Abstract
Objective
Chronic manganese (Mn) intoxication induces syndromes resembling Parkinson disease. The clinical intervention has largely been unsuccessful. We report a 17-year follow-up study of effective treatment of occupational Mn parkinsonism with sodium para-aminosalicylic acid (PAS).Methods
The patient, female and aged 50 at the time of treatment, was exposed to airborne Mn for 21 years (1963-1984). The patient had palpitations, hand tremor, lower limb myalgia, hypermyotonia, and a distinct festinating gait. She received 6 g PAS per day through an intravenous drip infusion for 4 days and rested for 3 days as one therapeutic course. Fifteen such courses were carried out between March and June 1987.Results
At the end of PAS treatment, her symptoms were significantly alleviated, and handwriting recovered to normal. Recent follow-up examination at age 67 years (in 2004) showed a general normal presentation in clinical, neurologic, brain magnetic resonance imaging, and handwriting examinations with a minor yet passable gait.Conclusions
This case study suggests that PAS appears to be an effective drug for treatment of severe chronic Mn poisoning with a promising prognosis.Free full text

Effective Treatment of Manganese-Induced Occupational Parkinsonism With p-Aminosalicylic Acid: A Case of 17-Year Follow-Up Study
Abstract
Objective
Chronic manganese (Mn) intoxication induces syndromes resembling Parkinson disease. The clinical intervention has largely been unsuccessful. We report a 17-year follow-up study of effective treatment of occupational Mn parkinsonism with sodium para-aminosalicylic acid (PAS).
Methods
The patient, female and aged 50 at the time of treatment, was exposed to airborne Mn for 21 years (1963–1984). The patient had palpitations, hand tremor, lower limb myalgia, hypermyotonia, and a distinct festinating gait. She received 6 g PAS per day through an intravenous drip infusion for 4 days and rested for 3 days as one therapeutic course. Fifteen such courses were carried out between March and June 1987.
Results
At the end of PAS treatment, her symptoms were significantly alleviated, and handwriting recovered to normal. Recent follow-up examination at age 67 years (in 2004) showed a general normal presentation in clinical, neurologic, brain magnetic resonance imaging, and handwriting examinations with a minor yet passable gait.
Conclusions
This case study suggests that PAS appears to be an effective drug for treatment of severe chronic Mn poisoning with a promising prognosis.
Occupational exposure to manganese (Mn) takes place in ore extraction and processing, steel and alloy production, welding, chemical synthesis, ceramic production, and dry battery fabrication. Mn is also used in water purification, as bactericidal and fungicide agents, and recently used as an antiknock agent in gasoline. Neurotoxicities resulting from occupational exposure to Mn have been well recognized and documented.1,2 However, the clinical intervention has been largely unsuccessful.
The clinical manifestations of Mn poisoning or manganism pertain to extrapyramidal syndrome in a pattern similar but not identical to Parkinson disease.1,3,4 The most relevant signs and symptoms of Mn-induced parkinsonism are frequently intentional tremor with absence or a low level of resting tremor, hypertonia, and adiadochokinesia. In severe cases, gait disturbance, particularly with difficulty in backward walking, can be seen.5–7 Mn poisoning has also been linked to distorted mental function such as memory loss, apathy, and even psychosis. One of the commonly referred criteria in clinics to differentiate manganism from idiopathic Parkinson disease is the lack of response of manganism patients to levodopa therapy.8 Prognosis of Mn toxicity intervention varies widely. In many cases, Mn concentrations in blood, urine, or hair may return to normal several years after withdrawal from the exposure scene. Patients’ symptoms, including disturbed rigor, muscle pain or cramps, sweating, fatigue, and anxiety, may be improved; however, most extrapyramidal syndromes in gait, rigidity, and writing remain unchanged.9–11 Some investigators suggest that clinical progression in patients with manganese parkinsonism can continue even 10 years after cessation of exposure.11 Recent evidence indicates that Mn intoxication may trigger a neurodegenerative process.12,13 Among career welders, exposure to airborne Mn has been associated with a higher prevalence of early onset of Parkinson disease.14,15
The chelation therapy has been used for treatment of severe cases of Mn intoxication. EDTA treatment has been shown to increase Mn excretion in urine and decrease Mn concentrations in blood; however, the clinical symptoms do not appear to be significantly improved among patients.2,3,16,17 In addition to chelation therapy, sodium para-aminosalicylic acid (PAS), an antibacterial drug for treatment of tuberculosis, has been found to be effective in treatment of severe chronic manganism.18–20 This group has previously reported that a patient received PAS treatment for 3.5 months showed much improved clinical symptoms with scarce relapse even 7 months after treatment.19 However, the longterm therapeutic prognosis of PAS in treatment of manganism was unknown. In this case report, we continued a 17-year follow-up clinical investigation on this patient who was reexamined in September 2004.
