Abstract
Free full text

Mitogenic response of canine fundic epithelial cells in short-term culture to transforming growth factor alpha and insulinlike growth factor I.
Abstract
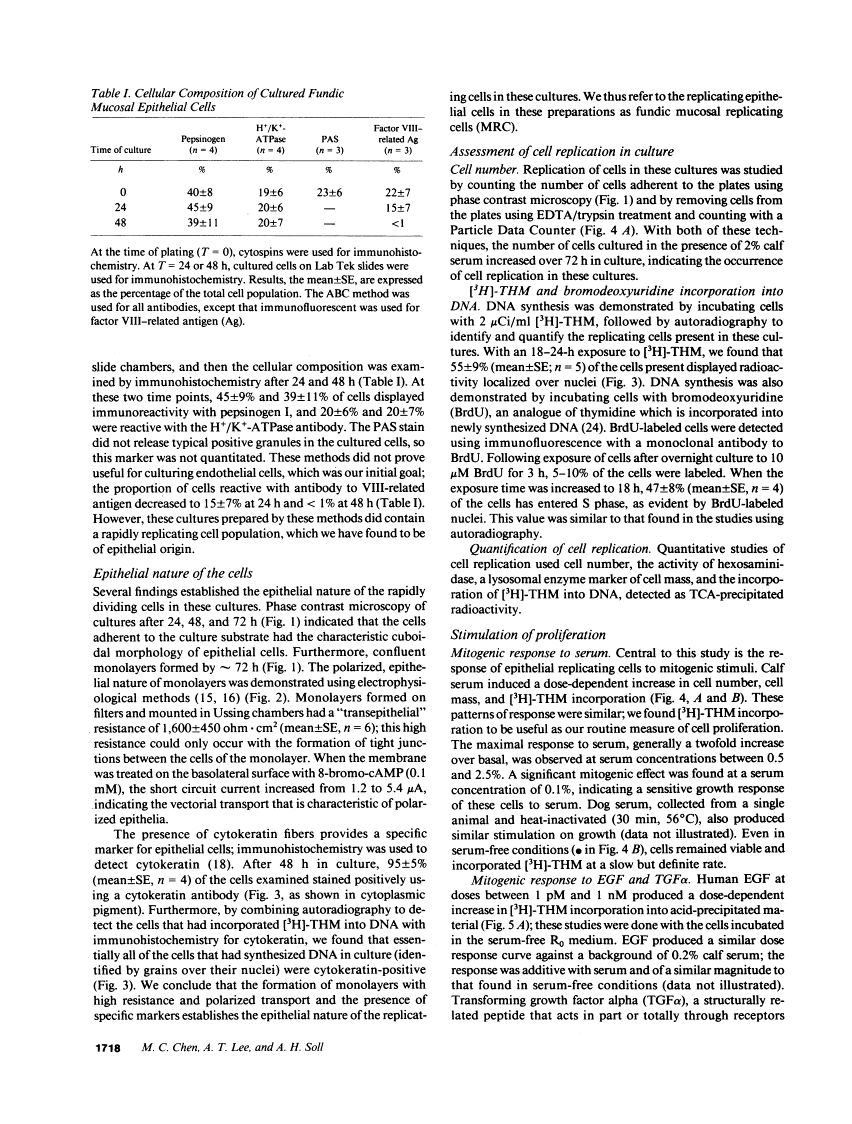
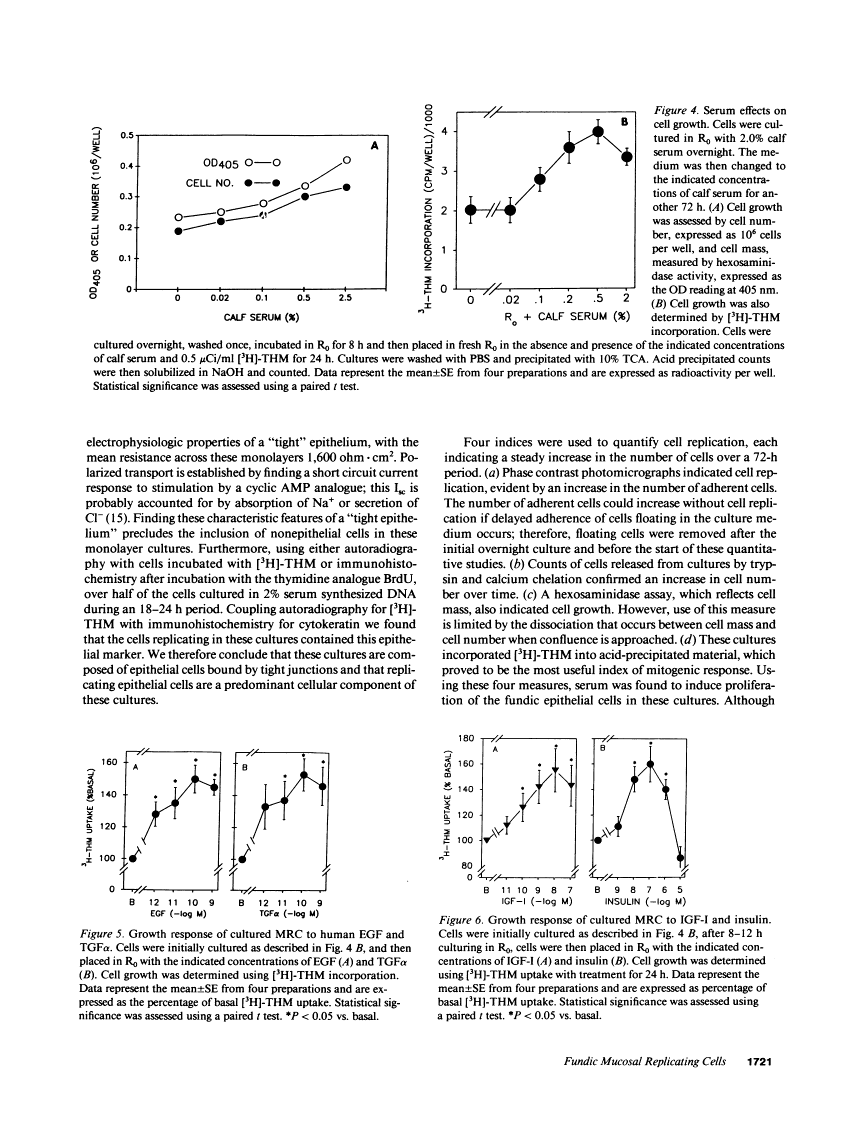
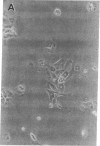
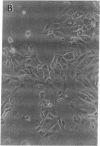
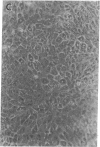
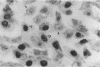
We report methods allowing the culture of rapidly dividing gastric epithelial cells to investigate the regulation of mucosal cell replication. Cells from canine fundic mucosa were dispersed by enzyme treatment, enriched by filtration and elutriation, and cultured on collagen gel in DMEM/F12 medium. After 48 h, greater than 95% of the cells displayed immunoreactivity with antibody to cytokeratin, an epithelial marker. The cells formed confluent monolayers by 72 h with a transmembrane resistance of 1,600 ohm.cm2 when mounted in a Ussing chamber indicating retention of epithelial cell characteristics. Calf serum (0.1-2%) produced a dose-dependent mitogenic effect evident by increases in [3H]-thymidine incorporation into acid-precipitated material and in cell number. After an 18-24-h incubation with [3H]-thymidine, approximately 55% of the cells cultured in 2% serum showed evidence of DNA synthesis by autoradiography and all of the replicating cells were cytokeratin positive. Using comparable culture conditions, a similar proportion of cells incubated for 18-24 h with bromodeoxyuridine displayed nuclear anti-bromodeoxyuridine immunoreactivity, thus indicating that over half of the cells in these cultures synthesized DNA during this period. As with serum, epidermal growth factor and transforming growth factor alpha (TGF alpha) (10 pM to 1 nM), insulin (10 nM to 1 microM) and insulinlike growth factor-I (IGF-I, 1-100 nM) increased [3H]-thymidine uptake. The greater potency of IGF-I, compared to insulin, suggests the presence of IGF-I receptors. We conclude that this culture preparation is composed of fundic mucosal epithelial cells and contains a predominance of dividing epithelial cells. EGF/TGF alpha and IGF-I are potential factors directly regulating proliferation of fundic mucosal cells.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hansen OH, Pedersen T, Larsen JK. Cell proliferation kinetics in normal human gastric mucosa. Studies on diurnal fluctuations and effect of food ingestion. Gastroenterology. 1976 Jun;70(6):1051–1054. [Abstract] [Google Scholar]
- Yeomans ND, Trier JS. Epithelial cell proliferation and migration in the developing rat gastric mucosa. Dev Biol. 1976 Oct 15;53(2):206–216. [Abstract] [Google Scholar]
- Chen KY, Withers HR. Proliferative capability of parietal and zymogen cells. J Anat. 1975 Dec;120(Pt 3):421–432. [Europe PMC free article] [Abstract] [Google Scholar]
