Abstract
Free full text

A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis
Abstract
The p38 mitogen-activated protein kinase (MAPK) signaling pathway plays an important role in stress-induced cell-fate decisions by orchestrating responses that go from cell-cycle arrest to apoptosis. We have identified a new p38 MAPK-regulated protein that we named p18Hamlet, which becomes stabilized and accumulates in response to certain genotoxic stresses such as UV or cisplatin treatment. Overexpression of p18Hamlet is sufficient to induce apoptosis, whereas its downregulation reduces the apoptotic response to these DNA damage-inducing agents. We show that p18Hamlet interacts with p53 and stimulates the transcription of several proapoptotic p53 target genes such as PUMA and NOXA. This correlates with enhanced p18Hamlet-induced recruitment of p53 to the promoters. In proliferating cells, low steady-state levels of p18Hamlet are probably maintained by a p53-dependent negative feedback loop. Therefore, p18Hamlet is a new cell-fate regulator that links the p38 MAPK and p53 pathways and contributes to the establishment of p53-regulated stress responses.
Introduction
Cells are continuously exposed to a variety of environmental stresses and, as a consequence, sometimes have to take the important decision whether to live or not to live. Several signaling pathways are involved in the stress-induced cell-fate decisions. One of these pathways leads to activation of the p38 mitogen-activated protein kinase (MAPK) cascade that coordinates cell responses to many types of stresses including UV, chemotherapeutic agents and oncogenes.
Four p38 MAPKs have been identified in higher eukaryotes. The most widely expressed and studied family member is p38α, which can be activated by the MAPK kinases MKK6, MKK3 and MKK4. Many proteins can be phosphorylated by p38 MAPKs, including protein kinases and a growing list of transcription factors. The set of substrates targeted by p38 MAPKs in each particular case is thought to be an important determinant for the specificity of the cellular responses, which can be as diverse as cytokine production, cell differentiation, cell-cycle arrest or apoptosis (Nebreda and Porras, 2000; Ono and Han, 2000; Bulavin and Fornace, 2004).
Activation of p38α in response to several anticancer agents is necessary and, in some cases, sufficient, to induce apoptosis in a variety of cancer cell lines (Sanchez-Prieto et al, 2000; Deacon et al, 2003; Poizat et al, 2005; Coltella et al, 2006). These results, together with the ability of p38α to positively regulate several tumor suppressor pathways and to attenuate oncogenic signals, have led to the proposal that this protein may function as a tumor suppressor (Bulavin and Fornace, 2004).
There is good evidence supporting a role for p38α in the regulation of the tumor suppressor protein p53, mainly through the phosphorylation of p53 induced by several types of stress (Bulavin et al, 1999; She et al, 2000). p53 is one of the most commonly mutated genes in human cancers and its loss of function is believed to result in increased genomic instability, with the subsequent acquisition of additional oncogenic mutations (Vousden and Prives, 2005). The protein level and transcriptional activity of p53 are upregulated in response to many stresses, including DNA damage. Upon activation, p53 coordinates a complex cellular response, which can lead to reversible cell-cycle arrest, an irreversible senescence-like state or apoptosis (Vousden and Lu, 2002). The role of p53 in the maintenance of genome integrity involves multiple control mechanisms, including various post-translational modifications, such as phosphorylation, acetylation, ubiquitination and sumoylation (Bode and Dong, 2004). These modifications may increase the half-life of the p53 protein, which results in a rapid rise in intracellular p53 levels and also enhances its ability to bind to specific promoter DNA sequences.
An additional and attractive mechanism of p53 regulation has emerged in the last years as a collection of transcriptional coactivators that influence p53 activity, usually without modifying the p53 protein (Coutts and La Thangue, 2005). These coactivators confer specificity to the p53 response as they are upregulated in response to certain types of stresses and, in some cases, enhance the ability of p53 to activate the transcription of genes involved in a particular response. This is the case of the ASPP (apoptotis-stimulating proteins of p53) family of proteins, which can interact with p53 and specifically stimulate the expression of the proapoptotic genes BAX and PIG3 (Samuels-Lev et al, 2001). Another example is hnRNP K (heterogeneous nuclear ribonucleoprotein K), recently identified as a transcriptional cofactor for p53 that has a crucial role in DNA damage-induced cell-cycle arrest (Moumen et al, 2005).
We report here a new p38α-regulated protein, which we named p18Hamlet, based on its ability to control life-or-death cell-fate decisions. p18Hamlet accumulates in response to genotoxic stresses and induces the transcriptional activation of several p53 target genes such as NOXA and PUMA.
Results
Identification of a new p38 MAPK substrate
We performed yeast two-hybrid screenings to identify new proteins that could mediate the biological responses of p38α (Cheung et al, 2003). In these experiments, we found that human p38α specifically interacted with a poorly characterized protein of 18 kDa (NP_006340). We named this protein p18Hamlet, based on its function, which is related to the regulation of cell-fate decisions, as described below. Two interesting features of p18Hamlet were a C-terminal zinc-finger-HIT1-type domain, which has been described as a protein–protein interaction domain in the Trip3 coactivator of hepatocyte nuclear factor-4a (Iwahashi et al, 2002), and a bipartite nuclear localization signal (Figure 1A). p18Hamlet was conserved along the evolutionary scale from yeast to human (Figure 1B).

p18Hamlet is an evolutionarily conserved substrate of the p38α and p38β MAPKs. (A) Amino-acid sequence alignment of p18Hamlet proteins. Identical and similar residues are indicated by asterisks and two dots, respectively. The domains corresponding to the nuclear localization signal (NLS) and the zinc-finger HIT type I domain (Znf-HIT) are boxed. The arrowhead indicates a Thr residue that was selected to generate specific phospho antibodies. Sequence alignment was performed using the ClustalW program. (B) Phylogenetic tree of p18Hamlet proteins from Homo sapiens (NM_006349), Mus musculus (BC026751), Xenopus (NM_001017056), Drosophila (NP_608895), Danio rerio (AAH67648), Caenorhabditis elegans (NP_504477), Schizosaccharomyces pombe (CAB60106) and Saccharomyces cerevisiae (NP_013671). (C) GST pull-down assays were performed with the indicated GST-fused proteins and 35S-labelled p18Hamlet. (D) HEK-293 cells were transfected with 5 μg of Myc-p38α, MKK6DD and p18Hamlet, as indicated. Forty-eight hours after transfection, total cell lysates were prepared and analyzed by Western blotting, together with Myc IP. (E) HEK-293 cells were transfected with Myc-p38α, and 48 h after transfection, p38α was immunoprecipitated with Myc and HA (as a negative control) antibodies. (F) Kinase assays were performed using activated p38α and p38β MAPKs (200 ng) and GST, GST-p18Hamlet and GST-ATF-2 (1 μg) in the presence of 32P-γ-ATP. Coomassie staining shows the proteins used.
