Abstract
Free full text

eIF2α phosphorylation bidirectionally regulates the switch from short to long-term synaptic plasticity and memory
Summary
The late phase of long-term potentiation (LTP) and memory (LTM) requires new gene expression but the molecular mechanisms that underlie these processes are not fully understood. Phosphorylation of eIF2α inhibits general translation and selectively stimulates translation of ATF4, a repressor of CREB-mediated late-LTP and LTM. We used a pharmacogenetic bidirectional approach to examine the role of eIF2α phosphorylation in synaptic plasticity and behavioral learning. We show that in eIF2α+/S51A mice, in which eIF2α phosphorylation is reduced, the threshold for eliciting L-LTP in hippocampal slices is lowered and memory is enhanced. In contrast, only early-LTP is evoked by repeated tetanic stimulation and LTM is impaired, when eIF2α phosphorylation is increased by injecting into the hippocampus a small molecule, Sal003, which prevents the dephosphorylation of eIF2α. These findings highlight the importance of a single phosphorylation site in eIF2α as a key regulator of L-LTP and LTM formation.
Introduction
Repeated synaptic activation results in sustained potentiation of synaptic transmission (LTP), a putative cellular model of learning and memory (Bliss and Collingridge, 1993; Chen and Tonegawa, 1997; Malenka and Nicoll, 1999; Pittenger and Kandel, 2003; Dudai, 2004). Both memory and synaptic plasticity have two components. One, evoked by weak training protocols or a single tetanic train, yields only transient phenomena, short-term memory (STM, lasting minutes to hours) and the early phase of LTP (E-LTP, lasting 1–3 hours). The second component which follows strong training or repeated tetanic trains, activates mechanisms that stabilize the memory and synaptic changes, and results in long-term memory (LTM, lasting days, weeks or years) and the late phase of LTP (L-LTP, lasting many hours), respectively. Quite different molecular machineries, widely conserved from sea slugs to rodents (Kandel, 2001), are thought to underlie these two components: modifications of pre-existing proteins are sufficient for the transient changes, whereas new gene expression (transcription and translation) is required for those that are sustained (Silva et al., 1998; Kandel, 2001; Dudai, 2004; Kelleher et al., 2004; Klann and Dever, 2004; Sutton and Schuman, 2006). For instance, LTM and L-LTP are suppressed by agents that block mRNA and protein synthesis and, conversely, both are induced more readily in transgenic mice in which gene expression is facilitated (Malleret et al., 2001; Chen et al., 2003; Wang et al., 2004). Although we still do not fully understand the molecular mechanism by which gene expression is turned on, there is good reason to believe that the removal of constraints on gene expression is a critical step (Kandel, 2001; Genoux et al., 2002).
In diverse phyla, the transcription factor ATF4 is a repressor of cAMP responsive element binding protein (CREB)-mediated gene expression, which is required for L-LTP and LTM (Bartsch et al., 1995; Chen et al., 2003). The expression of ATF4 is regulated at the level of translation (Harding et al., 2000). Phosphorylation of the α subunit of the translation initiation factor eIF2 suppresses general translation (Hinnebusch, 2000), but selectively stimulates the translation of ATF4 mRNA (Lu et al., 2004; Vattem and Wek, 2004). Neuronal activity-dependent modulation of eIF2α phosphorylation is likely to be important for sustained changes in synaptic transmission as induction of L-LTP in hippocampal slices, by either tetanic stimulation or treatment with forskolin or BDNF, is correlated with decreased eIF2α phosphorylation (Takei et al., 2001; Costa-Mattioli et al., 2005). In mice lacking the eIF2α kinase, GCN2, the reduction in phosphorylated eIF2α is associated with altered synaptic plasticity and memory (Costa-Mattioli et al., 2005). To investigate the role of eIF2α phosphorylation in long-term plasticity and behavioral memory, we used eIF2α heterozygous mutants (eIF2α+/S51A) in which the phosphorylation site is mutated. We report here that in eIF2α+/S51A mice L-LTP and LTM formation are facilitated, as determined by several behavioral tasks. Moreover, a small molecule inhibitor of eIF2α dephosphorylation, Sal003, blocks L-LTP and memory storage, thus further demonstrating that eIF2α phosphorylation is a critical step in L-LTP and memory formation.
Results
Brain morphology is not altered in eIF2α+/S51A mice
Newborn homozygous mutants (Ser to Ala at the phosphorylation site Ser51) are phenotypically indistinguishable from their wild type (WT) littermates. However, they die shortly after birth, owing to hypoglycemia (Scheuner et al., 2001). eIF2α heterozygous mutants (eIF2α+/S51A) are viable, fertile, of normal size and weight, and they develop normally (Scheuner et al. 2001, 2005). There were no detectable differences in the overall morphology of the brain or hippocampus between eIF2α+/S51A and WT mice, as determined by Nissl staining of coronal sections (Figures S1A and S1B) or with two imunohistochemical markers: a) GAP-43, a neural-specific growth-associated protein and marker of axonal growth and presynaptic terminals, that stains particularly the perforant pathway to dentate gyrus and the CA3 and CA1 regions (Figure S1C), and b) synaptophysin, a major synaptic vesicle protein that is a marker of presynaptic terminals, including those of the mossy fiber and Schaffer collateral projections (Figure S1D; (Small et al., 2000).
Hippocampal eIF2α phosphorylation is reduced by ~ 50% in eIF2α+/S51A mice relative to WT mice, as determined by immunohistochemistry and Western blotting (Figures S1E and S1F). The level of ATF4 is also reduced (~ 40%) in the hippocampus of eIF2α+/S51A mice, as compared to WT mice (Figure S1G).
Inhibition of eIF2α phosphorylation leads to enhancement of synaptic plasticity and memory
Late-LTP is facilitated in slices from eIF2α+/S51A mice
Previous studies showed an association between decreased eIF2α phosphorylation and a lowered threshold for eliciting L-LTP (Takei et al., 2001; Costa-Mattioli et al., 2005). Thus, we predicted that reduced eIF2α phosphorylation should facilitate L-LTP. To test this hypothesis, we compared LTP in CA1 of hippocampal slices from eIF2α+/S51A mice and WT littermates. In WT slices, a single train of high-frequency stimulation of Schaffer collateral/commissural fibers (100 Hz for 1 s) elicited the expected short-lasting LTP (early-LTP; E-LTP) (Figure 1A), which in contrast to L-LTP, does not require RNA or protein synthesis (Kandel, 2001; Kelleher et al., 2004). However, in slices from eIF2α+/S51A mice, such stimulation elicited a sustained L-LTP (Figure 1A), which was blocked by anisomycin (Figure 1B) or actinomycin-D (Figure 1C). Thus, the sustained L-LTP elicited by a single tetanic train in eIF2α+/S51A slices depended on translation and transcription. The input-output relation of field excitatory postsynaptic potentials (fEPSPs), plotted as a function of stimulus intensity, and paired-pulse facilitation (PPF) indicated no difference in basal transmission between slices from eIF2α+/S51A and WT mice (Figure S2). Therefore a nonspecific change in synaptic transmission cannot account for the facilitation of L-LTP observed in eIF2α+/S51A slices. These data demonstrate that a reduction in eIF2α phosphorylation in eIF2α+/S51A slices leads to the conversion of a transient E-LTP into a sustained L-LTP.