- Scheving LA, Yeh YC, Tsai TH, Scheving LE. Circadian phase-dependent stimulatory effects of epidermal growth factor on deoxyribonucleic acid synthesis in the tongue, esophagus, and stomach of the adult male mouse. Endocrinology. 1979 Dec;105(6):1475–1480. [Abstract] [Google Scholar]
- Feldman M, Walsh JH, Wong HC, Richardson CT. Role of gastrin heptadecapeptide in the acid secretory response to amino acids in man. J Clin Invest. 1978 Feb;61(2):308–313. [Europe PMC free article] [Abstract] [Google Scholar]
- Johnson LR, Guthrie PD. Stimulation of rat oxyntic gland mucosal growth by epidermal growth factor. Am J Physiol. 1980 Jan;238(1):G45–G49. [Abstract] [Google Scholar]
- Moyer MP. Culture of human gastrointestinal epithelial cells. Proc Soc Exp Biol Med. 1983 Oct;174(1):12–15. [Abstract] [Google Scholar]
- Matuoka K, Tanaka M, Mitsui Y, Murota SI. Cultured rabbit gastric epithelial cells producing prostaglandin I2. Gastroenterology. 1983 Mar;84(3):498–505. [Abstract] [Google Scholar]
- Kurokowa M, Lynch K, Podolsky DK. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. [Abstract] [Google Scholar]
- Chopra DP, Siddiqui KM, Cooney RA. Effects of insulin, transferrin, cholera toxin, and epidermal growth factor on growth and morphology of human fetal normal colon epithelial cells. Gastroenterology. 1987 Apr;92(4):891–904. [Abstract] [Google Scholar]
- Romano M, Razandi M, Sekhon S, Krause WJ, Ivey KJ. Human cell line for study of damage to gastric epithelial cells in vitro. J Lab Clin Med. 1988 Apr;111(4):430–440. [Abstract] [Google Scholar]
- Chen MC, Sanders MJ, Amirian DA, Thomas LP, Kauffman G, Soll AH. Prostaglandin E2 production by dispersed canine fundic mucosal cells. Contribution of macrophages and endothelial cells as major sources. J Clin Invest. 1989 Nov;84(5):1536–1549. [Europe PMC free article] [Abstract] [Google Scholar]
- Soll AH. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. [Europe PMC free article] [Abstract] [Google Scholar]
- Ayalon A, Sanders MJ, Thomas LP, Amirian DA, Soll AH. Electrical effects of histamine on monolayers formed in culture from enriched canine gastric chief cells. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7009–7013. [Europe PMC free article] [Abstract] [Google Scholar]
- Sanders MJ, Ayalon A, Roll M, Soll AH. The apical surface of canine chief cell monolayers resists H+ back-diffusion. Nature. 1985 Jan 3;313(5997):52–54. [Abstract] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. [Abstract] [Google Scholar]
- Sun TT, Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. [Abstract] [Google Scholar]
- Smolka A, Helander HF, Sachs G. Monoclonal antibodies against gastric H+ + K+ ATPase. Am J Physiol. 1983 Oct;245(4):G589–G596. [Abstract] [Google Scholar]
- Shiraishi T, Samloff IM, Taggart RT, Stemmermann GN. Slow moving proteinase in gastric cancer and its relationship to pepsinogens I and II. An immunohistochemical study. Dig Dis Sci. 1988 Nov;33(11):1466–1472. [Abstract] [Google Scholar]