We first investigated whether p18Hamlet was able to bind to different members of the p38 MAPK family. For this analysis, we performed in vitro pull-down assays with 35S-labelled p38 MAPKs and recombinant GST-fused p18Hamlet protein. As expected from the yeast two-hybrid results, p18Hamlet was able to interact with p38α and also with p38β but not with p38γ and p38δ, or with the p38 activator MKK6 (Figure 1C and Supplementary Figure 1). The interaction between p18Hamlet and p38α was also observed in transfected HEK-293T cells, and was independent of the activation loop phosphorylation of p38α (Figure 1D). We also detected interaction of endogenous p18Hamlet with Myc-p38α transfected in HEK-293T cells (Figure 1E), but we failed to detect interaction of both endogenous proteins. Of note, we could not detect interaction between the transfected proteins using p18Hamlet or p38α antibodies for immunoprecipitation (IP) (not shown), suggesting that the antibodies might sterically interfere with or somehow affect complex formation. We also confirmed that both p38α and p38β phosphorylated p18Hamlet in vitro with similar efficiencies (Figure 1F), whereas p38γ or p38δ did not phosphorylate p18Hamlet (Supplementary Figure 1).
The sequence of p18Hamlet contains only one consensus MAPK phosphorylation site (Ser/Thr–Pro) at Ser124. However, mutation of this Ser to Ala did not affect in vitro phosphorylation of p18Hamlet by p38α (not shown). Using generic phospho antibodies, we found that p18Hamlet was phosphorylated by p38α in vitro on Thr residues (Figure 2A). Based on this result, we individually mutated the nine Thr residues present in human p18Hamlet and found four (Thr6, Thr64, Thr71 and Thr103) that could be potentially phosphorylated by p38α. However, the quadruple mutant T6A, T64A, T71A and T103A (4 × T/A) was still partially phosphorylated by p38α (Supplementary Figure 2), suggesting that additional residues might be involved.
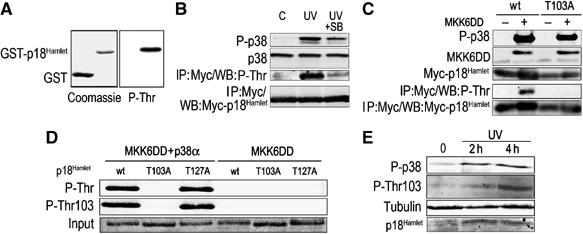
p38α phosphorylates several Thr residues in p18Hamlet. (A) GST-p18Hamlet or GST proteins (500 ng) were phosphorylated with p38α in vitro and then analyzed by Western blotting with phospho-Thr antibodies. (B) HEK-293 cells were transfected with Myc-p18Hamlet and 48 h later were treated with UV alone or in the presence of 10 μM SB203580. Three hours after irradiation, total cell lysates were prepared and analyzed by Western blotting, together with Myc immunoprecipitates. (C) HEK-293 cells were transfected with Myc-p18Hamlet wt and T103A either alone or together with MKK6DD, as indicated, and p18Hamlet phosphorylation was analyzed by Myc IP followed by Western blotting with phospho-Thr antibody. Total cell lysates were also analyzed by Western. (D) GST-p18Hamlet wt, T103A and T127A proteins were incubated with p38α and MKK6DD or with MKK6DD alone and then analyzed by Western blotting with both phospho-Thr103-p18Hamlet and generic phospho-Thr antibodies. (E) HeLa cells overexpressing p18Hamlet were UV irradiated, lysed at the indicated times after irradiation and analyzed by Western blotting with the phospho-Thr103-p18Hamlet antibody.
We found that p38 MAPK activation by UV treatment also correlated with Thr phosphorylation of p18Hamlet, and this phosphorylation was significantly reduced when the cells were pretreated with the p38 MAPK inhibitor SB203580 (Figure 2B). This suggests that p38 is involved in UV-induced p18Hamlet phosphorylation. The high conservation of Thr103 as a phosphorylation site in p18Hamlet proteins from different species (Figure 1A), together with the in vitro phosphorylation experiments, suggested that this residue could be an important target for p38 MAPK. Indeed, mutation of Thr103 impaired the p38 MAPK-mediated phosphorylation of p18Hamlet in cells, as determined by the reduced signal observed with the phospho-Thr antibody, when both wild-type (wt) and the T103A p18Hamlet proteins were coexpressed with the p38 activator MKK6DD (Figure 2C). We developed an antibody that specifically recognized phospho-Thr103 (Figure 2D) and confirmed that p18Hamlet was phosphorylated on this residue in UV-treated cells (Figure 2E). It is important to note that both the generic phospho-Thr and the specific phospho-Thr103 antibodies recognize p18Hamlet phosphorylated on Thr103. However, in vitro kinase assays indicate that this was not the only p38α-dependent phosphorylation residue present in p18Hamlet (Supplementary Figure 2).
p18Hamlet protein levels are regulated by p38 MAPK
The mRNA levels of p18Hamlet varied significantly among different human tissues and cell lines in contrast with the p18Hamlet protein, which was usually difficult to detect (Supplementary Figure 3 and data not shown). We therefore investigated the possibility that p18Hamlet protein stability could be regulated. As shown in Figure 3A, incubation with the proteasome inhibitor MG132 resulted in the accumulation of endogenous p18Hamlet protein in both mouse embryonic fibroblasts (MEFs) and human osteosarcoma U2OS cells. To demonstrate that p18Hamlet was a target of the ubiquitin–proteasome system, we transfected U2OS cells with Myc-p18Hamlet alone or in combination with HA-ubiquitin and then analyzed the Myc-p18Hamlet immunoprecipitates by immunoblotting with anti-p18Hamlet antibody. In this experiment, we detected a smear of slowly migrating p18Hamlet forms that were not observed in extracts from cells transfected with either HA-ubiquitin or p18Hamlet alone, suggesting that they probably correspond to p18Hamlet–ubiquitin conjugates. This was further supported by the recognition of the slowly migrating forms of p18Hamlet with an anti-HA antibody (Figure 3B). MG132 concentrations that inhibit the proteasome have been also reported to activate p38 MAPK (Wu et al, 2004). We confirmed that MG132-induced p18Hamlet accumulation correlated with the phosphorylation of p38 MAPK in MEFs, but this effect was abolished when the MG132 treatment was performed in the presence of the p38 MAPK inhibitor SB203580 (Supplementary Figure 4). These results strongly suggest that p18Hamlet accumulation requires the activation of p38 MAPK.
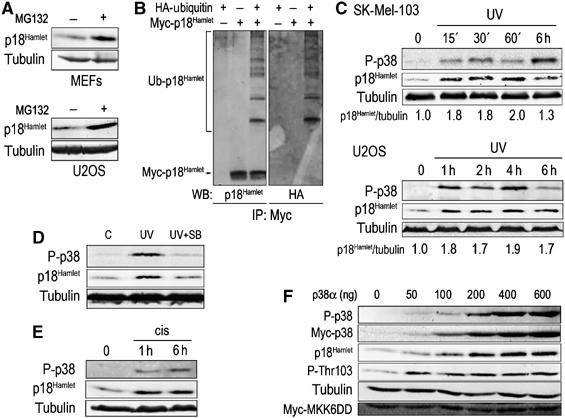
Accumulation of p18Hamlet protein in response to DNA damage-inducing agents. (A) MEFs and U2OS cells were treated with the proteasome inhibitor MG132 (25 μM) for 2 h and then lysed. Expression of endogenous p18Hamlet was analyzed by Western blotting. (B) U2OS cells were transfected with HA-ubiquitin and Myc-p18Hamlet, as indicated, and 16 h after transfection, were treated with MG132 (25 μM) for 5 h. Myc immunoprecipitates were analyzed by Western blotting using p18Hamlet and HA antibodies. (C) SK-Mel-103 and U2OS cells were treated with UV and cell lysates were analyzed by Western blotting using the indicated antibodies. (D) SK-Mel-103 cell lysates were prepared 3 h after UV treatment, either in the presence or absence of SB203580 (SB, 10 μM), and analyzed by Western blotting. (E) SK-Mel-103 cells were treated with cisplatin for the indicated times and p18Hamlet accumulation was analyzed by Western blotting. (F) U2OS cells were cotransfected with MKK6DD (600 ng), p18Hamlet (1 μg) and increasing amounts of p38α, as indicated. Twenty-four hours after transfection, lysates were prepared from both attached and floating cells (that express higher levels of p18Hamlet) and analyzed by Western blotting.