Case Report
The female patient, aged 45, who reported palpitations, hand tremor, headache, memory loss, lower limb myalgia, and hypermyotonia, visited the clinic in the summer of 1982. The hospital record showed that the patient had palpitations and hand tremor since 1968. In 1980, her symptoms progressed, including also headache, giddiness, salivation, lower limb myalgia, myasthenia, and frequent tetany and numbness in the arms and legs. The patient often suddenly knelt down while walking. Physical examination at that time showed hands, tongue, and eyelids to be tremulous, rigid, or hypertonic, with positive Houk’s sign and abnormal nose-pointing-test outcome. She had visible tremor while writing and a distinct festinating gait. The occupational history indicated that the patient had been working in milling Mn ore process for 19 years (1963–1982) and was still an employee in the same job at the time of the hospital visit. The patient was diagnosed with chronic mild Mn poisoning by the Occupational Diseases Diagnosis Group (ODDG) of the local hospital in May 1982 and admitted to the hospital at the end of 1982. A later occupational safety surveillance study in that manufacturer indicated that the range of airborne MnO2 concentration in her workplace was 1.2 to 31.3 mg/m3. According to the national standard set out by the Chinese Ministry of Public Health (TJ36–79), the maximum allowable concentration (MAC) of manganese in the workplace is 0.2 mg/m3.
Once hospitalized, the patient received CaNa2 EDTA treatment over two courses for the purpose of expelling Mn. Her symptoms appeared to be improved somewhat after treatment and thus discharged from the hospital. However, the symptoms relapsed and became intensified in early 1983. The patient felt tingling in the muscles of the extremities and asthenia. There was occasional cramping but pronounced trembling all over the limbs. She frequently visited the outpatient department of the hospital and was treated with Artane tablets, L-dopa, calcium gluconate, and vitamins for more than a year. Nonetheless, her symptoms became worse. She was admitted to the hospital for the second time in November 1984. During the hospitalization, the patient received the CaNa2 EDTA treatment for two courses in combination with L-dopa, Artanetablets, energy mixture, and various vitamins. Because her clinical symptoms were alleviated to some extent, the patient was discharged in early 1985. Six months later, however, her symptoms and signs reemerged and became more aggravated day by day. She was thus diagnosed with chronic severe Mn poisoning by ODDG in June 1986. At the time of her third hospital admission in March 1987, the patient exhibited such symptoms as tic at the angle of the lips, tongue biting while speaking, and profound trembling of the upper extremities while writing or carrying objectives. The body was unsteady when walking. The patient had significant difficulty in coping with daily life. The progress of the disease is chronologically summarized in Table 1.
