Abstract
Free full text

Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains.
Abstract
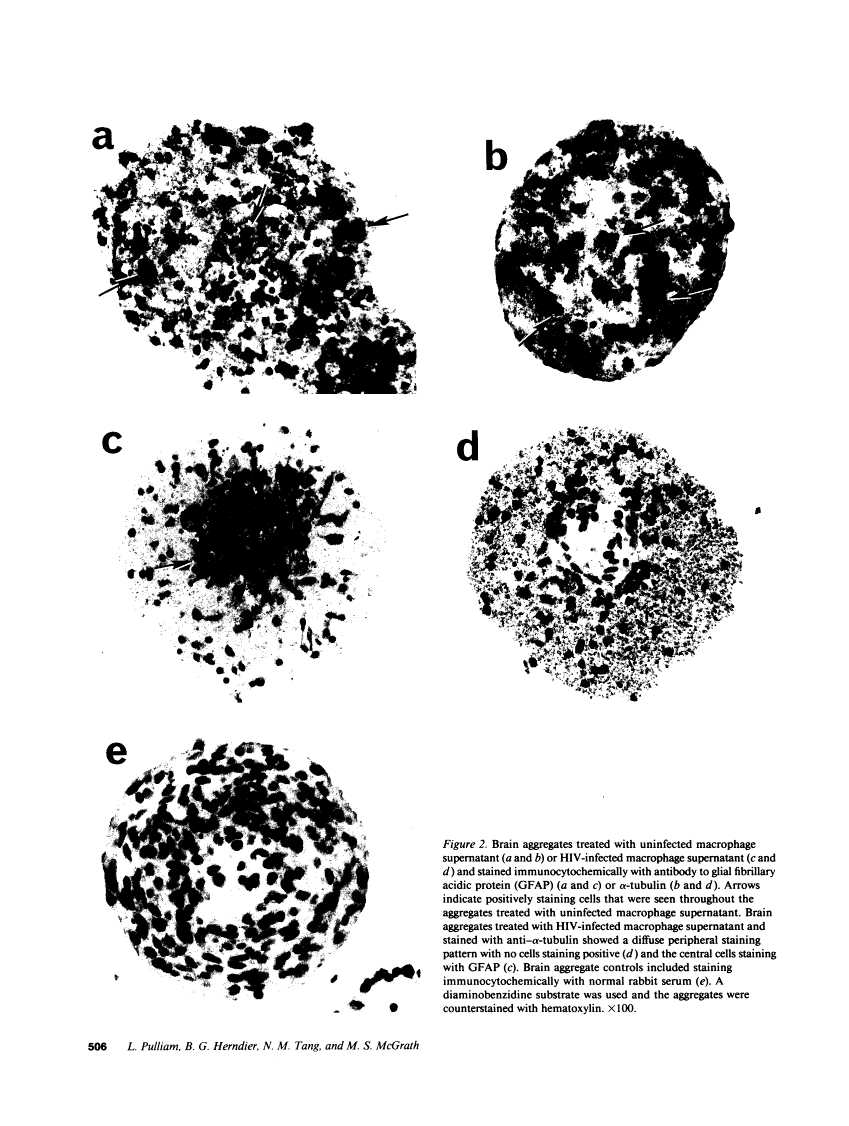
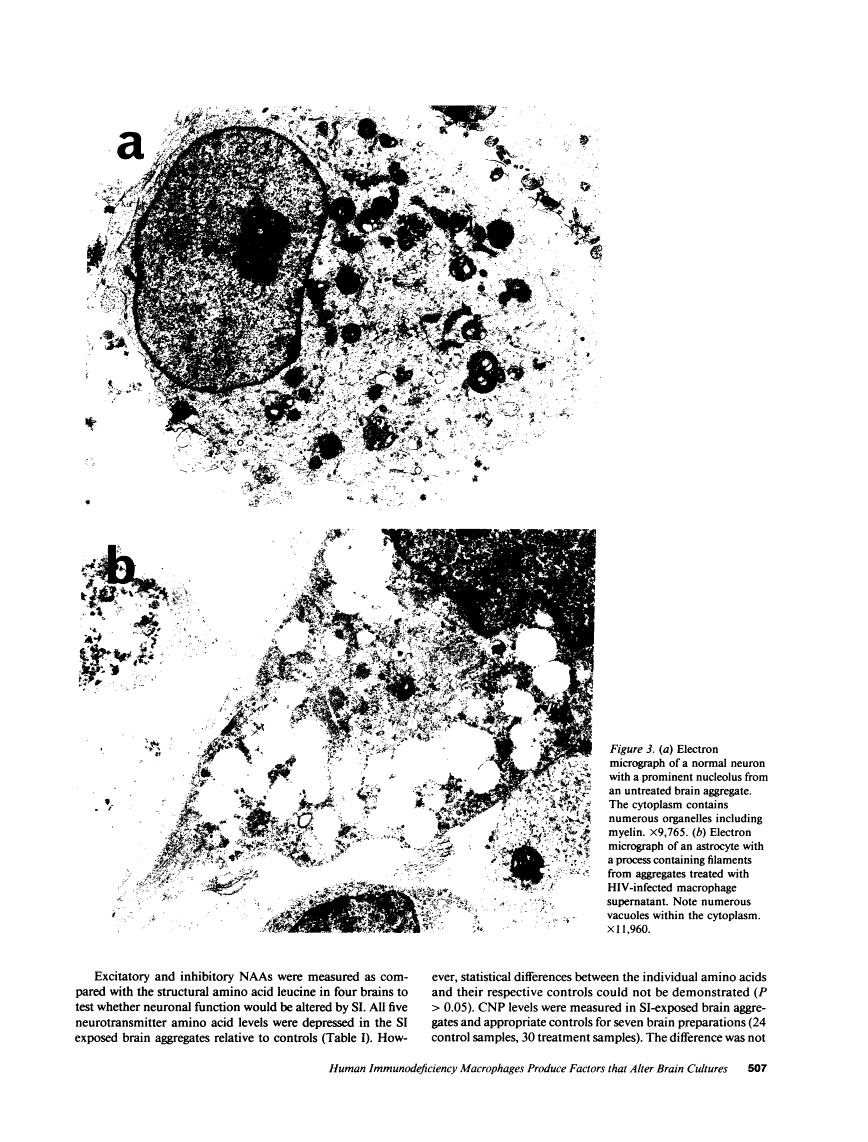
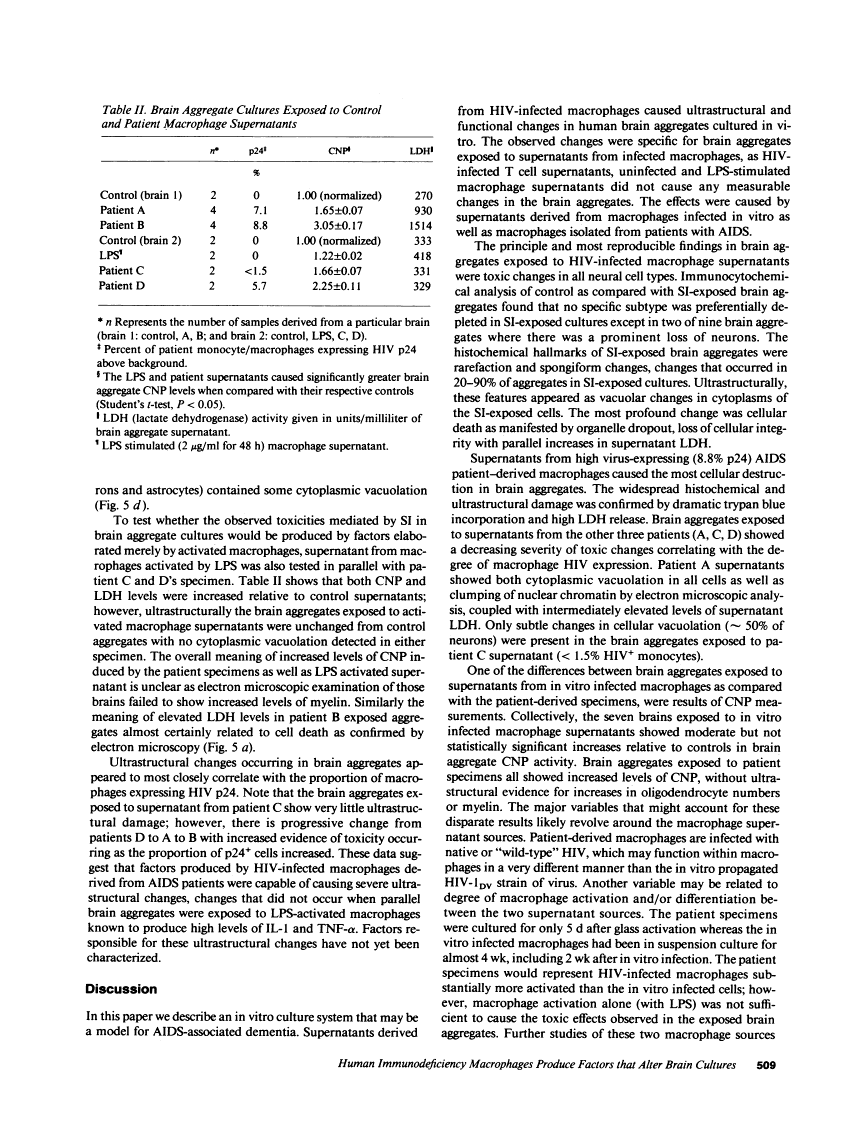
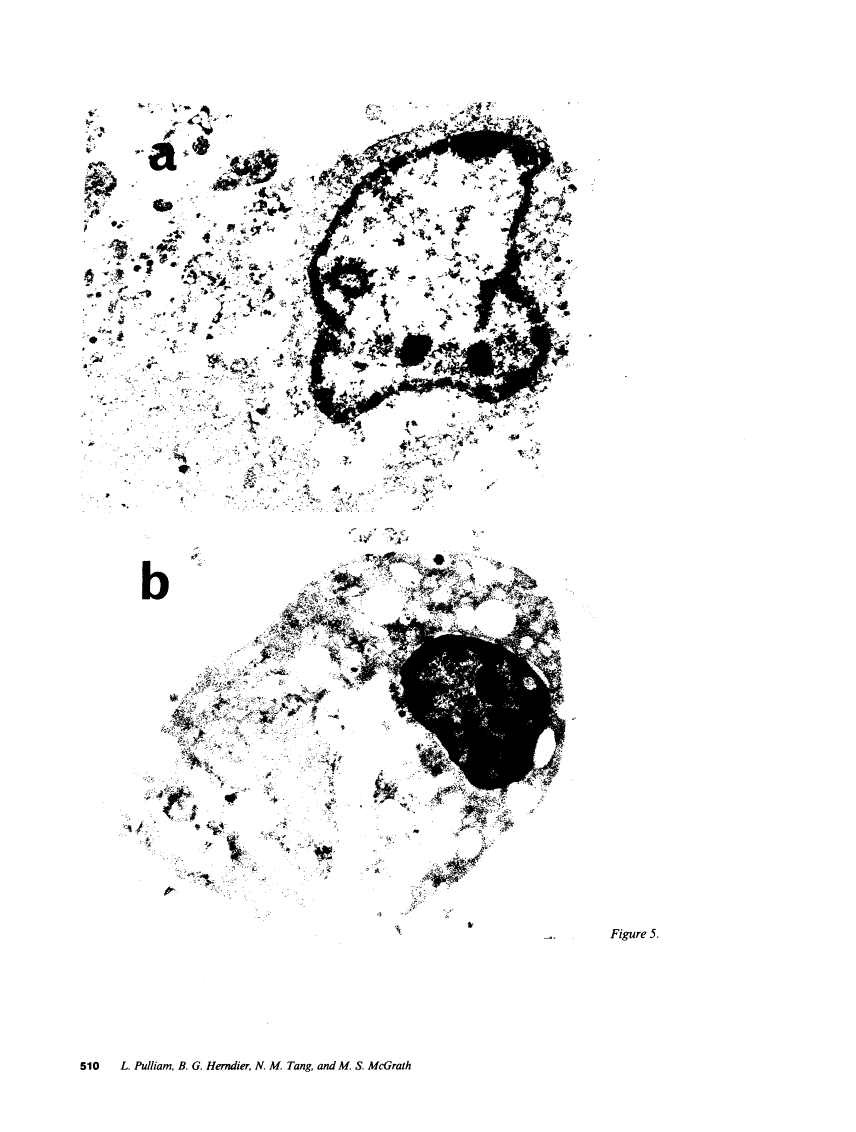
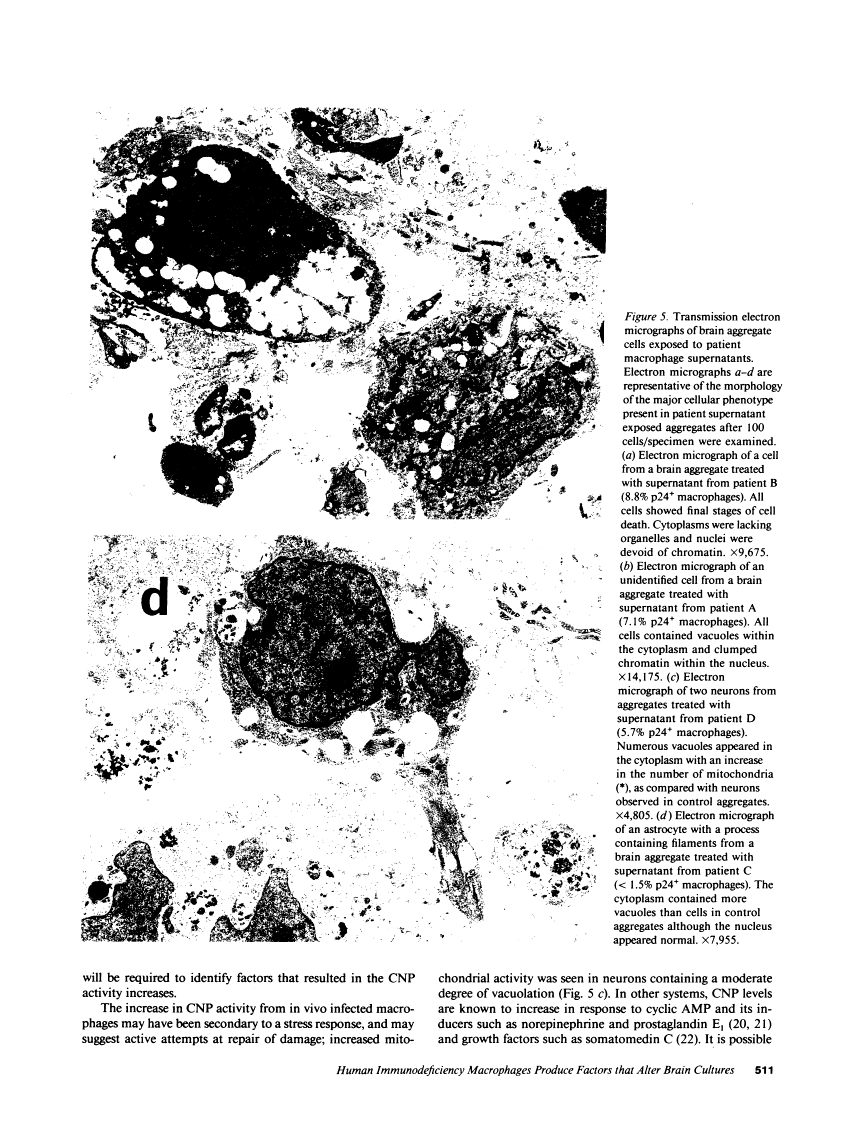
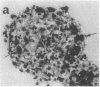
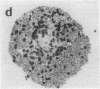
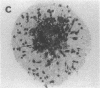
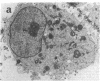
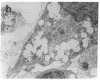
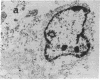
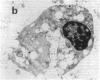
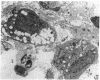
We wanted to establish an in vitro human model for AIDS-associated dementia and pursue the hypothesis that this disease process may be a result of soluble factors produced by HIV-infected macrophages. Human brain aggregates were prepared from nine different brain specimens, and were treated with supernatants from in vitro HIV-infected macrophages (SI), uninfected macrophages (SU), infected T cells, or macrophage-conditioned media from four AIDS patients. Seven of nine treated brains exposed to SI showed peripheral rarefaction after 1 wk of incubation that by ultrastructural analysis showed cytoplasmic vacuolation. Aggregates from two of three brain cultures treated with SI for 3 wk became smaller, an approximately 50% decrease in size. The degree