Next, we investigated the effect of genotoxic stresses that activate the p38 MAPK pathway, such as UV (Kyriakis and Avruch, 1996), on the endogenous p18Hamlet protein levels. In agreement with the above results, UV-induced p38 MAPK phosphorylation correlated with a small (about two-fold) but reproducible increase in endogenous p18Hamlet protein levels in different cell lines (Figure 3C). The amount of p18Hamlet protein typically peaked between 1 and 6 h after the treatment and later decreased to levels lower than those in untreated cells. Importantly, the UV-induced accumulation of p18Hamlet was prevented by pretreatment with SB203580 (Figure 3D).
We then investigated whether p18Hamlet accumulation was specific for UV-induced p38 MAPK activation or if it was a more general response to stress. Treatment of cells with the chemical DNA damage-inducing agent cisplatin induced p38 MAPK activation, which was accompanied by a significant increase in endogenous p18Hamlet levels (Figure 3E).
To further strengthen the connection between p38 MAPK activation and accumulation of p18Hamlet, we transfected U2OS cells with a low amount of p18Hamlet (to limit apoptosis induction, see below) together with increasing amounts of p38α and a constant amount of its activator MKK6DD (Figure 3F). In this experiment, we observed a direct correlation between p38α activation levels and the amount of p18Hamlet protein expressed. We also detected maximal p18Hamlet phosphorylation on Thr103 with the lowest concentration of p38α used, suggesting that this residue might be efficiently phosphorylated in situations of poor p38 MAPK activation. However, the T103A mutant protein was still able to accumulate upon p38α activation (Supplementary Figure 5), suggesting that Thr103 phosphorylation may contribute to, but is not essential for, p18Hamlet accumulation.
Finally, we confirmed that p18Hamlet was an unstable protein with a half-life of less than 3 h in cycloheximide-treated U2OS cells (Figure 4A). Interestingly, specific activation of p38α was sufficient to significantly increase the half-life of p18Hamlet (Figure 4B and C). In contrast, we could detect no changes in p18Hamlet mRNA levels when cells were treated with cisplatin or UV (Figure 4D). These results indicated that stress-induced accumulation of p18Hamlet was mainly regulated at the level of protein stability. To determine the importance of p38 MAPK-mediated phosphorylation in p18Hamlet protein stability, we performed cycloheximide-chase experiments in U2OS cells transfected with p18Hamlet wt, T103A or the quadruple mutant 4 × T/A and then UV irradiated the cells (Figure 4E). Whereas the T103A and wt proteins behaved similarly and were both significantly accumulated in response to UV, 4 × T/A mutant expression levels were not affected by this treatment. Moreover, the 4 × T/A mutant was expressed at levels lower than wt p18Hamlet, supporting the idea that phosphorylation of these sites could be important for the regulation of p18Hamlet protein stability.
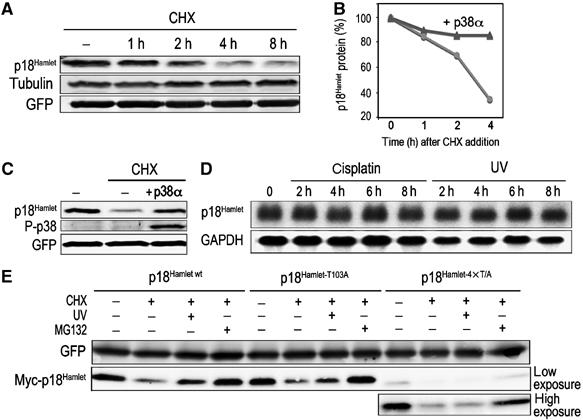
Stabilization of p18Hamlet protein in response to p38α activation. (A) U2OS cells were cotransfected with p18Hamlet (1 μg) and GFP (500 ng) and incubated with cycloheximide (CHX, 30 μg/ml) for the indicated times. Total cell lysates were analyzed by Western blotting. (B, C) U2OS cells were transfected with p18Hamlet, either alone or together with p38α and MKK6DD, and 24 h later were incubated with CHX for up to 4 h. Expression of p18Hamlet protein was determined by Western blotting. The blots corresponding to cells untreated or treated with CHX for 4 h, in the presence or absence of active p38α, are shown in (C). (D) Total RNAs were obtained from UV- or cisplatin-treated SK-Mel-103 cells and were analyzed by Northern blotting with a p18Hamlet probe. GAPDH was used to confirm equal RNA loading. (E) U2OS cells were transfected with 6 μg of p18Hamlet wt, T103A or 4 × T/A, as indicated. Twenty-four hours after transfection, cells were treated with CHX alone or in combination with MG132 for 4 h. Where indicated, cells were also UV irradiated 1 h before collection. A GFP expression vector (200 ng) was cotransfected to ensure equal efficiency of transfection.
Accumulation of p18Hamlet induces apoptosis
Once we established that p18Hamlet levels increased in response to DNA damage, we investigated the biological significance of this accumulation. U2OS cells were transfected with either GFP-p18Hamlet or GFP alone and then sorted to analyze the cell-cycle profile in the fluorescent cell population. We found that around 6% of the GFP-expressing cells were apoptotic (sub-G0/G1 population), whereas this amount increased to 18% in the GFP-p18Hamlet-positive cells, indicating that p18Hamlet overexpression was sufficient to induce apoptosis. Interestingly, GFP-p18Hamlet overexpression did not affect apoptosis levels in SAOS cells, a p53-deficient human osteosarcoma cell line (Figure 5A).