- Stemmermann GN, Samloff IM, Hayashi T. Pepsinogens I and II in carcinoma of the stomach: an immunohistochemical study. Appl Pathol. 1985;3(3):159–163. [Abstract] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. [Abstract] [Google Scholar]
- Soll AH, Amirian DA, Thomas LP, Reedy TJ, Elashoff JD. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984 May;73(5):1434–1447. [Europe PMC free article] [Abstract] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. [Abstract] [Google Scholar]
- Taub M, Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. [Abstract] [Google Scholar]
- Deuel TF. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. [Abstract] [Google Scholar]
- Coffey RJ, Jr, Goustin AS, Soderquist AM, Shipley GD, Wolfshohl J, Carpenter G, Moses HL. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [Abstract] [Google Scholar]
- Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 328(6133):817–820. [Abstract] [Google Scholar]
- Malden LT, Novak U, Burgess AW. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989 Mar 15;43(3):380–384. [Abstract] [Google Scholar]
- Koyama SY, Podolsky DK. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989 May;83(5):1768–1773. [Europe PMC free article] [Abstract] [Google Scholar]
- Beauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ., Jr Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. Implications for a regulatory role in acid secretion and mucosal renewal. J Clin Invest. 1989 Sep;84(3):1017–1023. [Europe PMC free article] [Abstract] [Google Scholar]
- D'Ercole AJ, Stiles AD, Underwood LE. Tissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci U S A. 1984 Feb;81(3):935–939. [Europe PMC free article] [Abstract] [Google Scholar]
- King GL, Kahn CR, Rechler MM, Nissley SP. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. [Europe PMC free article] [Abstract] [Google Scholar]
- Vostrejs M, Moran PL, Seligman PA. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. [Europe PMC free article] [Abstract] [Google Scholar]
- Trowbridge IS, Domingo DL. Anti-transferrin receptor monoclonal antibody and toxin-antibody conjugates affect growth of human tumour cells. Nature. 1981 Nov 12;294(5837):171–173. [Abstract] [Google Scholar]
- Ristow HJ, Messmer TO. Basic fibroblast growth factor and insulin-like growth factor I are strong mitogens for cultured mouse keratinocytes. J Cell Physiol. 1988 Nov;137(2):277–284. [Abstract] [Google Scholar]
- Bar RS, Boes M, Booth BA, Dake BL, Henley S, Hart MN. The effects of platelet-derived growth factor in cultured microvessel endothelial cells. Endocrinology. 1989 Apr;124(4):1841–1848. [Abstract] [Google Scholar]
- Lynch SE, Nixon JC, Colvin RB, Antoniades HN. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. [Europe PMC free article] [Abstract] [Google Scholar]