TABLE 1
Timeline of Disease Onset, Progression, and Chelation Therapy
| Time | Symptoms and Treatment | Work Activity |
|---|---|---|
| 1963–1982 | Worked on a job to mill Mn ore | |
| 1968 | Complained palpitation, hand tremor | Active in job |
| 1980 | Lower limb myalgia, sudden knelt down, myasthenia | Active in job |
| May 1982 | Tremor in hands, tongue, and eyelids; diagnosed as light manganism | Active in job |
| November 1982 | Hospitalized, first EDTA therapy, symptoms/signs somewhat improved, discharged | Active in job after treatment |
| 1983–1984 | Symptoms/signs relapsed, further developed and worsened | Worked on and off until next hospitalization |
| November 1984 | Hospitalized, second EDTA therapy, symptoms/signs somewhat alleviated, discharged | Not work |
| 1985–1986 | Symptoms/signs relapsed | Not work |
| March 1987 | Diagnosed as severe manganism, difficult in coping with daily life, hospitalized, PAS chelation initiated | Not work |
| June 1987 | Symptoms/signs significantly alleviated, discharged from hospital | Not work |
| February 1988 | First follow-up examination | Part-time work after follow-up examination |
| 1988–1992 | Symptoms stable | Part-time work, retired in 1992 |
| September 2004 | Second follow-up examination | Not work |
Mn indicates manganese; PAS, para-aminosalicylic acid.
Tables 2 and and33 summarize the general symptoms and characteristic signs as examined at the third admission. Physical examination showed a pulse of 80 beats/min, blood pressure of 120/80 mm Hg, and body weight of 48.5 kg. No abnormal signs were found in the heart, lungs, and abdomen; however, her lips often twitched. Handwriting was characteristically irregular and letters were progressively becoming small (Fig. 1A). Chest x-ray showed that lung markings had increased slightly. The electrocardiogram revealed occasionally premature ventricular beats. Electroencephalogram was normal. Laboratory tests demonstrated normal hepatic and renal functions. Urinary Mn concentration was 3.11 μg/24 hours; the concentrations of 17-hydroxysteroid and 17-ketosteroid in urine were 2.16 mg/24 hours and 6.7 mg/24 hours, respectively. The diagnosis of the patient with manganism was based on the following facts: 1) the patient’s occupational history of Mn exposure, 2) the detected high airborne Mn concentration in the working site, and 3) typical clinical evidence, including “mask-like” face, speech disorder, dystonia (cogwheel-like muscular tension, positive Houk’s sign), resting tremor in the hands, tongue, and eyelids, positive Romberg’s sign, failure in finger-to-finger and finger-to-nose pointing tests, micro-graphia, difficulty in walking backward, unable to take a sudden turn, abnormal gait, failure in response to L-dopa therapy, and psychiatric features such as purposeless weeping and laughing.

Handwriting of a manganism patient receiving para-aminosalicylic acid (PAS) therapy. (A) Handwriting at the time of hospital admission before PAS therapy; (B) handwriting at the end of 15-week PAS treatment; and (C) handwriting after 17 years of PAS treatment.
TABLE 2
General Symptoms of the Manganism Case Before and After Para-Aminosalicylic Acid Treatment
| Before Treatment | After Treatment | First Follow Up | Second Follow Up | |
|---|---|---|---|---|
| Headache | ++ | − | − | − |
| Giddiness | ++ | − | − | + |
| Weariness | + | − | − | − |
| Insomnia | + | − | − | + |
| Amnesia | ++ | ± | − | ± |
| Purposeless weeping and laughing | ++ | − | − | ± |
| Speech disorders | ++ | − | − | − |
| Asthenia in all four limbs | + | − | − | − |
| Arthralgia | + | − | − | − |