of apparent toxicity in brains exposed to patient-derived macrophage supernatants paralleled the proportion of macrophages found to be expressing HIV p24. Ultrastructural abnormalities were not observed in brains treated with supernatants from HIV-infected T cells, uninfected macrophages, or LPS-activated macrophages. Levels of five neurotransmitter amino acids were decreased in comparison to the structural amino acid leucine. These findings suggest that HIV-infected macrophages, infected both in vitro as well as derived from AIDS patients' peripheral blood, produce factors that cause reproducible histochemical, ultrastructural, and functional abnormalities in human brain aggregates.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (5.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988 Nov;158(5):1079–1083. [Abstract] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986 Jun;19(6):525–535. [Abstract] [Google Scholar]
- Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, et al. AIDS subacute encephalitis. Identification of HIV-infected cells. Am J Pathol. 1987 Mar;126(3):403–410. [Europe PMC free article] [Abstract] [Google Scholar]
- Shaw GM, Harper ME, Hahn BH, Epstein LG, Gajdusek DC, Price RW, Navia BA, Petito CK, O'Hara CJ, Groopman JE, et al. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science. 1985 Jan 11;227(4683):177–182. [Abstract] [Google Scholar]
- Gonda MA, Wong-Staal F, Gallo RC, Clements JE, Narayan O, Gilden RV. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. [Abstract] [Google Scholar]
- Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985 Sep 14;2(8455):586–588. [Abstract] [Google Scholar]
- Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985 Dec 12;313(24):1493–1497. [Abstract] [Google Scholar]
- Hollander H, Levy JA. Neurologic abnormalities and recovery of human immunodeficiency virus from cerebrospinal fluid. Ann Intern Med. 1987 May;106(5):692–695. [Abstract] [Google Scholar]
- Cheng-Mayer C, Rutka JT, Rosenblum ML, McHugh T, Stites DP, Levy JA. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. [Europe PMC free article] [Abstract] [Google Scholar]
- Chiodi F, Fuerstenberg S, Gidlund M, Asjö B, Fenyö EM. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987 Apr;61(4):1244–1247. [Europe PMC free article] [Abstract] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988 Oct 13;335(6191):639–642. [Abstract] [Google Scholar]
- Wahl LM, Corcoran ML, Pyle SW, Arthur LO, Harel-Bellan A, Farrar WL. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci U S A. 1989 Jan;86(2):621–625. [Europe PMC free article] [Abstract] [Google Scholar]
- Gurney ME, Heinrich SP, Lee MR, Yin HS. Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science. 1986 Oct 31;234(4776):566–574. [Abstract] [Google Scholar]