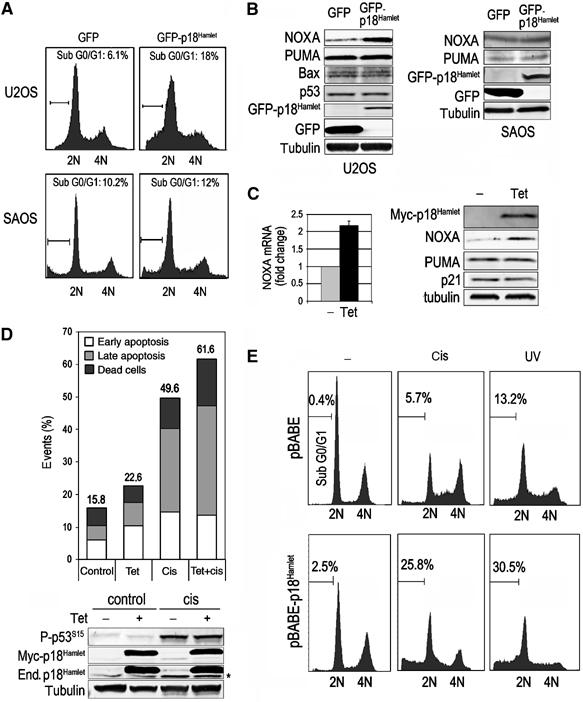
Induction of apoptosis by p18Hamlet overexpression. (A) U2OS and SAOS cells were transfected with GFP or GFP-p18Hamlet and analyzed by flow cytometry. The percentage of cells with a sub-G0/G1 DNA content in a representative experiment is shown. (B) U2OS and SAOS cells were transfected as indicated in (A) and the expression of the indicated proteins was analyzed by Western blotting. (C) U2OS cells expressing inducible p18Hamlet were treated with tetracycline for 24 h before RNA and protein extraction. Samples were analyzed by quantitative RT–PCRs (left) and Western blotting (right). (D) U2OS cells expressing tetracycline-inducible p18Hamlet were incubated with tet for 24 h and then treated with cisplatin for another 24 h before analyzing apoptosis by annexin V staining. Numbers on top of the bars indicate total percentage of early and late apoptotic events as well as dead cells. The lower panel shows the protein levels of overexpressed Myc-p18Hamlet, endogenous (End.) p18Hamlet (marked with an asterisk) and phospho-Ser15-p53. (E) Primary MEFs were infected with pBABE puro or p18Hamlet-expressing pBABE puro retroviruses. After puromycin selection, cells were treated for 24 h with cisplatin or UV as indicated, and the percentage of sub-G0/G1 cell population, as a measure of apoptosis levels, was analyzed by FACS.
Given the key role of p53 in the apoptotic response induced by DNA damage, we investigated if p18Hamlet could regulate p53. In U2OS cells, GFP-p18Hamlet overexpression did not affect the total levels of p53 (Figure 5B), but resulted in higher levels of the proapoptotic p53 target gene NOXA, whereas it had no effect on other p53-dependent proapoptotic genes such as PUMA or Bax. In contrast, NOXA levels were not affected by GFP-p18Hamlet overexpression in SAOS cells. To study the induction of apoptosis by p18Hamlet in more detail, we generated a tetracycline-inducible system in which p18Hamlet protein expression peaked at about 16 h after addition of tetracycline to U2OS cells (Supplementary Figure 6). Consistent with the above results, tetracycline-induced p18Hamlet expression was accompanied by an increase in both NOXA mRNA and protein levels (Figure 5C), whereas we could detect no significant changes in the expression of other p53-dependent targets, such as PUMA, Bax, p21 or Hdm2 (Figure 5C and data not shown). We also confirmed that p18Hamlet induction was sufficient on its own to significantly increase the early and late apoptotic populations. In addition, p18Hamlet cooperates with cisplatin treatment in apoptosis induction (Figure 5D). To confirm the proapoptotic function of p18Hamlet in a more physiological system, we overexpressed p18Hamlet in primary MEFs. As shown in Figure 5E, p18Hamlet overexpression was sufficient to increase apoptosis levels in non-stressed cells, and it also strongly promoted apoptosis induced by cisplatin or UV. Taken together, these results support a role for p18Hamlet in p53-mediated apoptosis induction.
To confirm the role of endogenous p18Hamlet as an apoptosis mediator, we designed siRNA oligonucleotides that efficiently downregulated p18Hamlet (Figure 6A and Supplementary Figures 7 and 8). Previous work has shown that p53 contributes to apoptosis induced by UV or cisplatin in both U2OS and MCF7 cells (Bergamaschi et al, 2003). Using a highly sensitive, quantitative method that detects apoptotic nucleosomes (see Materials and methods), we found that p18Hamlet downregulation did not affect basal apoptosis levels, but significantly impaired apoptosis induced by either UV or cisplatin in U2OS cells (Figure 6A). The same effect was observed in MCF7 cells treated with cisplatin (Supplementary Figure 7). We then analyzed the effect of p18Hamlet downregulation on the expression of p53-dependent target genes upon cisplatin and UV treatment. Interestingly, only certain p53 target genes were induced in response to these two types of stress. In particular, NOXA and Hdm2 responded to cisplatin, whereas p21, Bax and PUMA remained unaffected. In the case of UV, only NOXA, and also slightly PUMA, protein levels were upregulated, whereas p21, Hdm2 and Bax levels did not increase (Figure 6B). This result suggests that specific programs of gene expression account for the p53-dependent apoptosis in response to each particular type of stress. Downregulation of p18Hamlet prevented NOXA and, in the case of UV irradiation, also PUMA protein accumulation, but had no effect on Hdm2 protein induction. Taken together, the results support an important role for p18Hamlet in stress-induced apoptosis.

p18Hamlet is required for apoptosis induction in response to DNA damage. (A) U2OS cells were transfected with p18Hamlet or control siRNAs and the levels of endogenous p18Hamlet were analyzed by Western blotting (upper). Forty-eight hours after transfection, cells were treated with UV or cisplatin for 24 h and apoptosis was quantified by measuring DNA fragmentation in a colorimetric assay. Means±standard deviations of three independent experiments are represented. Statistical significance was evaluated with the Student's t-test (P-values are shown). (B) U2OS cells were treated with siRNAs and UV or cisplatin as described in (A). Expression of the indicated proteins was detected by Western blotting.
p18Hamlet activates p53-dependent gene promoters
The ability of p18Hamlet to upregulate p53-dependent proapoptotic genes prompted us to investigate whether both proteins could interact. We found that tetracycline-induced p18Hamlet co-immunoprecipitated with endogenous p53 from U2OS cells (Figure 7A, upper panel), and we also managed to co-immunoprecipitate the two endogenous proteins (Figure 7A, lower panel). The interaction was also observed in pull-down assays using recombinant GST-p53 and 35S-labelled p18Hamlet and it involves the zinc-finger domain of p18Hamlet and the C-terminal region of p53 (see below, Figure 9D and Supplementary Figure 9). In contrast, overexpression of p18Hamlet did not affect phosphorylation of p53 on Ser15 and Ser46, two common p53 phosphorylation events in response to stress (Figure 7B).