| Myalgia in all four limbs | + | − | − | − |
| Myoclonus in all four limbs | ++ | ± | ± | ± |
| Numbness in all four limbs | + | − | − | − |
| Heavy feeling in lower limbs | ++ | − | − | − |
| Palpitation | + | − | − | − |
| Salivation | + | − | − | − |
| Excessive perspiration | ++ | − | − | − |
TABLE 3
Characteristic Signs of the Manganism Case Before and After Para-Aminosalicylic Acid Treatment
| Before Treatment | After Treatment | First Follow Up | Second Follow Up | |
|---|---|---|---|---|
| “Mask-like” face | + | − | − | − |
| Speech disorder | + | − | − | − |
| Excessive perspiration | ++ | − | − | − |
| Tremor in hands | ++ | − | − | ± |
| Tremor in tongue | ++ | − | − | − |
| Tremor in eyelids | ++ | ± | − | − |
| Muscular tension (cogwheel-like) | + | − | − | ± |
| Houk’s sign | ++ | + | + | + |
| Romberg’s sign | + | − | − | − |
| Finger to finger test | + | − | − | − |
| Nose pointing test | ++ | − | − | − |
| Sitting down suddenly test | ++ | − | − | − |
| Hend’s observation test | +++ | − | − | − |
| Handwriting disorder | ++ | − | − | − |
| Backing difficulty | ++ | − | − | − |
| Unable to take a sudden turn | + | − | − | − |
| Gait | Abnormal | Normal | Normal | Passable |
Treatment with PAS began on March 11, 1987. The patient received 6 g sodium salt of PAS (dissolved in 500 mL of 10% glucose) per day through an intravenous drip infusion for 4 days and rested for 3 days as one therapeutic course. Fifteen such courses were carried out between March and June 1987. At the end of therapy, most of her symptoms and signs were significantly alleviated and some disappeared (Tables 2 and and3).3). Her morale and mental state were upright, her appetite improved, and her handwriting ability recovered to normal (Fig. 1B). The patient was able to copy with daily life. During the treatment, the mean of urinary Mn concentration was 6.8 μg/24 hours, more than twofold increase as compared with that before treatment. Other clinical laboratory tests showed no abnormalities. She was then considered as clinically recovered by ODDG and thus subsequently discharged from the hospital.
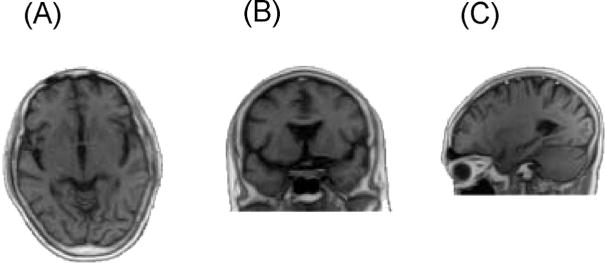
The first follow-up study was conducted 7 months post-PAS therapy. The patient essentially exhibited nearly the same health status as when she was discharged from the hospital. Her condition was normal; the symptoms and signs of manganism did not relapse. Her urinary Mn level was 2.8 μg/24 hours. The second follow-up study was conducted in September 2004, approximately 17 years after PAS therapy. The patient at the second follow-up study was 67 years old. Examinations included clinical and neurologic testing, brain magnetic resonance imaging (MRI), handwriting ability test, and the content of Mn in whole blood, Mn, Fe, Al, Zn, and Se in hair were conducted. The patient was alert, well-oriented, and coherent, yet slow in mentation, speech, and walking. No significant clinical symptoms and signs of manganism were discovered (Tables 2 and and3).3). The gait was passable. Although her handwriting was slightly trembling, the letters did not become smaller (Fig. 1C). T1-weighted MRI images did not detect Mn-related high intensity in the globus pallidus (Fig. 2). The content of Mn in whole blood was 10 μg/L, which was within the range of local surveillance reference values (30–100 μg/L). The contents of Mn, Fe, Al, Zn, and Se in hair were 0.64, 50.2, 26.6, 132.7, and 0.57 μg/g dry weight (dw), respectively, most of which were within the ranges of local surveillance reference values, ie, Mn: 0.5–4, Al: 5–55, Zn: 100–200, and Se: 0.39–0.63 μg/g dw, except for Fe, which is 15–40 μg/g dw.