A) A single 100 Hz train of stimulation elicits only E-LTP in control slices (open circles), whereas LTP is sustained in eIF2α+/S51A slices (closed circles; at 180 min, p < 0.01). The sustained L-LTP elicited in eIF2α+/S51A slices is suppressed by B) anisomycin (ANISO, closed circles; at 180 min p < 0.05) or C) actinomycin-D (ACTD, closed circles; at 180 min p < 0.01). Horizontal bars indicate the period of incubation with anisomycin and actinomycin-D. D) Similar L-LTP is induced by four tetanic trains in slices from WT and eIF2α+/S51A mice (at 240 min p > 0.05). Data are means ± S.E.M; numbers of tests are indicated. Scale bar 5 ms, 100μV.
In further experiments we investigated the effect of eIF2α phosphorylation on L-LTP. In hippocampal slices stimulated with four trains at 100 Hz, which normally induce L-LTP in WT slices (Kandel, 2001; Kelleher et al., 2004), a similar L-LTP was observed in eIF2α+/S51A and WT mice (Figure 1D). Forskolin, an activator of adenylate cyclase (Seamon et al., 1981), elicited a virtually identical L-LTP in slices from eIF2α+/S51A and WT mice (Figure S3). These data indicate that decreased eIF2α phosphorylation does not interfere with the elicitation of L-LTP.
Spatial learning and memory are enhanced in eIF2α+/S51A mice
Hippocampus-dependent spatial learning and memory were first studied in the Morris water maze (Morris et al., 1982). Because weak stimulation was sufficient to induce L-LTP in eIF2α+/S51A slices, mice were first trained only once per day, for six days, in a weaker training protocol than the standard three/four trials per day protocol (Chen et al., 2003; Costa-Mattioli et al., 2005). The time required for the mice to find the hidden platform (‘escape latencies’) did not differ until day 6, when eIF2α+/S51A mice reached the platform significantly faster than did WT littermates (Figure 2A). Another indication of enhanced spatial learning by eIF2α+/S51A mice was their significantly greater preference for the target quadrant, during the probe test conducted at the end of the sessions (Figure 2B). Next, mice were trained three times per day (strong protocol) for 6 days. The escape latencies of eIF2α+/S51A mice were already significantly shorter on training days 2 and 3, as compared to control mice (Figure 2C). During the first probe test (PT1) after 3 days of training, eIF2α+/S51A mice, unlike their WT littermates, spent significantly more time in the target quadrant, thus confirming their superior ability in spatial learning (Figure 2D). At the end of training on day 6, however, escape latencies (Figure 2C) or the preference for the target quadrant in the second probe test (PT2 in Figure 2E) were similar for eIF2α+/S51A and WT mice. We conclude that, relative to WT mice, eIF2α+/S51A mice show enhanced hippocampus-dependent spatial learning and memory.
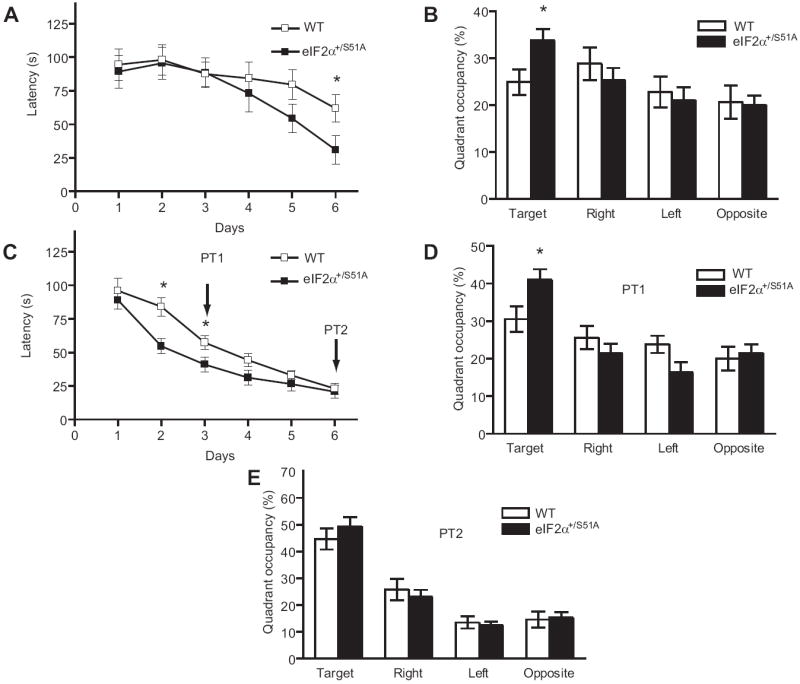
Data (means ± S.E.M.) were obtained either (A, B) in a weak version of the Morris water maze (1 trial per day) or (C, D) in the more intensive, standard version (3 trials per day). A) On day 6, escape latencies, plotted as function of training days, were significantly shorter for eIF2α+/S51A (closed squares, n = 13) as compared to WT mice (open squares, n=14; P < 0.05). B) In the probe test performed after the completion of training, unlike WT mice (open columns, p > 0.05) eIF2α+/S51A mutants (closed columns) showed significant preference for the target quadrant (p < 0.01). C) Escape latencies on day 2 and 3 after a strong training protocol (3 trials per day) show that eIF2α+/S51A mice (n=13) learned significantly faster than control littermates (n=13) (p < 0.05). D) In the first probe test (PT1) performed after three training days, only eIF2α+/S51A mice spent significantly more time in the target quadrant (p < 0.01). E) In the second probe test (PT2), after six days of training, both groups showed a similar preference for the target quadrant (p > 0.05).
Contextual and auditory fear conditioning are enhanced in eIF2α+/S51A mice
Pairing tone presentation with a foot-shock in a particular environmental context leads to both auditory and contextual fear conditioning. In the latter, the context is represented in the hippocampus and acts as the conditioned stimulus (CS), which is associated with the foot-shock, the unconditioned stimulus (US). Auditory fear conditioning, which associates the tone (CS) and the foot-shock (US), depends on the amygdala but not the hippocampus (Fanselow and LeDoux, 1999; LeDoux, 2000). Both types of fear conditioning require new protein synthesis (Bourtchouladze et al., 1998; Schafe et al., 1999).