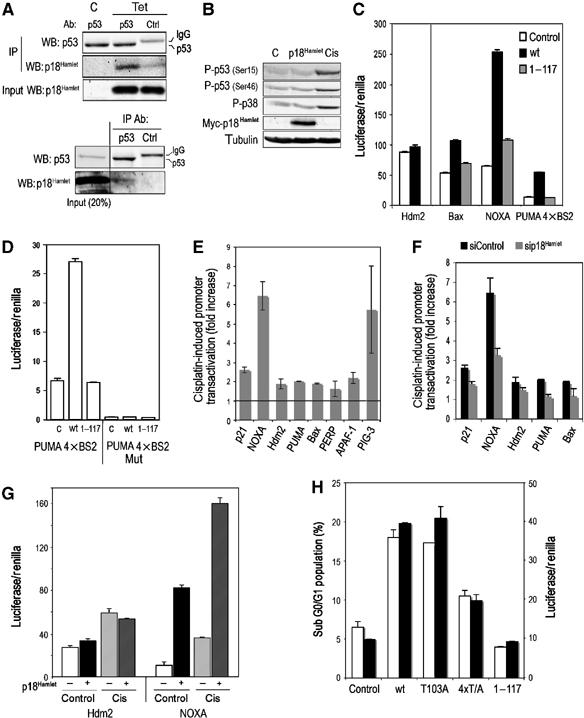
p18Hamlet stimulates some p53-regulated genes. (A) Upper: U2OS cells with inducible p18Hamlet were treated with tetracycline (tet) for 24 h and total cell lysates were immunoprecipitated with p53 or control antibodies and then blotted with p18Hamlet antibodies. Lower: total lysates of MG132-treated U2OS cells were immunoprecipitated and blotted as above to detect interaction between to endogenous p18Hamlet and p53. (B) U2OS cells expressing tet-inducible p18Hamlet were treated with tet or cisplatin and then analyzed by Western blotting. (C) U2OS cells were transfected with empty vector (control) and either p18Hamlet wt or p18Hamlet(1–117), together with reporter constructs containing different p53-responsive promoters upstream of the luciferase gene, as indicated. Luciferase activity was analyzed 16 h later and transfection efficiency was normalized to Renilla activity. Means±s.d. of three independent experiments are represented. (D) Transfections and luciferase assays were performed exactly as in (C) to test the wt and mutant PUMA 4 × BS2 reporters. (E) U2OS cells were transfected with the indicated reporter constructs. Twenty-four hours later, cells were treated with cisplatin and after 16 h, luciferase activity was measured and normalized to Renilla. (F) U2OS cells were transfected with p18Hamlet or control siRNAs and 24 h later cotransfected with the indicated luciferase reporters. Twenty-four hours after transfection, cells were incubated with cisplatin for 16 h and luciferase activity was measured. (G) U2OS cells were transfected with p18Hamlet or empty vector (control) in combination with Hdm2 and NOXA promoter reporters. Twenty four hours after transfection, cells were mock treated or treated with cisplatin and luciferase activity was measured 10 h later. Means±s.d. of three independent experiments are represented. (H) U2OS cells were transfected with GFP alone (control) or the indicated GFP-tagged p18Hamlet proteins and 48 h later the sub G0/G1 percentage in the fluorescent population was determined by FACS (white bars). U2OS cells were transfected with empty vector (control) or Myc-tagged wt and mutant p18Hamlet proteins, together with the PUMA 4 × BS2 reporter, and luciferase activity was measured 16 h later (black bars). Expression levels of Myc- and GFP-tagged p18Hamlet proteins are shown in Supplementary Figure 12.

The p53 target gene cyclin G1 controls p18Hamlet protein levels under normal growing conditions. (A) MEFs (wt and p53−/−) were immunostained with p18Hamlet antibodies. Nuclear localization was confirmed by DAPI staining. (B) Expression of the indicated proteins was analyzed by Western blotting in wt and p53−/− MEFs. (C) U2OS cells with inducible p18Hamlet were treated with tetracycline (tet) and 24 h later were transfected with p53 and analyzed by Western blotting. (D) GST pull-down assay was performed by incubation of 35S-labelled p18Hamlet wt and 1–117 with GST and GST-fused p38α, cyclin G1 or p53, as indicated. (E) HEK-293 cells were cotransfected with Myc-p18Hamlet (5 μg) and increasing amounts of HA-cyclin G1 (0–10 μg). Twenty-four hours after transfection, the expression levels of the indicated proteins were analyzed by Western blotting using HA and Myc antibodies. Transfection efficiency was evaluated by cotransfection with GFP (500 ng). (F) HEK-293 cells were cotransfected with p18Hamlet (1 μg) and YFP-cyclin G1 (9 μg) and 16 h after transfection, cells were treated for 5 h with MG132. The expression levels of the indicated proteins were analyzed by Western blotting. (G) U2OS cells expressing tet-inducible p18Hamlet were transfected with HA-cyclin G1. Cellular localization was analyzed by immunostaining with HA and p18Hamlet antibodies. Colocalization areas are indicated in yellow (merge).
We then analyzed whether p18Hamlet could regulate the transactivation function of p53. For these experiments, we expressed the p53-regulated promoters of Hdm2, Bax, NOXA and PUMA in U2OS cells, which contain wt p53 protein. As shown in Figure 7B, p18Hamlet stimulated the transcription of the three p53-regulated proapoptotic genes (only a minor effect was observed in the case of Bax), but had no effect on the Hdm2 promoter. We confirmed that both Bax and Hdm2 promoters were indeed able to respond to p53 (Supplementary Figure 10). Importantly, stimulation of p53 transcriptional activity by p18Hamlet was dependent upon the integrity of its C-terminal zinc-finger HIT domain, as a p18Hamlet derivative lacking the last 37 amino acids (p18Hamlet(1–117)) had no significant effect on any of the p53-regulated promoters (Figure 7C). The C-terminally truncated p18Hamlet(1–117) protein was still able to bind to p38α and localized to the nucleus, as the full-length p18Hamlet, but failed to interact with p53 (Figure 9D and Supplementary Figure 9). It therefore appears that p38α and p53 interact with different parts of the p18Hamlet protein and that the C-terminal domain of p18Hamlet is required for p53 transactivation.
To confirm that the effect of p18Hamlet was indeed p53 dependent, we used a reporter plasmid with the PUMA minimal promoter (PUMA 4 × BS2) consisting of four tandem repeats of the p53 binding site or a mutant version of this reporter, which does not bind to p53 (Yu et al, 2001). Full-length p18Hamlet increased PUMA 4 × BS2 transcription by about four-fold, whereas the mutant p18Hamlet(1–117) had no effect. In contrast, neither full-length nor truncated p18Hamlet proteins were able to stimulate transcription of the mutated PUMA 4 × BS2 promoter (Figure 7D). Based on these results, we conclude that p18Hamlet can activate, through its C-terminal domain, the transcription of several p53-dependent genes.
Consistent with the results shown in Figure 6B, cisplatin was able to differentially transactivate several p53-dependent promoters, being more effective in the cases of NOXA and PIG-3 (Figure 7E) when compared with other genes such as Bax or PUMA. The downregulation of p18Hamlet inhibited by about 50% the ability of cisplatin to transactivate the NOXA and PUMA promoters, whereas it had a more moderate effect on p21 and Bax promoters (Figure 7F). In addition, when p18Hamlet overexpression was combined with cisplatin treatment, we observed an additive effect on the NOXA promoter, without affecting the Hdm2 promoter, leading to an imbalance that was clearly favorable to the transcription of the proapoptotic gene NOXA (Figure 7G). Of note, overexpression of p18Hamlet in p53-deficient SAOS cells had no effect on the PUMA 4 × BS2-reporter construct, unless the cells were cotransfected with p53 (Supplementary Figure 11).
To determine the importance of p38 MAPK-mediated phosphorylation in p18Hamlet function, we analyzed the ability of different p18Hamlet mutants to transactivate the PUMA 4 × BS2 promoter and to induce apoptosis. The three p18Hamlet mutants were able to bind to p38α to a similar extent, but phosphorylation was significantly reduced in p18Hamlet-T103A and specially in the quadruple mutant p18Hamlet-4 × T/A (Supplementary Figure 2). However, only the p18Hamlet-T103A mutant recapitulated the activity of the wt protein regarding PUMA promoter transactivation and apoptosis induction, consistent with the idea that phosphorylation of this residue was not essential for p18Hamlet function and accumulation (Figure 7H). In contrast, truncated p18Hamlet(1–117) was not able to induce cell death, supporting the importance of p53 interaction for the induction of apoptosis by p18Hamlet. Finally, p18Hamlet-4 × T/A showed a significant decrease in both PUMA transactivation and apoptosis induction, suggesting that p18Hamlet phosphorylation is essential for its activity (Figure 7H).