Discussion
Lack of effective treatment for chronic manganism has been a major barricade in clinical management of occupational Mn intoxication. Chelating therapy with EDTA is reportedly of conflicting benefits but does seem to lower the body burden of Mn.2,3,16,17 The lack of symptomatic improvement after EDTA chelation is not entirely unexpected. Four carboxyl groups in EDTA structure, although essential to its chelating property, render the molecule poorly lipophilic and thus prevent it from effectively crossing the blood– brain barrier. In fact, radiolabeled EDTA has been used as an extracellular tracer to monitor the leakage of the blood– brain barrier.21–23 Thus, EDTA appears likely to chelate and therefore remove mainly the extracellular Mn ions; the intracellular Mn level may be reduced as a consequence of the mass balance of the metal between the cell member. A rather poor bioavailability of EDTA to brain parenchyma, where most of Mn ions accumulate, may limit its efficacy in reducing brain burden of Mn.2,24 Because EDTA unlikely possesses the ability to repair the damaged neurons, it is essentially of no practical benefit for more advanced neurologic signs and symptoms in the late stage of severe Mn poisoning. It has been recommended that for symptomatic treatment, L-dopa or 5-hydroxytryptophan may relieve some Parkinson type of syndromes such as lessened muscular tension during the course of drug application, yet the original signs recurred soon after discontinuation of the drugs.9
To the best of our knowledge, this group is the first in the literature to demonstrate an effective treatment of severe Mn intoxication in humans by using antibacterial PAS.18–20 More importantly, our 17-year follow-up study reported here clearly shows that PAS treatment not only significantly alleviates clinical symptoms and signs of manganism at the time of treatment, but appears also to have a beneficial prognostic effect. Several recent clinical studies in China adapting the similar PAS therapy strategy in treatment of occupational manganism (more than 85 cases involved) have also come to the similar conclusion.25,26,32–34 The outcomes of these case reports appear largely in Chinese literature and now are summarized in Table 4. In view of the benefit of PAS in treatment of manganism, a double-blind clinical trial with a larger patient population is well warranted.
TABLE 4
Summary of Reported Chelation Therapy with Para-Aminosalicylic Acid (PAS) and/or EDTA in Chinese Literature
| Author (year) | Chelating Agent | No. of Cases | Courses of Treatment | Effectiveness |
|---|---|---|---|---|
| Ky et al18 (1982) | PAS | 6 | 21–28 d* | + + |
| EDTA | 4 | 4 | + | |
| PAS + EDTA | 6 | 21–28 d* + 4 | + | |
| Li et al20 (1986) | PAS | 6 | 4 | + + |
| EDTA | 4 | 4 | + | |
| Wu32 (1991) | PAS | 1 | 6 | + + |
| Ky et al19 (1992) | PAS | 2 | 15 | + + |
| Zhao et al33 (1995) | PAS | 12 | 6 | + + |
| Dong et al25 (2001) | PAS | 30 | 8 | + + |
| Gao et al26 (2001)) | PAS | 20 | 90 d† | + + |
| 4 | 6–21 | + + | ||
| 4 | 2–16‡ | + + | ||
| EDTA | 24 | 1–4 | + | |
| Shi et al34 (2002) | PAS | 1 | 13 | + + |
Cases without mark: intravenous drip infusion for 4 d and rest for 3 d as one therapeutic course.
How then does PAS cure Mn intoxication? Two putative mechanisms may explain its effectiveness. First, PAS may act as a Mn-chelating agent. In biologic matrices, Mn ions can exist in more than five valent states, with a majority in Mn(II) and Mn(III).24 The high-spin Mn(III) is a trivalent metal ion with the ionic radii of 0.645 Å. As a hard Lewis acid, by virtue of its high charge density, Mn(III) can form a stable complex with hard donor atoms such as oxygen donors in PAS structure. In contrast, the Mn(II) cation has a lower charge density and thus prefers relatively softer donors such as nitrogen, which is also present in PAS structure.27 The exact coordination chemistry of Mn-PAS complex is unknown. It is possible that PAS may form a reasonably stable Mn(II) complex that can be rapidly oxidized to a more stable Mn(III) complex and subsequently remove both Mn(II) and Mn(III) from the intracellular matrix under physiological conditions. In fact, Tandon et al have shown that PAS is capable of removing Mn from the liver and testis in Mn-exposed rats.28 The same investigator has further demonstrated that PAS treatment can facilitate Mn excretion, which is higher in feces than urine, in Mn-exposed rabbits.29 Noticeably, Mn has a relatively short blood t1/2, but a prolonged tissue retention.24 Whether PAS chelates physiological Mn or excessive Mn is unknown and whether PAS has a better brain barrier permeability than EDTA is also unknown. These outstanding questions merit further investigation.