Naïve WT and eIF2α+/S51A mice showed similar freezing responses prior to training and before the delivery of the foot shock. However, eIF2α+/S51A mice froze nearly twice as often as did WT mice 24 hr after a weak training protocol (a single pairing of a tone with a 0.35 mA foot-shock) (Figure 3A). A similar result was obtained when mice where tested for auditory fear conditioning (Figure 3B). The auditory tests were performed in a chamber that differed from the training chamber to minimize the influence of contextual memory.
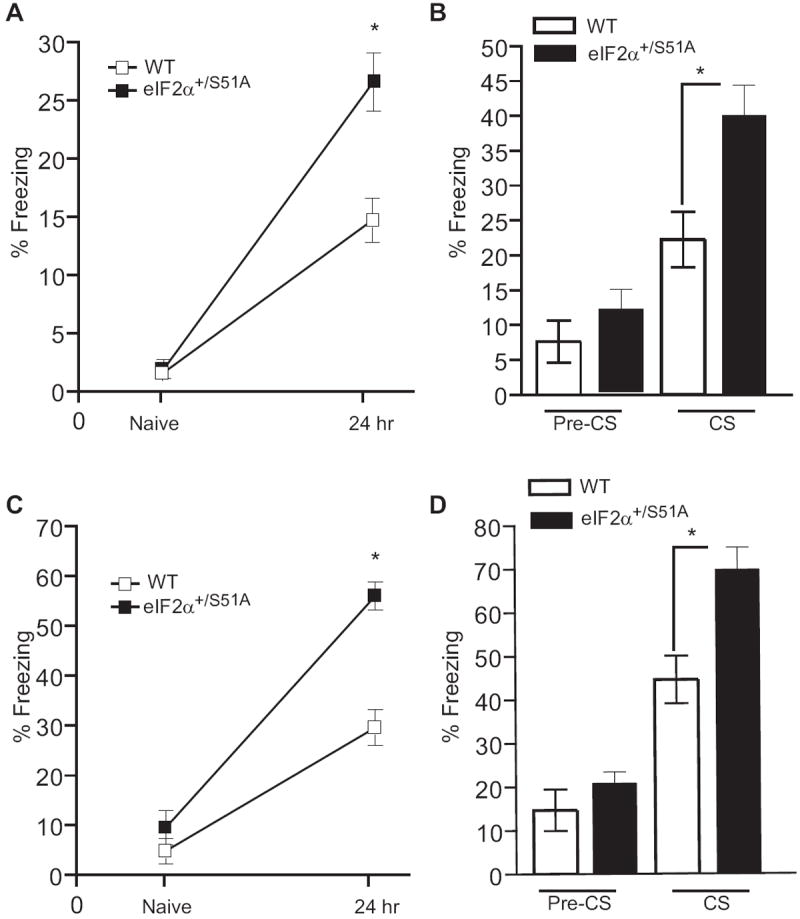
For contextual fear conditioning, freezing was first assessed during a two min control period in the training context prior to the conditioning (Naïve) and then during a 5 min period 24 hr after training. Freezing in response to the tone was assessed 24 hr after training during a two min period before the tone (pre-CS) and during the tone (CS) presentation. Data (means ± SEM) were obtained after either a weak protocol (single training with weak foot-shock; n=12 for each group; A, B) or a strong protocol (two trainings with stronger foot-shock; n=13 for each group; C, D). When tested 24 hr after training, eIF2α+/S51A mice froze more than the WT controls in response to the context after a weak (A; p<0.001) or a strong (C; p<0.0001) training protocol. They also had an enhanced freezing in response to the tone in both weak (B; p<0.05) and strong (D; p<0.01) protocols.
WT and eIF2α+/S51A mice exhibited equivalent levels of freezing to cued and fear conditioning when tested 1 hr after a strong training protocol (two pairings of a tone with a 0.7 mA foot shock) (Figure S4). However, after a delay of 24 hr, both contextual and auditory fear conditioning were enhanced in eIF2α+/S51A mice (Figures 3C and 3D). The finding that in eIF2α+/S51A mice, short-term contextual and auditory memory are normal (Figure S4) argues against non-specific responses to fear. In addition, the normal incidence of freezing in control tests (Figure 3) and in the elevated plus maze (data not shown) indicates a physiological level of anxiety in eIF2α+/S51A mice. Taken together, these data suggest that long-term memory is selectively enhanced in eIF2α+/S51A mice, as compared to WT littermates, in both contextual and auditory fear learning.
Long-term taste memory and extinction are enhanced in eIF2α+/S51A mice
Mice were subjected to two additional learning tasks: conditioned taste aversion (CTA) and latent inhibition (LI) of CTA. In CTA, a novel taste (CS) is followed by an unpleasant experience (US). A salient feature of this type of conditioning is that the CS and US can be separated by many hours. Like other long-term memories, CTA depends on protein synthesis, in this instance within the gustatory insular cortex (Rosenblum et al., 1993). Latent inhibition (LI), the weakening of CTA produced by pre-exposure to the same CS, is also a form of learning which is dependent on new protein synthesis (Rosenblum et al., 1993).
WT and eIF2α+/S51A mice did not differ in their natural preference for saccharin (Figure S5A) and aversion to quinine (Figure S5B), thus showing no indication of any non-specific impairment that would compromise their ability to perform this task. For CTA, after three days of water restriction, the naïve mice were offered saccharin followed by a malaise-inducing injection of LiCl. Two days after this aversive conditioning to saccharin, the aversion index did not significantly differ between control and eIF2α+/S51A mice (Figure 4A). The lack of difference between WT and eIF2α+/S51A mice in CTA may be explained by a ceiling effect of the very high aversion index or by enhanced negative aversion to the naturally preferred saccharine. In contrast, learning of a novel taste using a milder protocol, in the LI paradigm was significantly enhanced in eIF2α+/S51A mice as shown by the lower aversion index in comparison to the WT mice (Figure 4A).

An index > 0.5 means an aversion to, and a score < 0.5 means a preference for, that flavor. A) CTA induced by linking taste of saccharin to LiCl-induced malaise, was similar in eIF2+/S51A (n=5) and WT (n=4) mice (p > 0.05). An index > 0.5 means an aversion to, and a score < 0.5 means a preference for, that flavor. However, pre-exposure to saccharin caused a greater fall in aversion index (latent inhibition (LI)) in eIF2+/S51A mice (p<0.01; eIF2α+/S51A CTA: n=5, eIF2α+/S51A LI: n=7) as compared to WT mice. Further measurements during five days showed a similar decay of CTA with and without LI for WT mice (for all days p>0.05; WT CTA: n=4, WT LI: n=6) (B) and a much accelerated drop after LI in mutants (for all days p<0.01; eIF2α+/S51A CTA: n=5, eIF2α+/S51A LI: n=7), (C). All results are means ± S.E.M.