Our results indicated that the ability of p18Hamlet to stimulate p53-induced transcription was not related to p53 phosphorylation or stabilization. We therefore performed chromatin IP (ChIP) experiments to investigate the recruitment of p53 to the promoter of its target genes. We found that p18Hamlet overexpression was sufficient to enhance p53 binding to both the PUMA and NOXA promoters, whereas it had no effect on the ability of p53 to bind to the Hdm2 promoter (Figure 8A). In addition, p18Hamlet itself was also bound to the NOXA promoter, even in unstimulated U2OS cells, and the binding was increased in response to cisplatin treatment (Figure 8B). Interestingly, p18Hamlet knockdown had a profound effect on p53 loading onto the NOXA promoter (Figure 8C), supporting a key role for p18Hamlet in the recruitment of p53 to certain target promoters.

Recruitment of p53 and p18Hamlet to p53-regulated promoters. (A) U2OS cells expressing tetracycline (tet)-inducible p18Hamlet were treated with tet for 24 h or UV irradiated for 8 h and then subjected to ChIP analysis. The DNA associated with the p53 immunoprecipitates was subjected to PCR with primers specific for the Hdm2, PUMA and NOXA promoters. (B) U2OS cells were treated with cisplatin for 6 h and then subjected to ChIP analysis using both p53 and p18Hamlet antibodies and NOXA primers. (C) p53 recruitment to NOXA promoter was analyzed by ChIP assay in U2OS cells 72 h after incubation with control and p18Hamlet siRNAs.
p18Hamlet levels can be regulated by cyclin G1 in normally proliferating cells
The ability of p18Hamlet to induce apoptosis suggests that the levels of this protein should be strictly regulated under normal growing conditions in order to avoid improper biological responses. We analyzed the subcellular localization of endogenous p18Hamlet protein by immunofluorescence and found a nuclear pattern with a clear and well-defined perinucleolar distribution (Figure 9A, upper panels). The same pattern was observed for transfected Myc-p18Hamlet using either Myc or p18Hamlet antibodies (Supplementary Figure 13). Interestingly, p18Hamlet was expressed at higher levels in p53-deficient MEFs than in their wt counterparts, whereas cyclin G1 was downregulated (Figure 9B) and p18Hamlet expression was more disorganized in the absence of p53, being uniformly distributed all over the nuclear compartment (Figure 9A, lower panels). This suggested that p53 downstream effectors could be normally required to maintain p18Hamlet protein at low levels and in the right subcellular localization. In fact, p53 overexpression resulted in a decreased accumulation of p18Hamlet protein in U2OS cells (Figure 9C).
A potential candidate regulator of p18Hamlet was the p53 target gene cyclin G1, which has been reported to interact in vitro with p18Hamlet (Xu et al, 2000). It has been reported that p53−/− MEFs express lower levels of cyclin G1 protein than wt MEFs (Reimer et al, 1999). In contrast, as mentioned above, p18Hamlet was expressed at higher levels in p53-deficient than in wt MEFs (Figure 9B), suggesting that both proteins could be subjected to opposite regulation. We confirmed that cyclin G1 and p18Hamlet proteins interacted in vitro (Figure 9D) and colocalized in vivo (Figure 9G). Interestingly, cotransfection of increasing amounts of cyclin G1 with a constant amount of p18Hamlet efficiently decreased the expression of p18Hamlet (Figure 9E), and this effect could be mediated by the ubiquitin–proteasome system (Figure 9F). In contrast, the subcellular localization of p18Hamlet was not affected by cyclin G1 overexpression (data not shown). Thus, cyclin G1 induces degradation of p18Hamlet and can potentially control its expression levels in normally growing cells.
Discussion
Mammalian cells have evolved a complex network of DNA damage responses to ensure the integrity of their genomes. These mechanisms enable injured cells either to arrest the cell cycle and establish a DNA repair program or to undergo cell death by apoptosis, depending on the severity of the damage. We have identified p18Hamlet as a new protein regulated by the stress-activated p38 MAPK pathway and have established its implication in p53-induced apoptosis. Specifically, p18Hamlet protein accumulates in response to genotoxic agents and behaves as a p53 transcriptional coactivator that promotes the expression of genes such as NOXA and PUMA, helping cells to undergo apoptosis.
p18Hamlet links the p38 MAPK and p53 pathways
Our results indicate that p38 MAPK plays an important role in the regulation of p18Hamlet half-life. In particular, p38 MAPK activation is required for the accumulation of p18Hamlet induced by DNA damage-inducing agents such as UV. We also showed that several sites that are phosphorylated by p38α in vitro are also important for p18Hamlet protein stability in cells. However, the exact contribution of specific phosphorylation sites to p18Hamlet protein stability remains to be elucidated. It is also possible that p38 MAPK might regulate the stabilization of the p18Hamlet protein by other mechanisms, in addition to direct phosphorylation.
Previous studies have documented that activation of the p38 MAPK pathway may lead to p53-induced apoptosis (Bulavin and Fornace, 2004). The connection between p38 MAPK activation and increased transcription from p53-regulated promoters has been classically attributed to the ability of p38 MAPK to directly phosphorylate p53 on Ser33 and Ser46 (Bulavin et al, 1999; Sanchez-Prieto et al, 2000). Our findings provide a new mechanism by which p38 MAPK can contribute to p53-induced apoptosis, namely by contributing to the stabilization of p18Hamlet, a p53 coactivator that stimulates p53-dependent transcription.
We have shown that the stimulatory effect of p18Hamlet on p53-regulated genes is mediated by the p53 binding sites in the promoters. Nevertheless, we cannot rule out that p18Hamlet could also have p53-independent functions. The biological significance of p18Hamlet homologues in yeast, which lack p53-related proteins, is as yet unclear but might support this possibility.
p18Hamlet as a determinant for p53 response specificity
There are several mechanisms by which p18Hamlet could provide specificity to p53-regulated stress responses. First, p18Hamlet does not seem to be a ubiquitously expressed protein, in contrast with p38 MAPK and p53, and might therefore provide tissue-specific responses. Second, p18Hamlet accumulates only in response to certain types of stresses such as UV and cisplatin but not in response to γ irradiation (not shown). Finally, p18Hamlet can specifically stimulate the transcription of certain p53-dependent promoters. In particular, in response to cisplatin, p18Hamlet contributes to the p53-dependent transcriptional activation of NOXA but not Hdm2, suggesting that the effects of p18Hamlet on p53-mediated transcription are promoter specific. This property of p18Hamlet is shared by other p53 coactivators. For example, ASPP proteins can stimulate transcription of the proapoptotic genes Bax and PIG3, but not Mdm2 or p21Cip1 (Samuels-Lev et al, 2001), whereas hDaxx specifically represses p53-mediated induction of genes involved in cell-cycle arrest such as p21Cip1 (Gostissa et al, 2004). Other p53 coactivators, such as the p300/CBP cofactor JMY, can efficiently upregulate Bax but not p21Cip1 (Shikama et al, 1999).