- Crowe S, Mills J, McGrath MS. Quantitative immunocytofluorographic analysis of CD4 surface antigen expression and HIV infection of human peripheral blood monocyte/macrophages. AIDS Res Hum Retroviruses. 1987 Summer;3(2):135–145. [Abstract] [Google Scholar]
- McGrath MS, Hwang KM, Caldwell SE, Gaston I, Luk KC, Wu P, Ng VL, Crowe S, Daniels J, Marsh J, et al. GLQ223: an inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2844–2848. [Europe PMC free article] [Abstract] [Google Scholar]
- Locksley RM, Crowe S, Sadick MD, Heinzel FP, Gardner KD, Jr, McGrath MS, Mills J. Release of interleukin 1 inhibitory activity (contra-IL-1) by human monocyte-derived macrophages infected with human immunodeficiency virus in vitro and in vivo. J Clin Invest. 1988 Dec;82(6):2097–2105. [Europe PMC free article] [Abstract] [Google Scholar]
- Pulliam L, Berens ME, Rosenblum ML. A normal human brain cell aggregate model for neurobiological studies. J Neurosci Res. 1988 Oct-Dec;21(2-4):521–530. [Abstract] [Google Scholar]
- Kurihara T, Tsukada Y. The regional and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphohydrolase in the central nervous system. J Neurochem. 1967 Dec;14(12):1167–1174. [Abstract] [Google Scholar]
- Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods. 1987 May;20(1):83–90. [Abstract] [Google Scholar]