Repeated application of a CS without reinforcement by the US leads to a progressive extinction of the conditioned reflex (Pavlov, 1927). The rate of extinction of CTA, indicated by the gradual drop in aversion index on subsequent days, was similar for control and eIF2α+/S51A mice (Figures 4B and 4C). However, there was a sharp difference between WT and eIF2α+/S51A mice with regard to the effect of LI, which was induced by pre-exposure to saccharin for two days preceding the CTA protocol. Throughout the period of testing, CTA of control mice was not significantly reduced by LI (Figure 4B), whereas the decay of the aversion index in eIF2α+/S51A mice was remarkably accelerated, the aversion being already abolished by day 1 and replaced by a strong preference for saccharin by day 2 (Figure 4C). These data suggest that the much more rapid extinction of CTA in eIF2α+/S51A mice reflects accelerated learning (reinforcement) of the new taste (saccharin) (Sheffield and Roby, 1950).
Increased eIF2α phosphorylation leads to impairment of synaptic plasticity and memory
The small molecule Sal003 promotes eIF2α phosphorylation
Our hypothesis predicts that preventing eIF2α dephosphorylation should block L-LTP and LTM. To test this prediction, we used a potent derivative of salubrinal, Sal003 (Robert et al., 2006), which specifically prevents dephosphorylation of eIF2α by blocking eIF2α phosphatases (Boyce et al., 2005). As expected, Sal003 sharply increased eIF2α phosphorylation in mouse embryonic fibroblasts (MEFs) (Figure 5A). As noted above, phosphorylation of eIF2α should decrease general translation, but stimulate that of ATF4 mRNA (Harding et al., 2000). In Sal003-treated cells polysomes dissociated (Figure 5B, right panel) and consistent with this β-actin mRNA shifted to lighter fractions (Figure 5D), reflecting the inhibition of general translation. In contrast, ATF4 mRNA shifted to heavier polysomal fractions (Figure 5C), indicating the selective translation de-repression of this mRNA.

A) Mouse embryonic fibroblasts (MEFs) were treated with Sal003 (20 μM) or vehicle (DMSO) and incubated for the indicated periods of time. The phosphorylation state of eIF2α (at serine 51) was determined in cell lysates with a specific polyclonal antibody. B) MEFs were incubated with vehicle or Sal003 (10 μM) for 8 hr and lysates were layered on a 10-ml continuous sucrose gradient (10 to 50%). Polysomes were analyzed as described in Methods. Left, vehicle-treated. Right, Sal003-treated cells. The positions of the polysomes and ribosomes are indicated. C, D) RT-PCR of ATF4 and β-actin mRNAs from separate fractions of sucrose gradients. Sal003 (10 μM) prevents the induction of L-LTP by four trains of tetanic stimulation in slices from WT mice (E; at 240 min, p < 0.05), but not in hippocampal slices from ATF4 -/- mice (F; at 240 min, p > 0.05). Open circles are means (± S.E.M.) from slices treated with vehicle alone and closed circles from slices treated with Sal003 at the times indicated by horizontal bars. Scales represent 5 ms and 100 μV.
Sal003 impairs L-LTP in WT slices but not in slices from ATF4 knockout (-/-) mice
In accordance with our prediction, L-LTP induced by 4 trains in hippocampal slices from WT mice was prevented by Sal003 (Figure 5E), whereas E-LTP was unaffected (Figure S6A). The effect of Sal003 was specific to the potentiated synapses, as basal synaptic transmission was not altered (Figure S6B). An important finding was that when Sal003 was applied 45 min after the high-frequency tetani (when LTP was fully established), L-LTP was not suppressed (Figure S6C). Thus, enhanced eIF2α phosphorylation interferes with the induction but not the maintenance of L-LTP. These results demonstrate that increased eIF2α phosphorylation blocks the conversion of E-LTP to L-LTP.
To what extent does the impairment of L-LTP caused by Sal003 depend on ATF4? We addressed this question by examining the effect of Sal003 in hippocampal slices from ATF4 -/- mice (Masuoka and Townes, 2002). Comparable L-LTP was elicited by four trains of 100 Hz stimulation in slices from ATF4 -/- and WT mice (data not shown). However, in sharp contrast to WT slices, L-LTP induction was not prevented by Sal003 in slices from ATF4 -/- mice (Figure 5F). It is noteworthy that Sal003 inhibited translation to the same extent (~ 20%) in WT and ATF4 -/- hippocampal slices, as determined by 35S-methionine incorporation followed by TCA precipitation (data not shown). This indicates that the effect of Sal003 on L-LTP is not due to disparate inhibition of translation in WT and ATF4 -/- slices. These data constitute compelling evidence that Sal003 blocks L-LTP by specifically increasing the translation of ATF4 mRNA, a mechanism that cannot occur in ATF4 -/- mice.
Sal003 impairs long-term memory
Given that L-LTP-inducing stimulation results in decreased in eIF2α phosphorylation (Takei et al., 2001; Costa-Mattioli et al., 2005), we investigated whether eIF2α phosphorylation is altered during LTM formation in the hippocampus. At different times after a contextual fear conditioning, rats were euthanized, the hippocampi isolated, and the level of eIF2α phosphorylation was determined by Western blotting. Already by 30 min after training, eIF2α phosphorylation was reduced approximately by half (Figures 6A and B).

A) Western blots from hippocampal tissue show decreased phosphorylation of eIF2α after contextual fear conditioning. Dorsal hippocampi of rats were removed at different times after training. B) Quantification of normalized p-eIF2α following training (n=4 per time point). C) Diagram of the experimental protocol. Sal003 (40 μM) or Vehicle was infused into the hippocampus immediately after the strong training protocol and dorsal hippocampi were dissected 30 min after training. D) A representative Western blot showing phosphorylation of hippocampal eIF2α after infusion of Sal003. E) Quantification of normalized p- eIF2α using total eIF2α as a loading control (n=4 for vehicle and Sal003 group). F) Diagram of the experimental protocol. Bilateral infusion of Sal003 into the hippocampus immediately after training reduced long-term contextual fear conditioning (G, p < 0.05) but did not affect auditory fear conditioning (H, p > 0.05), both assessed 24 hr after training. Data (means ± S.E.M) from vehicle (n=7) and Sal003-injected rats (n=9) are represented by open and closed columns or symbols, respectively.
To examine the effect of eIF2α phosphorylation on the consolidation of fear memories, Sal003 was bilaterally infused into the dorsal hippocampus (Figure S7), immediately after training. The dorsal hippocampus was removed 30 min later (Figure 6C) and the levels of eIF2α phosphorylation were determined. Strikingly, Sal003-infusion blocked the decrease in eIF2α phosphorylation normally observed after training (Figures 6D and 6E). In analogous experiments (Figure 6F), Sal003-infused rats showed a significant reduction (~ 50%) in freezing response when tested for contextual fear conditioning 24 hr after training (Figure 6G). These data are consistent with previous reports that inhibitors of gene expression administered immediately after fear conditioning disrupt long-term memory (Bourtchouladze et al., 1998). In contrast, Sal003 did not affect fear memory associated with a tone (Figure 6H), as expected, since the drug was injected only into the hippocampus, and auditory fear conditioning is independent of the hippocampus. The normal auditory fear conditioning shows that the suppression of contextual freezing by Sal003 was not caused by non-specific impairment of the animals’ ability to perform the task.