It has been clearly established that p21Cip1 has a key role in p53-induced cell-cycle arrest, but the molecular pathways involved in p53-mediated apoptosis are not fully understood. Several proapoptotic molecules can be transcriptionally induced by p53, but the contribution of each factor to the apoptotic response depends on both the cell type and nature of the stress. We have found that p18Hamlet can stimulate the recruitment of p53 to the p21Cip1 promoter (not shown), but we could not observe p21Cip1 protein induction in response to UV or cisplatin, most likely due to post-translational downregulation (Fotedar et al, 2004). Thus, the contribution of p18Hamlet to the regulation of the p21Cip1 promoter needs to be further investigated.
The function of p18Hamlet as a transcriptional coactivator is further supported by recent work, which has identified this protein as a potential subunit of the SRCAP (SNF2-related CBP-activating protein) complex (Cai et al, 2005). Interestingly, SRCAP may contribute to the recruitment of histone acetyltransferase CBP to certain promoters (Eissenberg et al, 2005). The p300/CBP proteins are well-established regulators of the p53 response that control p53 acetylation and its DNA binding activity (Barlev et al, 2001; Espinosa and Emerson, 2001). It is therefore tempting to speculate that p53 modulation by p18Hamlet could involve the regulation of p300/CBP.
Control of p18Hamlet expression in proliferating cells
The accumulation of p18Hamlet can potently trigger apoptosis, suggesting that its expression should be tightly controlled. We have identified a p53-dependent negative feedback loop that normally maintains p18Hamlet at low steady-state levels. Regulatory loops are a common feature of the p53 pathway. The best characterized one involves the E3 ubiquitin ligase Hdm2, a p53 target gene that is responsible for maintaining low basal levels of p53 activity under normal proliferating conditions (Haupt et al, 1997; Kubbutat et al, 1997). Our results show that p18Hamlet levels are increased in p53-deficient cells but downregulated when p53 is overexpressed. This negative effect of p53 on p18Hamlet expression may be mediated by cyclin G1, a p53 target gene whose overexpression suffices to interfere with p18Hamlet accumulation and that can associate with this protein in vitro as well as colocalize in cells.
In summary, our results support a link between p18Hamlet and p53 function at two different levels. On the one hand, the half-life of p18Hamlet increases in response to DNA damaging agents and this is mediated at least in part by p38 MAPK. Accumulation of p18Hamlet leads to apoptosis, by increasing the ability of p53 to bind to specific promoters such as the proapoptotic genes NOXA and PUMA. In addition, low steady-state levels of p18Hamlet are maintained by a p53-dependent mechanism, probably mediated by cyclin G1. Therefore, p18Hamlet functions as a new cell-fate regulator, which contributes to the implementation of p53-regulated cellular responses.
Materials and methods
DNA cloning and mutagenesis
The human p18Hamlet cDNA was obtained from a Gal4 fusion-expressing clone identified in yeast two-hybrid screenings using p38α as bait (Cheung et al, 2003). Expression constructs for Escherichia coli and mammalian cells are described in Supplementary data. All p18Hamlet mutants were prepared using the QuickChange® site-directed mutagenesis kit (Stratagene) and were verified by DNA sequencing.
Cell culture
HEK-293, HeLa, SAOS, MCF7, U2OS and melanoma SK-Mel-103 cells as well as wt and p53−/− MEFs were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. FuGene reagent (Roche Applied Science) was used for cell transfection according to the manufacturer's protocol. Cells were treated with UV (50–100 J/m2) and cisplatin (5–10 μg/ml), as indicated.
The generation of stable cell lines expressing inducible p18Hamlet and the retroviral infections were performed as indicated in Supplementary data.
Transfection of siRNA and apoptosis measurement
The siRNA oligonucleotide for p18Hamlet (UGCGGACACUGGAAAGAAAUU) was obtained from Dharmacon (Lafayette, CO). U2OS and MCF7 cells were grown to 50% of confluency and transfected with Dharmafect reagent 1 (Dharmacon) according to the manufacturer's protocol. Cells were treated with UV or cisplatin 48 h after siRNA transfection. Human Lamin A siRNA (siGLO™, Dharmacon) was used as a control. Apoptosis was analyzed using the cell death detection ELISAPLUS kit (Roche Applied Science).
Flow cytometry analysis
Cells were trypsinized, washed with PBS, fixed with chilled 70% ethanol for 30 min at 4°C and then incubated in PBS containing 30 mg/ml of RNAse and stained for 30 min at 37°C with propidium iodide (25 μg/ml). Apoptotic cells were determined by their hypochromic subdiploid staining profiles. To estimate early apoptotic cells, Alexa 488-conjugated annexin V was used together with propidium iodide counterstain following the manufacturer's recommendations (Molecular Probes Inc.).
Luciferase expression analysis
U2OS and SAOS cells (2 × 105) were plated 24 h before transfection in six multiwell dishes. Transactivation assays contained 30 ng of the Renilla expression construct phRL-TK (Promega), as a transfection control, 10 ng of p53, 300 ng of promoter reporter and 700 ng of full-length or mutated p18Hamlet, as indicated. Cells were lysed in reporter lysis buffer 24 h after transfection. In the case of cisplatin treatments, cells were treated with the drug 24 h after transfection and collected 10–16 h later. Luciferase and Renilla activities were measured using the Dual-Luciferase® Reporter kit (Promega).
Antibodies, Western blotting, IP, pull-down and kinase assays
Western blot analysis was performed using 40–60 μg of cell lysates prepared in ice-cold IP lysis buffer. Buffers and antibodies are described in Supplementary data.
For IPs, 20 μl of anti-Myc or anti-HA agarose conjugates were incubated with 250–500 μg of protein lysates for 14 h at 4°C. The beads were then washed three times in IP buffer and analyzed by immunoblotting or further washed in kinase buffer and used for kinase assays (Alonso et al, 2000).
GST pull-downs and in vitro kinase assays were performed as described in Supplementary data.
Quantitative RT–PCR, Northern blot, ChIP analysis, ubiquitination assays, immunofluorescence and confocal microscopy
These protocols are described in Supplementary data.
Acknowledgments
This work was initiated while AC, ID and ARN were working at the EMBL (Heidelberg, Germany). We thank Manuel Serrano, Marisol Soengas, Ivan Dikic and Pedro Lazo for providing advice and reagents and Esther Seco and Sara Sánchez for technical support. AC acknowledges a post-doctoral fellowship from Fundación Ramón Areces (Spain). ARN is supported by grants from MEC and the Fundación Científica de la AECC (Spain) and PC by the UK MRC and the Royal Society.