- Prohaska JR, Clark DA, Wells WW. Improved rapidity and precision in the determination of brain 2',3'-cyclic nucleotide 3'-phosphohydrolase. Anal Biochem. 1973 Nov;56(1):275–282. [Abstract] [Google Scholar]
- McMorris FA, Smith TM, Sprinkle TJ, Auszmann JM. Induction of myelin components: cyclic AMP increases the synthesis rate of 2',3'-cyclic nucleotide 3'-phosphohydrolase in C6 glioma cells. J Neurochem. 1985 Apr;44(4):1242–1251. [Abstract] [Google Scholar]
- McMorris FA. Cyclic AMP induction of the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphohydrolase in rat oligodendrocytes. J Neurochem. 1983 Aug;41(2):506–515. [Abstract] [Google Scholar]
- McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986 Feb;83(3):822–826. [Europe PMC free article] [Abstract] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989 May 19;244(4906):798–800. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci115024
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/115024/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1172/jci115024
Article citations
Extracellular vesicles released from macrophages modulates interleukin-1β in astrocytic and neuronal cells.
Sci Rep, 13(1):3005, 21 Feb 2023
Cited by: 5 articles | PMID: 36810605 | PMCID: PMC9944928
HIV Dementia: A Bibliometric Analysis and Brief Review of the Top 100 Cited Articles.
Cureus, 14(5):e25148, 19 May 2022