To examine whether Sal003-treatment of WT rats is a reciprocal model for the eIF2α+/S51A mutation, experiments were performed in the Morris water maze. The rats were trained by the standard training protocol (three trials per day, for six days). Sal003 or Vehicle were bilaterally infused into the hippocampus within 5 min of the end of each day’s training (Morris et al., 2006) over sessions 1–6 (Figure S8). The significant increase in escape latency (Figure S8A) and the reduced quadrant occupancy (Figure S8B) clearly indicate that Sal003 impaired spatial long-term memory formation. These findings demonstrate that a local increase in eIF2α phosphorylation counteracts the physiological post-training decrease in eIF2α phosphorylation and impairs hippocampus-dependent long-term contextual memory.
Discussion
eIF2α phosphorylation regulates gene expression-dependent synaptic plasticity and memory
De novo gene expression (via translational and transcriptional mechanisms) is required for long-lasting synaptic plasticity and memory (Silva et al., 1998; Kandel, 2001; Dudai, 2004; Kelleher et al., 2004; Klann and Dever, 2004; Sutton and Schuman, 2006). Our results provide new insight into the mechanism of mnemonic processes by showing that changes at a single phosphorylation site of a key translation initiation factor bidirectionaly modulate synaptic plasticity and memory storage. Specifically, we found that reduced phosphorylation of eIF2α in eIF2α+/S51A mice is associated with enhanced synaptic plasticity (Figure 1), learning and memory (Figures 2, ,33 and and4).4). Conversely, when dephosphorylation of eIF2α is blocked by Sal003, both L-LTP induction (but not maintenance) and long-term memory are impaired (Figures. 5, ,66 and S8). Thus, our study provides genetic, chemical, physiological, behavioral and molecular evidence that eIF2α dephosphorylation is essential for the induction of L-LTP and LTM.
Other lines of evidence support the hypothesis that dephosphorylation of eIF2α is critical for the induction of gene expression leading to L-LTP and LTM. First, eIF2α phosphorylation promotes the translation of ATF4 mRNA, which encodes an inhibitor of CREB-driven gene expression, long-term synaptic and memory storage in different phyla (Bartsch et al., 1995; Chen et al., 2003). Second, eIF2α phosphorylation regulates protein synthesis, which is required for long-lasting synaptic plasticity and memory consolidation (Kelleher et al., 2004; Klann and Dever, 2004; Sutton and Schuman, 2006). Third, eIF2α phosphorylation is decreased by procedures that induce L-LTP (Takei et al., 2001; Costa-Mattioli et al., 2005) and memory formation. Fourth, both L-LTP and LTM are more readily induced in mice lacking GCN2, the major kinase responsible for the phosphorylation of eIF2α in the brain (Costa-Mattioli et al., 2005).
eIF2α Phosphorylation: A Molecular Master Switch for L-LTP and Memory Consolidation
How does eIF2α phosphorylation mediate the switch from short-term to long-term synaptic changes and memory? Our model is based on the regulation of gene expression by eIF2α phosphorylation (Figure 7). Under basal conditions, when eIF2α is partly phosphorylated by GCN2, ATF4 acts as a brake on the expression of CREB-dependent genes and protein synthesis is diminished. By reducing eIF2α phosphorylation, repeated training or tetanic stimulation lowers the level of ATF4 and removes the inhibitory constraint, and also increases protein synthesis. Both mechanisms lead to the expression of genes required for long-term synaptic plasticity and memory. As a result of these changes in gene expression the threshold for eliciting L-LTP and LTM is lowered. For instance, in eIF2α+/S51A and GCN2 -/- mice, in which both eIF2α phosphorylation and ATF4 levels are reduced, the threshold for eliciting L-LTP and LTM is lowered (Costa-Mattioli et al., 2005 and Figures 1--4).4). This model is supported by the increase in ATF4 expression upon treatment with Sal003 (Figure 5C), which leads to an impairment of L-LTP and LTM (Figures 5, ,66 and S8). The critical role of ATF4 in these processes is emphasized by the preservation of L-LTP in Sal003-treated ATF4 -/- slices (Figure 5F). Thus, these data provide direct genetic evidence that the impairment of L-LTP caused by Sal003 is dependent on ATF4’s repressor action.
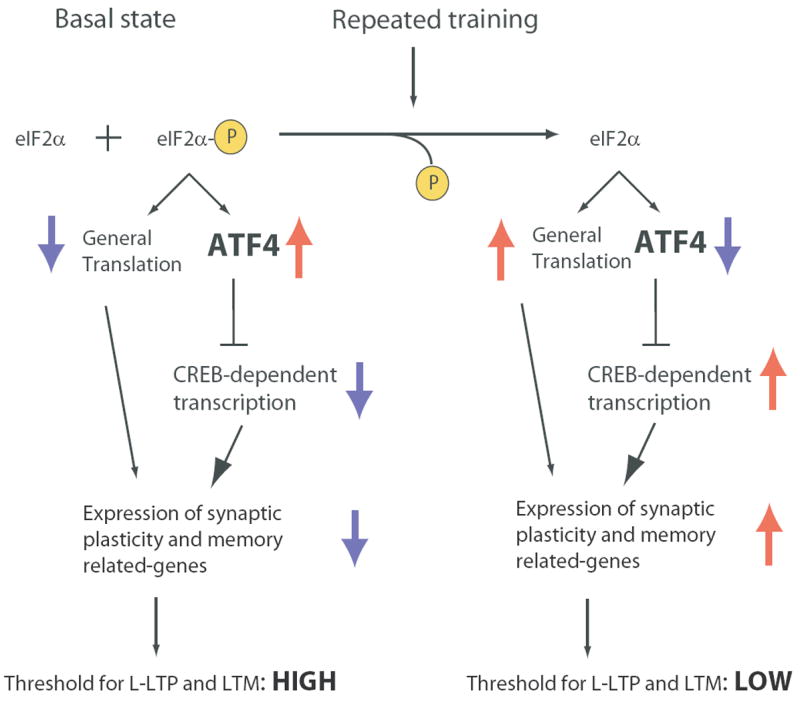
Under basal conditions, general translation is reduced and ATF4 mRNA translation is augmented, due to partial phosphoryation of eIF2α. As a consequence, expression of synaptic plasticity and memory-related genes is depressed, late-LTP has a high threshold and mnemonic function is poor. Decreased eIF2α phosphorylation reduces ATF4 mRNA translation and enhances general mRNA translation, thus facilitating the induction of gene expression which leads to L-LTP and long-term-memory (LTM) consolidation.