Second, the salicylate moiety in PAS structure, which possesses an antiinflammatory effect, may contribute to therapeutic effectiveness of PAS in treatment of neurodegenerative manganism. Recent studies have suggested that nonsteroidal anti-inflammatory drugs, including sodium salicylic acid, may have neuroprotective benefit, because the inflammatory processes have been shown to play a role in the pathogenesis of neurodegenerative diseases such as Alzheimer disease, Parkinson disease, and amyotrophic lateral sclerosis.30,31 These drugs may facilitate regulation of neurotransmitters, suppress nitric oxide synthesis, and protect against oxidative stress in neurons and neuroglia. Nonetheless, the hypothetical neuroprotective effects of PAS in treatment of Mn-induced neurodegenerative damage await in-depth experimental exploration.
In summary, occupational exposure to Mn causes manganism with symptoms similar to those of Parkinson disease. Our 17-year follow-up study suggests that antibacterial drug PAS appears to be effective in treatment of severe chronic Mn intoxication with a promising long-term prognosis. Although the exact mechanism of drug action is unknown, a further study of PAS and its analog in treatment of Mn intoxication is deemed necessary.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/01.jom.0000204114.01893.3e
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4180660?pdf=render
Citations & impact
Impact metrics
Article citations
Metabolic Derangement of Essential Transition Metals and Potential Antioxidant Therapies.
Int J Mol Sci, 25(14):7880, 18 Jul 2024
Cited by: 0 articles | PMID: 39063122 | PMCID: PMC11277342
Review Free full text in Europe PMC
Changes in serum TIM-3 and complement C3 expression in workers due to Mn exposure.
Front Public Health, 11:1289838, 09 Nov 2023
Cited by: 0 articles | PMID: 38026392 | PMCID: PMC10666638
Manganese-Induced Parkinsonism: Evidence from Epidemiological and Experimental Studies.
Biomolecules, 13(8):1190, 30 Jul 2023
Cited by: 8 articles | PMID: 37627255 | PMCID: PMC10452806
Review Free full text in Europe PMC
Mechanisms of manganese-induced neurotoxicity and the pursuit of neurotherapeutic strategies.
Front Pharmacol, 13:1011947, 20 Dec 2022
Cited by: 9 articles | PMID: 36605395 | PMCID: PMC9808094
Review Free full text in Europe PMC
Chelation Combination-A Strategy to Mitigate the Neurotoxicity of Manganese, Iron, and Copper?
Biomolecules, 12(11):1713, 18 Nov 2022
Cited by: 0 articles | PMID: 36421727 | PMCID: PMC9687779
Review Free full text in Europe PMC
Go to all (77) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A report of two cases of chronic serious manganese poisoning treated with sodium para-aminosalicylic acid.
Br J Ind Med, 49(1):66-69, 01 Jan 1992
Cited by: 34 articles | PMID: 1733459 | PMCID: PMC1039237
Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats.
Neurotoxicology, 30(2):240-248, 25 Dec 2008
Cited by: 57 articles | PMID: 19150464 | PMCID: PMC2677987
Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2-EDTA chelation.
Neurotoxicology, 21(5):863-866, 01 Oct 2000
Cited by: 47 articles | PMID: 11130292
Manganese-Induced Parkinsonism and Parkinson's Disease: Shared and Distinguishable Features.
Int J Environ Res Public Health, 12(7):7519-7540, 06 Jul 2015
Cited by: 151 articles | PMID: 26154659 | PMCID: PMC4515672
Review Free full text in Europe PMC