- Larsson H, Carlsson E, Mattsson H, Lundell L, Sundler F, Sundell G, Wallmark B, Watanabe T, Håkanson R. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986 Feb;90(2):391–399. [Abstract] [Google Scholar]
- Håkanson R, Vallgren S, Ekelund M, Rehfeld JF, Sundler F. The vagus exerts trophic control of the stomach in the rat. Gastroenterology. 1984 Jan;86(1):28–32. [Abstract] [Google Scholar]
- Lehy T, Puccio F. Influence of bombesin on gastrointestinal and pancreatic cell growth in adult and suckling animals. Ann N Y Acad Sci. 1988;547:255–267. [Abstract] [Google Scholar]
- Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 316(6031):823–826. [Abstract] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation
Full text links
Read article at publisher's site: https://doi.org/10.1172/jci115189
Read article for free, from open access legal sources, via Unpaywall:
http://www.jci.org/articles/view/115189/files/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Perturbed meibomian gland and tarsal plate morphogenesis by excess TGFα in eyelid stroma.
Dev Biol, 406(2):147-157, 10 Sep 2015
Cited by: 6 articles | PMID: 26363126 | PMCID: PMC4996271
Establishment of a gastric epithelial progenitor cell line from a transgenic mouse expressing the simian virus 40 large T antigen gene in the parietal cell lineage.
Cell Prolif, 41(2):310-320, 01 Apr 2008
Cited by: 4 articles | PMID: 18336475 | PMCID: PMC6495901
Ménétrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach.
J Clin Invest, 117(1):70-80, 01 Jan 2007
Cited by: 62 articles | PMID: 17200708 | PMCID: PMC1716220
Review Free full text in Europe PMC
Integrative roles of transforming growth factor-alpha in the cytoprotection mechanisms of gastric mucosal injury.
BMC Gastroenterol, 6:22, 01 Aug 2006
Cited by: 9 articles | PMID: 16879752 | PMCID: PMC1552080
Expression patterns of transforming growth factor-beta and its receptors in gastric mucosa of patients with refractory gastric ulcer.
World J Gastroenterol, 11(1):136-141, 01 Jan 2005
Cited by: 11 articles | PMID: 15609413 | PMCID: PMC4205373
Go to all (58) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Hepatocyte growth factor stimulates fetal gastric epithelial cell growth in vitro.
J Surg Res, 78(2):161-168, 01 Aug 1998
Cited by: 9 articles | PMID: 9733635
Effects of transforming growth factor alpha and beta on rabbit gastric epithelial cell proliferation: a preliminary report.
J Clin Gastroenterol, 17 Suppl 1:S121-4, 01 Jan 1993
Cited by: 3 articles | PMID: 8283006
Effects of transforming growth factor-beta, transforming growth factor-alpha, and other growth factors on renal proximal tubule cells.
Lab Invest, 64(4):538-545, 01 Apr 1991
Cited by: 56 articles | PMID: 2016859
Paracrine control of gastric epithelial cell growth in culture by transforming growth factor-alpha.
Am J Physiol, 264(2 pt 1):G390-6, 01 Feb 1993
Cited by: 24 articles | PMID: 8447422