Additional evidence that ATF4 regulates L-LTP and LTM comes from the study of Chen and colleagues (2003). They reported that in a transgenic mouse expressing a dominant negative inhibitor (EGFP-AZIP) that targets both C/EBP proteins and ATF4, long-term synaptic plasticity and memory were facilitated, under weak training protocols (Chen et al., 2003). This scheme is also consistent with previous studies on the ATF4 homologue in Aplysia (ApCREB2). After injection of anti-ApCREB2 antibodies into Aplysia sensory neurons, a single pulse of serotonin (5-HT), which normally induces short-term facilitation, evoked a gene expression-dependent facilitation that lasted more than one day (Bartsch et al., 1995). Thus, activation of gene expression is critical for long-term synaptic plasticity and memory formation.
Dephosphorylation of eIF2α could also lead to an increase in general protein synthesis. Since an increase in translation could facilitate LTP, it might in principle contribute to the enhancement of LTP and LTM in eIF2α+/S51A mice. However, the lack of effect of Sal003 in slices from ATF4 -/- mice demonstrates that eIF2α phosphorylation acts primarily through regulation of ATF4 levels. It is likely that treatment with Sal003 for short times affects ATF4 mRNA translation to a greater extent than general translation. Indeed, in yeast it is known that levels of eIF2α phosphorylation that do not inhibit general translation, are sufficient for the enhanced translation of the GCN4 mRNA, which encodes a transcription factor of the same b-ZIP family, which contains ATF4 (Hinnebusch, 2000, 2005). Though a significant block in general translation initiation was observed when Sal003 was applied for a long period (~ 8 hr; Figure 5B), there was only a modest decrease in general translation when slices were incubated in the presence of Sal003 for 1 hr (Figures 5E and 5F). Taken together, these data strongly suggest that eIF2α acts as a switch for both LTP and LTM through modulation of ATF4 mRNA translation.
Differences between GCN2 -/- and eIF2α+/S51A mice
Hippocampal ATF4 levels are regulated through GCN2-mediated phosphorylation of eIF2α (Costa-Mattioli et al., 2005). Accordingly, L-LTP and LTM are enhanced in eIF2α+/S51A mice following weak tetanic stimulation or weak training in various behavioral tasks. However, in contrast to GCN2 -/- mice, in which L-LTP and LTM are impaired in response to repeated tetani or strong behavioral training, L-LTP and LTM are enhanced in eIF2α+/S51A mice. A possible explanation for this difference is that strong training or tetanic stimulation cause GCN2 to phosphorylate other targets in the brain (in addition to eIF2α) which interfere with L-LTP and LTM formation. Clearly, there must be limits to the extent to which the threshold for LTP induction can be manipulated. Therefore it is conceivable that a mild induction of translation would facilitate L-LTP and LTM. However, too much translation may interfere with the optimal pattern of synaptic weight changes and may even induce an excess of proteins that antagonize L-LTP and LTM (such as depotentiation-related proteins). Another difference between GCN2 -/- and eIF2α+/S51A mice is that auditory fear conditioning is normal in GCN2 -/- mice whereas it is enhanced in eIF2α+/S51A mice, after both weak and strong training protocols. Thus, in the amygdala, decreased eIF2α phosphorylation enhances memory formation but this may occur in a GCN2-independent manner. Thus, either GCN2 is not the major eIF2α kinase in the amygdala or the pathway which turns off GCN2 activity is not activated by auditory fear conditioning.
In conclusion, we have shown that the induction of L-LTP and LTM is facilitated by decreased eIF2α phosphorylation and impaired by increased eIF2α phosphorylation. Taken together, these data strongly support the notion that, under physiological conditions, a decrease in eIF2α phosphorylation constitutes a critical step for the activation of gene expression that leads to the long-term synaptic changes required for memory formation. Our data suggest that ATF4 is an important regulator of these processes. These findings also raise the interesting possibility that regulators of translation could serve as therapeutic targets for the improvement of memory, for instance in human disorders associated with memory loss.
Experimental Procedures
eIF2α+/S51A mice
eIF2α+/S51A mice were back-crossed for fifteen generations to C57Bl/6J mice. They were weaned at the fifth postnatal week and their genotypes determined (Scheuner et al., 2001; Scheuner et al., 2005). ATF4 -/- mice were kindly provided by Dr. Tim Townes (Masuoka and Townes, 2002). All experiments were done with 2-4 month old males. Mice were kept on a 12 h light/dark cycle, and the behavioral experiments were always done during the light phase of the cycle. Mice had access to food and water ad libitum, except during tests. All the procedures followed the guidelines of the Canadian Council on Animal Care and were approved by the McGill Facility Animal Care Committee.
Cell culture and Sal003 treatment
Mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle’s medium (BioWhittaker), 100 units/ml penicillin, 10% fetal bovine serum, and 100 μg/ml streptomycin. MEFs were treated with 10 μM Sal003 for the indicated time periods, washed twice with ice-cold phosphate-buffered solution and lysed as previously described (Costa-Mattioli et al., 2005).
Immunohistochemistry and Western Blotting
Hippocampal cell lysates and Western blots, using total and p-eIF2α antibodies, were performed as described ((Costa-Mattioli et al., 2005). Immunohistochemistry was performed as described (Côté et al., 1993). Digital photos were taken using the Axiovision 4 Imaging program (Zeiss).
Electrophysiology
Transverse hippocampal slices (400 μm) were cut from brains of WT or eIF2α+/S51A age-matched littermates (6-12 weeks old) as described earlier (Costa-Mattioli et al., 2005). Briefly, slices were kept submerged at 27-28°C and superfused (1-2 ml/min) with oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (ACSF) containing: 124 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl2, 26 mM NaHCO3 and 10 mM glucose. Bipolar tungsten stimulating electrodes were placed in the CA1 stratum radiatum to stimulate the Schaffer collateral and commissural fibers, and extracellular field EPSPs (fEPSPs) were recorded with a glass microelectrode (2-3 MΩ, filled with 2 M NaCl) positioned in the stratum radiatum. Baseline stimulation frequency was 2 min-1 and the intensity of the 0.1 ms pulses was adjusted to evoke 35-40% maximal fEPSPs.
Tetanic LTP was induced by high frequency stimulation in brief trains (100 Hz, 1 s), applied either as a single train or four trains separated by 5 min intervals. When indicated, ACSF contained anisomycin (40 μM, Calbiochem), actinomycin-D (40 μM, Calbiochem) or Sal003 (20 μM) for 30 min before the onset of tetanic stimulation. To reduce day-to-day variability simultaneous recordings were obtained from two slices. On a given day, data were recorded from WT, eIF2α+/S51A slices, and slices treated with drugs or vehicle. The experimenter was blind to the mouse genotype. Statistical analyses were done by t-tests and two-way ANOVA. All electrophysiological data are presented as means ± SEM and “n” indicates the number of slices.