References
- Alonso G, Ambrosino C, Jones M, Nebreda AR (2000) Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem 275: 40641–40648 [Abstract] [Google Scholar]
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL (2001) Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 8: 1243–1254 [Abstract] [Google Scholar]
- Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, Hsieh JK, O'Connor DJ, Zhong S, Campargue I, Tomlinson ML, Kuwabara PE, Lu X (2003) iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33: 162–167 [Abstract] [Google Scholar]
- Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4: 793–805 [Abstract] [Google Scholar]
- Bulavin DV, Fornace AJ Jr (2004) p38 MAP kinase's emerging role as a tumor suppressor. Adv Cancer Res 92: 95–118 [Abstract] [Google Scholar]
- Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ Jr (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18: 6845–6854 [Europe PMC free article] [Abstract] [Google Scholar]
- Cai Y, Jin J, Florens L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW (2005) The mammalian YL1 protein is a shared subunit of the TRRAP/TIP60 histone acetyltransferase and SRCAP complexes. J Biol Chem 280: 13665–13670 [Abstract] [Google Scholar]
- Cheung PC, Campbell DG, Nebreda AR, Cohen P (2003) Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J 22: 5793–5805 [Europe PMC free article] [Abstract] [Google Scholar]
- Coltella N, Rasola A, Nano E, Bardella C, Fassetta M, Filigheddu N, Graziani A, Comoglio PM, Di Renzo MF (2006) p38 MAPK turns hepatocyte growth factor to a death signal that commits ovarian cancer cells to chemotherapy-induced apoptosis. Int J Cancer 118: 2981–2990 [Abstract] [Google Scholar]
- Coutts AS, La Thangue NB (2005) The p53 response: emerging levels of co-factor complexity. Biochem Biophys Res Commun 331: 778–785 [Abstract] [Google Scholar]
- Deacon K, Mistry P, Chernoff J, Blank JL, Patel R (2003) p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell 14: 2071–2087 [Europe PMC free article] [Abstract] [Google Scholar]
- Eissenberg JC, Wong M, Chrivia JC (2005) Human SRCAP and Drosophila melanogaster DOM are homologs that function in the notch signaling pathway. Mol Cell Biol 25: 6559–6569 [Europe PMC free article] [Abstract] [Google Scholar]
- Espinosa JM, Emerson BM (2001) Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell 8: 57–69 [Abstract] [Google Scholar]
- Fotedar R, Bendjennat M, Fotedar A (2004) Role of p21WAF1 in the cellular response to UV. Cell Cycle 3: 134–137 [Abstract] [Google Scholar]
- Gostissa M, Morelli M, Mantovani F, Guida E, Piazza S, Collavin L, Brancolini C, Schneider C, Del Sal G (2004) The transcriptional repressor hDaxx potentiates p53-dependent apoptosis. J Biol Chem 279: 48013–48023 [Abstract] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299 [Abstract] [Google Scholar]
- Iwahashi H, Yamagata K, Yoshiuchi I, Terasaki J, Yang Q, Fukui K, Ihara A, Zhu Q, Asakura T, Cao Y, Imagawa A, Namba M, Hanafusa T, Miyagawa J, Matsuzawa Y (2002) Thyroid hormone receptor interacting protein 3 (trip3) is a novel coactivator of hepatocyte nuclear factor-4alpha. Diabetes 51: 910–914 [Abstract] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH (1997) Regulation of p53 stability by Mdm2. Nature 387: 299–303 [Abstract] [Google Scholar]
- Kyriakis JM, Avruch J (1996) Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem 271: 24313–24316 [Abstract] [Google Scholar]
- Moumen A, Masterson P, O'Connor MJ, Jackson SP (2005) hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell 123: 1065–1078 [Abstract] [Google Scholar]
- Nebreda AR, Porras A (2000) p38 MAP kinases: beyond the stress response. Trends Biochem Sci 25: 257–260 [Abstract] [Google Scholar]
- Ono K, Han J (2000) The p38 signal transduction pathway: activation and function. Cell Signal 12: 1–13 [Abstract] [Google Scholar]
- Poizat C, Puri PL, Bai Y, Kedes L (2005) Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol 25: 2673–2687 [Europe PMC free article] [Abstract] [Google Scholar]
- Reimer CL, Borras AM, Kurdistani SK, Garreau JR, Chung M, Aaronson SA, Lee SW (1999) Altered regulation of cyclin G in human breast cancer and its specific localization at replication foci in response to DNA damage in p53+/+ cells. J Biol Chem 274: 11022–11029 [Abstract] [Google Scholar]
- Samuels-Lev Y, O'Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X (2001) ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8: 781–794 [Abstract] [Google Scholar]
- Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS (2000) A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res 60: 2464–2472 [Abstract] [Google Scholar]
- She QB, Chen N, Dong Z (2000) ERKs and p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem 275: 20444–20449 [Abstract] [Google Scholar]
- Shikama N, Lee CW, France S, Delavaine L, Lyon J, Krstic-Demonacos M, La Thangue NB (1999) A novel cofactor for p300 that regulates the p53 response. Mol Cell 4: 365–376 [Abstract] [Google Scholar]
- Vousden KH, Lu X (2002) Live or let die: the cell's response to p53. Nat Rev Cancer 2: 594–604 [Abstract] [Google Scholar]
- Vousden KH, Prives C (2005) P53 and prognosis: new insights and further complexity. Cell 120: 7–10 [Abstract] [Google Scholar]
- Wu WT, Chi KH, Ho FM, Tsao WC, Lin WW (2004) Proteasome inhibitors up-regulate haem oxygenase-1 gene expression: requirement of p38 MAPK (mitogen-activated protein kinase) activation but not of NF-kappaB (nuclear factor kappaB) inhibition. Biochem J 379: 587–593 [Europe PMC free article] [Abstract] [Google Scholar]
- Xu F, Wang Y, Hall FL (2000) Molecular cloning and characterization of FX3, a novel zinc-finger protein. Oncol Rep 7: 995–1001 [Abstract] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7: 673–682 [Abstract] [Google Scholar]
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1038/sj.emboj.7601657
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1852783?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/sj.emboj.7601657
Article citations
Targeting carbohydrate metabolism in colorectal cancer - synergy between DNA-damaging agents, cannabinoids, and intermittent serum starvation.
Oncoscience, 11:99-105, 12 Nov 2024
Cited by: 0 articles | PMID: 39534512
Review
Reversal of Platinum-based Chemotherapy Resistance in Ovarian Cancer by Naringin Through Modulation of The Gut Microbiota in a Humanized Nude Mouse Model.
J Cancer, 15(13):4430-4447, 11 Jun 2024
Cited by: 0 articles | PMID: 38947385
Metal-Organic Frameworks for Cisplatin Delivery to Cancer Cells: A Molecular Dynamics Simulation.
ACS Omega, 9(17):19627-19636, 22 Apr 2024
Cited by: 0 articles | PMID: 38708264 | PMCID: PMC11064028
Targeting Colorectal Cancer: Unravelling the Transcriptomic Impact of Cisplatin and High-THC Cannabis Extract.
Int J Mol Sci, 25(8):4439, 18 Apr 2024
Cited by: 1 article | PMID: 38674023 | PMCID: PMC11050262
Editorial: Celebrating the 200th mendel's anniversary: gene-targeted diagnostics and therapies for cancer.
Front Mol Med, 4:1366963, 27 Feb 2024
Cited by: 0 articles | PMID: 39086436 | PMCID: PMC11285536
Go to all (62) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
p18(Hamlet) mediates different p53-dependent responses to DNA-damage inducing agents.
Cell Cycle, 6(19):2319-2322, 12 Jul 2007
Cited by: 16 articles | PMID: 17700068
Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis.
Cell Death Dis, 12(7):659, 30 Jun 2021
Cited by: 28 articles | PMID: 34193827 | PMCID: PMC8245518
Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53.
J Biol Chem, 281(11):7260-7270, 06 Jan 2006
Cited by: 328 articles | PMID: 16407291
A systematic review of p53 regulation of oxidative stress in skeletal muscle.
Redox Rep, 23(1):100-117, 03 Jan 2018
Cited by: 111 articles | PMID: 29298131 | PMCID: PMC6748683
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Medical Research Council (1)
The signaling pathways that regulate the production of pro-inflammatory cytokines
Sir Philip Cohen, MRC Protein Phosphorylation Unit
Grant ID: MC_U127084348