Cited by: 3 articles | PMID: 35733470 | PMCID: PMC9205453
Review Free full text in Europe PMC
Suppression of HIV-associated Macrophage Activation by a p75 Neurotrophin Receptor Ligand.
J Neuroimmune Pharmacol, 17(1-2):242-260, 22 Jul 2021
Cited by: 4 articles | PMID: 34296391 | PMCID: PMC9386897
Alzheimer's-Like Pathology at the Crossroads of HIV-Associated Neurological Disorders.
Vaccines (Basel), 9(8):930, 21 Aug 2021
Cited by: 8 articles | PMID: 34452054 | PMCID: PMC8402792
Review Free full text in Europe PMC
Neurocognitive Complications of Pediatric HIV Infections.
Curr Top Behav Neurosci, 50:147-174, 01 Jan 2021
Cited by: 7 articles | PMID: 31522375
Go to all (176) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier.
Ann Neurol, 34(3):339-350, 01 Sep 1993
Cited by: 202 articles | PMID: 7689819
Concurrent infection of human macrophages with HIV-1 and Mycobacterium avium results in decreased cell viability, increased M. avium multiplication and altered cytokine production.
J Immunol, 151(4):2261-2272, 01 Aug 1993
Cited by: 25 articles | PMID: 8345208
Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms.
Ann Neurol, 33(5):429-436, 01 May 1993
Cited by: 216 articles | PMID: 8498818
Review
OTK18 expression in brain mononuclear phagocytes parallels the severity of HIV-1 encephalitis.
J Neuroimmunol, 150(1-2):186-198, 01 May 2004
Cited by: 15 articles | PMID: 15081264