively rapid and easy method of strongly associating gene and function. This is a particularly important advance for P.berghei transfection because of the paucity of selectable markers, which hitherto has hampered confirmation of phenotype, as it requires the introduction of transgenes that complement the loss of gene function. In this study the role of the P48/45 protein in the process of fertilization in Plasmodium was investigated (20). We used positive–negative selection to first disrupt p48/45 and observe the phenotype of the mutants, i.e. the lack of fertilization, followed by restoration of the gene which led to complete recovery of the fertilization capacity shown by the formation of zygotes/ookinetes at wt levels.
In addition to the above, this system can be a useful tool in other reverse genetics technologies (see Introduction). For example, due to the homologous recombination during the negatively selected reversion of integration a ‘Hit and Run’ approach can be used to introduce point mutations into targeted genes. In such experiments the negatively-selected population would be a mixture of wt and mutants and would require parasite cloning and verification before proceeding further with analysis of additional manipulation [see (33) for a review]. Additionally negative selection can be used to recycle positive selectable markers by excision of the selection marker cassettes from the genome of mutant parasites and will of great benefit for Plasmodium transfection given the limited number of selection markers. Recycling would be achieved by placing two repeated DNA elements flanking the selectable marker cassette and their recombination eliminates the flanked marker. In our studies, such recombination was mediated by two 0.5 kb copies of the pbdhfr-ts 3′ region (highlighted in red in Figures 1 and and3,3, mutants m1 and m4 respectively, asterisked). Vector formats that would allow marker recycling have been constructed and in initial tests appear to be function as predicted (J.A.M.B., unpublished data). Negative selection may also be useful in the production of live parasites for vaccination that have been attenuated by genetic modification (34). In this instance negative selection would permit the production of modified parasites that may not contain foreign DNA (e.g. drug-resistant markers) before they may be used as a vaccine. For such more advanced applications of negative selection systems it will be essential to optimize the configuration of constructs containing the positive and negative selectable markers. An immediate improvement stems from the use of the fusion gene of the positive and negative selectable markers as reported in this study that significantly reduced the size of the selectable marker cassettes in the construct (by up to 4.0 kb). This change should improve plasmid stability and allow larger DNA fragments to be cloned. In addition, the plasmids containing the fused selectable markers lack the duplicated elements that mediated unwanted recombination in the original vectors. Unfortunately, parasites containing these fusion genes showed a reduced sensitivity to 5-FC and using the fusion tgdhfr-ts-yfcu we were not able to select for populations enriched for parasites that had lost the fusion gene. However, with the hdhfr–yfcu fusion gene we could accomplish selection for parasites that had lost the selectable marker cassette if the dose of 5-FC was significantly increased. Although we did not observe toxic side effects with the increased amounts of 5-FC the effective dose was limited by the low solubility of the drug. Possible future improvements of the hdhfr-yfcu selectable marker cassette involve either the use of alternative linkers between the open reading frames of the two fusion partners or the use of a bidirectional promoter that drives the expression of both individual selectable markers.
This negative selection system offers significant improvements over both the hsvtk and bcd systems that have been developed in P.falciparum and work is currently ongoing to apply the yfcu-based vectors to this parasite (A. Cowman, personal communication). Lastly, this is the first report of the general use of yfcu in combination with a positive selectable marker in reverse genetics procedures. Although dhfr cannot be used as a positive selectable marker in most biological systems, fusion of yfcu with a more widely applicable positive selectable marker such as pac should prove useful for general use in reverse genetics procedures.
Acknowledgments
The authors are grateful to Dr P. Erbs for providing plasmid pCI-neoFCU1, to Dr M. E. Black for plasmid pET23d:HSVTK-SR39 and Dr C. Karreman for plasmid pCMVCODA. The authors would like to thank J. Ramesar for excellent technical assistance. This work was supported by the Wellcome Trust Functional Genomics Initiative and EC INCO-DEV grant ICA4-1999-10022. Funding to pay the Open Access publication charges for this article was provided by LUMC.
Conflict of interest statement. None declared.
REFERENCES
Articles from Nucleic Acids Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/nar/gnj033
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1401515?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1093/nar/gnj033
Article citations
Intracellular Plasmodium aquaporin 2 is important for sporozoite production in the mosquito vector and malaria transmission.
Proc Natl Acad Sci U S A, 120(44):e2304339120, 26 Oct 2023
Cited by: 1 article | PMID: 37883438 | PMCID: PMC10622946
Significance of Plasmodium berghei Amino Acid Transporter 1 in Food Vacuole Functionality and Its Association with Cerebral Pathogenesis.
Microbiol Spectr, e0494322, 28 Mar 2023
Cited by: 0 articles | PMID: 36976018 | PMCID: PMC10101031
Creation and preclinical evaluation of genetically attenuated malaria parasites arresting growth late in the liver.
NPJ Vaccines, 7(1):139, 04 Nov 2022
Cited by: 11 articles | PMID: 36333336 | PMCID: PMC9636417
Phosphatase inhibitors BVT-948 and alexidine dihydrochloride inhibit sexual development of the malaria parasite Plasmodium berghei.
Int J Parasitol Drugs Drug Resist, 19:81-88, 30 Jun 2022
Cited by: 4 articles | PMID: 35792443 | PMCID: PMC9260261
Plasmodium sporozoite disintegration during skin passage limits malaria parasite transmission.
EMBO Rep, 23(7):e54719, 11 Apr 2022
Cited by: 5 articles | PMID: 35403820 | PMCID: PMC9253755
Go to all (57) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Improved negative selection protocol for Plasmodium berghei in the rodent malarial model.
Malar J, 11:103, 31 Mar 2012
Cited by: 34 articles | PMID: 22463060 | PMCID: PMC3364864
The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome.
Mol Biochem Parasitol, 106(2):199-212, 01 Mar 2000
Cited by: 71 articles | PMID: 10699250
A case of 'hit-and-run' in Plasmodium genetics.
Trends Parasitol, 22(11):493-495, 12 Sep 2006
Cited by: 0 articles | PMID: 16971181
Towards genome-wide experimental genetics in the in vivo malaria model parasite Plasmodium berghei.
Pathog Glob Health, 109(2):46-60, 19 Mar 2015
Cited by: 20 articles | PMID: 25789828 | PMCID: PMC4571819
Review Free full text in Europe PMC
Funding
Funders who supported this work.