Contextual and auditory fear conditioning
Tests were performed on 2–4 month old male eIF2α+/S51A and WT littermates by an experimenter blind to mouse genotype. Mice were handled for 3-4 days before the start of the experiment. They were habituated to two distinct contexts (here named A and B) for 20 min for 3 days. Within a single day, the habituation sessions were separated by at least 4 hr. After three days of habituation, mice were trained in chamber A. Training consisted of a 2 min period of acclimatizing to the context, followed by one pairing of a tone (2800 Hz, 85 dB, 30 s) with a co-terminating foot-shock (0.35 mA, 1 s) for the weak training protocol, or two pairings of a tone (2800 Hz, 85 dB, 30 s) with a co-terminating foot-shock (0.7 mA, 2 s) for the strong training protocol. The mice remained in the chamber for an additional 1 min after the end of the last pairing, after which they were returned to their home cages. Contextual fear conditioning was assayed 1 h and 24 hr after training by replacing the animals in the conditioning context (chamber A) for a 5 min period, during which the incidence of freezing (immobile except for respiration) was recorded. The tests of auditory fear conditioning consisted of a 2 min acclimatizing period to the context (chamber B; pre-CS period), followed by a 3 min presentation of the same tone (2800 Hz, 85 dB, 30 s) (CS). Mice were returned to their cages 30 s after the end of the tone. For all tests, each mouse was judged at 5 s intervals as either freezing or not freezing. Data are expressed as the percent of 5 s intervals in which freezing was observed. Tests of responses to the training context (chamber A) and to the tone (chamber B) were done in a counterbalanced manner. Statistical analysis was based on repeated measures ANOVA and between-group comparisons by Tukey’s Test.
Morris water maze
The task was performed as described (Morris et al., 1982; Costa-Mattioli et al., 2005). Mice were trained in one trial per day (1T/d), or three trials per day (3T/d) with 30 min inter-trial intervals, over six consecutive days. Learning was evaluated by monitoring escape latencies to a hidden (submerged) platform with an automated video tracking system (HVS Image, Buckingham, UK). For probe trials, the platform was removed from the maze and the animals were allowed to search for 60 s. The percentage of time spent in each quadrant of the maze (quadrant occupancy) was recorded. Swimming speed was similar in WT and control and mutant animals (data not shown). The experimenter was blind to the genotype for all behavioural tests and trials were performed at the same time of day (±1 hr) during the animal’s light phase. Statistical analysis was based on repeated measures ANOVA. In between-group comparisons were performed by Tukey’s Test. A level of 5% was considered significant. The p value is reported.
Conditioned taste aversion and latent inhibition
Conditioned taste aversion (CTA) was studied as described by (Rosenblum et al., 1993), with a few modifications. Saccharin (0.5% w/v) was the unfamiliar taste (conditioned stimulus (CS)), and 0.14 M LiCl i.p. (volume 2% of body weight) the malaise-inducing agent (unconditioned stimulus (US)). Mice were trained for three days to get their daily water ration (once only, for 20 min) from two pipettes, each containing 5 ml of water (‘water restriction’). On the fourth day, the conditioning day, mice were given the saccharin solution instead of water (for 20 min), and 60 min later, received the LiCl injection. Two days after training, in a multiple choice test (one pipette with 5 ml of saccharin solution and one with 5 ml of water) the conditioned mice preferred water to saccharin, whereas non-conditioned mice preferred saccharin to water. The results are presented as the aversion index, which is defined as the volume of water consumed as a fraction of total fluid intake [ml water/(ml water + ml saccharin solution)]: an index of 0.5 indicates no preference; the higher the index, the greater the preference for water instead of the negatively conditioned taste.
In the LI experiments, two days before CTA training, the mice were exposed on two consecutive days to saccharin for 20 min (experimental) or tap water (control). The taste aversion index was determined for five consecutive days.
Tests of the natural reactions to bitter quinine and sweet saccharin were done on mice habituated to drinking from pipettes (conditioning was not needed). In three consecutive drinking sessions, mice had access to two pipettes simultaneously, one containing 5 ml of either 0.04% quinine or 0.5% saccharin and the other 5 ml of water. The aversion index was defined as in the CTA tests.
Cannulation and Sal003 infusion
Rats (300-325g body weight) were cannulated as described previously (Debiec et al., 2002). Briefly, rats were anesthetized with a 1ml/kg of body weight i.p. injection of a mixture of ketamine (55.5 mg/ml), xylazine (3.33 mg/ml) and medetomidine hydrochloride (0.27 mg/ml). Anesthetized rats were mounted in a stereotaxic frame and guide cannulae (22 gauge) were bilaterally implanted into the dorsal hippocampus, angled at 10° away from the midline. The tips of the cannulae were located 3.6 mm posterior to bregma, 3.1 mm lateral to the midline and 2.4 mm ventral from the skull surface (Paxinos and Watson, 1986) Three jewelry screws were inserted into the skull and acrylic cement was applied to stabilize the cannulae. A 28-gauge probe was inserted into the guide to prevent clogging. Rats were housed individually and allowed a week to recover from the surgery before the experiments. During the recovery period, animals were handled daily. Sal003 was dissolved in DMSO and further diluted in 0.9 % NaCl (saline) to a final DMSO concentration of 0.1 %. Two μl of 20μM Sal003 or vehicle (0.1% DMSO in saline) were infused bilaterally into the cannulae through a 28-gauge injection needles (injectors) immediately after behavioral training. The infusions were driven by using a motorized syringe pump (kdScientific) at a rate of 0.25 μl/min. Following 8 min of infusion, the injector remained in the guide cannula for an additional minute, to ensure adequate diffusion of the solutes from the tip of the injector. With only minor modifications, the contextual fear conditioning protocol was as described above for mice. Briefly, the training consisted of two pairings of a tone (2800 Hz, 85 dB, 30 s) with a stronger co-terminating foot-shock (1.5 mA, 2 s), and freezing was recorded over the whole 5 min period of interest (not at 5 s intervals).
After completion of the behavioral experiments, animals were anesthetized with urethane (0.5 g/ml i.p.) and decapitated. The brains were then fixed in a 10% formalin-saline, 20% sucrose solution. Brain sections (50 μm thick) were stained with formal-thionin to identify the placements of the cannulae. Statistical analysis was based on repeated measures ANOVA and between-group comparisons by Tukey’s Test.
Polysome profile analysis and RT-PCR
Polysome profiles were obtained as previously described (Costa-Mattioli et al., 2005). MEFs were treated with Sal003 for 8 hr and washed twice with cold PBS + 100 μg/ml cycloheximide, suspended in lysis buffer, homogenized with 15 strokes (7ml Wheaton Dounce) on ice, and then centrifuged for 2 min at 14,000g. The supernatant was loaded onto a 10%–50% sucrose gradient prepared in 20 mM HEPES-KOH (pH 7.6), 100 mM KCl and 5 mM MgCl2 and was centrifuged in an SW40 rotor at 35,000 rpm for 2 hr. Gradients were analyzed by piercing the tube with a Brandel tube piercer, passing 60% sucrose through the bottom of the tube, and monitoring the absorbance of the eluting material with an ISCO UA-6 UV detector. Total RNA from individual fractions was isolated with Trizol (Invitrogen) according to manufacturer’s instructions. ATF4 and β-actin mRNAs were amplified in one-tube RT-PCR reactions which were optimized to detect the exponential phase on the amplification curve (3).
Acknowledgments
We thank R. Darnell, A. Fine, R. Blitzer, R. Kelleher, A. Koromilas, C. Alberini, Y. Dudai and M. Fabian for comments on the manuscript. T. Townes for providing the ATF4 -/- mice. A. LaFrance for assisting in the maintenance of the eIF2α+/S51A mice, T. Lawson, N. Taheri and C. Lister for excellent assistance. This work was supported by a Team grant from the Canadian Institute of Health Research (CIHR) to N. S, K. N, J. P, J-C. L and M. C-M, a Howard Hughes Medical Institute (HHMI) grant to N.S, an NIH grant to R.K, an ISF for K.R and a Natural Sciences and Engineering Research Council of Canada (NSERC), the Volkswagen, Alfred P. Sloan and EJLB foundations to K. N. N.S. is a CIHR Distinguished Scientist and an HHMI International scholar. M.C-M is supported by a CIHR post-doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. [Abstract] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. [Abstract] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [Europe PMC free article] [Abstract] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. [Abstract] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. [Abstract] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annu Rev Neurosci. 1997;20:157–184. [Abstract] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, et al. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. [Europe PMC free article] [Abstract] [Google Scholar]
- Côté SL, Ribeiro-da-Silva A, Cuello AC. Current protocols for light microscopy immunocytochemistry. In: Cuello AC II, editor. Immunohistochemistry. West Sussex: Wiley; 1993. [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. [Abstract] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. [Abstract] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. [Abstract] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. [Abstract] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. [Abstract] [Google Scholar]
- Hinnebusch AG. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N, Hershey JW, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 185–244. [Google Scholar]
- Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annual review of microbiology. 2005;59:407–450. [Abstract] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. [Abstract] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. [Abstract] [Google Scholar]
- Klann E, Dever TE. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. [Abstract] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. [Abstract] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. [Europe PMC free article] [Abstract] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. [Abstract] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–686. [Abstract] [Google Scholar]
- Masuoka HC, Townes TM. Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood. 2002;99:736–745. [Abstract] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. [Abstract] [Google Scholar]
- Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kelly PA. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. [Abstract] [Google Scholar]
- Pavlov I. Conditioned reflexes. 1960 NY: Dover Publications; 1927. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1986. [Abstract] [Google Scholar]
- Pittenger C, Kandel ER. In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2003;358:757–763. [Europe PMC free article] [Abstract] [Google Scholar]
- Robert F, Kapp LD, Khan SN, Acker MG, Kolitz S, Kazemi S, Kaufman RJ, Merrick WC, Koromilas AE, Lorsch JR, et al. Initiation of Protein Synthesis by Hepatitis C Virus Is Refractory to Reduced eIF2 {middle dot} GTP {middle dot} Met-tRNAiMet Ternary Complex Availability. Mol Biol Cell. 2006;17:4632–4644. [Europe PMC free article] [Abstract] [Google Scholar]
- Rosenblum K, Meiri N, Dudai Y. Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol. 1993;59:49–56. [Abstract] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [Europe PMC free article] [Abstract] [Google Scholar]
- Scheuner D, Mierde DV, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. [Abstract] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. [Abstract] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. [Europe PMC free article] [Abstract] [Google Scholar]
- Sheffield FD, Roby TB. Reward value of a non-nutritive sweet-taste. J Comp Physiol Psychol. 1950;43:471–481. [Abstract] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. [Abstract] [Google Scholar]
- Small SA, Wu EX, Bartsch D, Perera GM, Lacefield CO, DeLaPaz R, Mayeux R, Stern Y, Kandel ER. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron. 2000;28:653–664. [Abstract] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. [Abstract] [Google Scholar]
- Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor enhances neuronal translation by activating multiple initiation processes: comparison with the effects of insulin. J Biol Chem. 2001;276:42818–42825. [Abstract] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 2004 [Europe PMC free article] [Abstract] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.cell.2007.01.050
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S0092867407003248/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102243178
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.cell.2007.01.050
Article citations
Defective regulation of the eIF2-eIF2B translational axis underlies depressive-like behavior in mice and correlates with major depressive disorder in humans.
Transl Psychiatry, 14(1):397, 01 Oct 2024
Cited by: 0 articles | PMID: 39349438 | PMCID: PMC11442801
Behavioral screening reveals a conserved residue in Y-Box RNA-binding protein required for associative learning and memory in C. elegans.
PLoS Genet, 20(10):e1011443, 18 Oct 2024
Cited by: 0 articles | PMID: 39423228 | PMCID: PMC11524487
Quintessential Synergy: Concurrent Transient Administration of Integrated Stress Response Inhibitors and BACE1 and/or BACE2 Activators as the Optimal Therapeutic Strategy for Alzheimer's Disease.
Int J Mol Sci, 25(18):9913, 13 Sep 2024
Cited by: 0 articles | PMID: 39337400 | PMCID: PMC11432332
Neurodegenerative diseases reflect the reciprocal roles played by retroelements in regulating memory and immunity.
Front Neurosci, 18:1445540, 20 Sep 2024
Cited by: 0 articles | PMID: 39371608 | PMCID: PMC11451048
The ISR downstream target ATF4 represses long-term memory in a cell type-specific manner.
Proc Natl Acad Sci U S A, 121(31):e2407472121, 24 Jul 2024
Cited by: 0 articles | PMID: 39047038
Go to all (324) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2.
Nature, 436(7054):1166-1173, 01 Aug 2005
Cited by: 267 articles | PMID: 16121183 | PMCID: PMC1464117
Promoter-Specific Effects of DREADD Modulation on Hippocampal Synaptic Plasticity and Memory Formation.
J Neurosci, 36(12):3588-3599, 01 Mar 2016
Cited by: 53 articles | PMID: 27013687 | PMCID: PMC4804014
eIF2alpha Phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation.
J Neurosci, 30(7):2582-2594, 01 Feb 2010
Cited by: 85 articles | PMID: 20164343 | PMCID: PMC2836228
BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory?
Neurobiol Learn Mem, 89(3):312-323, 17 Oct 2007
Cited by: 456 articles | PMID: 17942328 | PMCID: PMC2387254
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Canadian Institutes of Health Research (1)
Grant ID: 15121
NCI NIH HHS (1)
Grant ID: R01 CA154916




