Abstract
Free full text

Vascular Endothelial Growth Factor: Biology and Therapeutic Applications
Abstract
While the development of anti-angiogenic therapy, as it pertains to cancer treatment, may still be in its infancy relative to well-established modalities such as chemotherapy, radiation, and surgery, major strides made in the past several decades have allowed translation of basic science discoveries in this field into clinical reality. The discovery of key molecular modulators of angiogenesis, notably Vascular Endothelial Growth Factor (VEGF), has catalyzed the development of numerous neutralizing therapeutic agents. The validity of VEGF inhibition as a therapeutic strategy has been well supported in randomized clinical trials, as well as U.S. Food and Drug Administration approval of the VEGF antagonists bevacizumab, sunitinib, sorafinib, pegaptinib and ranibizumab. Accordingly, this review will 1) briefly review the basic molecular biology of VEGF and 2) summarize recent progress in targeting the VEGF molecular pathway as therapy for angiogenic diseases such as cancer and age-related macular degeneration.
INTRODUCTION
The concept of tumor angiogenesis may have originated as early as a century ago with the observation of tumors as well-vascularized entities. However, the concept of angiogenesis inhibition as a therapeutic strategy against angiogenesis-dependent diseases, such as cancer and ocular disorders is comparatively new. Judah Folkman hypothesized in 1971 that tumor growth is dependent on angiogenesis, the process by which new blood vessels form from preexisting vasculature (Folkman, 1971). Based on observations that tumor growth does not occur continuously but rather as an abrupt and rapid growth occurring after extended periods (up to years) of non-neovascularized tumor dormancy, an ‘angiogenic switch’ was postulated wherein, neovascularization proceeds and is required for tumor growth beyond the pre-angiogenic dimensions of a few millimeters (Folkman & Kalluri, 2004).
This new concept in cancer biology implied the existence of biological modulators of angiogenesis and therapeutic uses thereof. In the three ensuing decades, intensive research has yielded a wealth of knowledge regarding both activators and inhibitors of angiogenesis. Chief among these was the purification in 1989 by Napoleone Ferrara of Vascular Endothelial Growth Factor (VEGF), also known as VEGF-A, as a potent endothelial mitogen from bovine pituitary follicular cell conditioned media (Ferrara & Henzel, 1989). Notably, the same polypeptide had been previously isolated as Vascular Permeability Factor (VPF) by Harold Dvorak and colleagues (Senger et al., 1983). It is now known that VEGF plays a critical role in angiogenesis, vasculogenesis, and lymphangiogenesis, during embryonic and early post-natal development. Moreover, the role of VEGF as a key mediator of tumor angiogenesis is now well-established, and forms the basis for using anti-VEGF drugs to treat neovascular diseases. In light of these important advances, this article will 1) review the role of VEGF in tumor angiogenesis, and 2) summarize current clinical progress in therapeutic targeting of VEGF.
Structure of VEGF
The VEGF gene family consists of numerous members including VEGF-A, -B, -C, -D and PlGF. The most well-characterized member, VEGF-A, exists as a homodimeric glycoprotein comprised of two identical 23 kDa subunits (Ferrara & Henzel, 1989). Of the numerous alternatively spliced isoforms of human VEGF-A, the 23 kDa monomer most closely corresponds to VEGF165 (ie. 165 residues beyond the signal sequence), which is the most abundant and mitogenic VEGF isoform. Other main VEGF isoforms, including VEGF121, VEGF189, and VEGF206, arise from alternative splicing of the human VEGF-A gene which comprises eight exons and seven intervening introns (Fig. 1) (Ferrara et al., 2003). The longer VEGF189 and VEGF206 bind avidly to heparin and heparin-like moieties in the extracellular matrix, while VEGF121 lacks basic residues corresponding to exon 6 and 7, leading to loss of extracellular matrix sequestration. VEGF165, lacking exon 6, exists as both bound and freely diffusible protein with the bound fraction undergoing proteolysis by plasmin and matrix metalloproteinases (MMPs). Beside differences in bioavailability and bioactivity, these isoforms also have different receptor specificities. VEGF165, through exon 7-encoded domains, binds to the tyrosine kinase receptors VEGFR1/Flt1 and VEGFR2/KDR/Flk1, as well as Neuropilin (NRP)-1 and NRP-2, while VEGF145 binds exclusively to the latter (Soker et al., 1996) (Fig. 2).
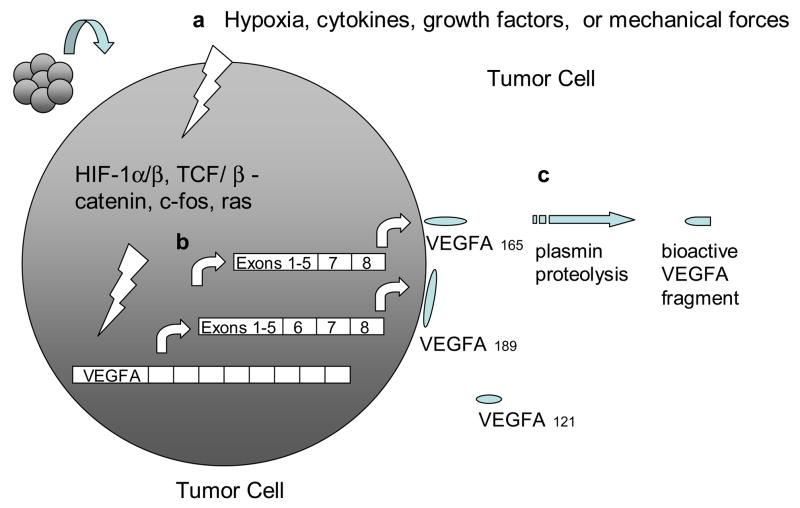
a, VEGFA gene transcription is induced by various extra- and intracellular signals, which ultimately target the regulatory elements within the VEGFA promoter region. b, alternative splicing of VEGFA transcript gives rise to VEGFA isoforms/polypeptides with disparate solubilities. c, proteolysis of matrix-bound VEGFA polypeptides releases bioactive fragments.
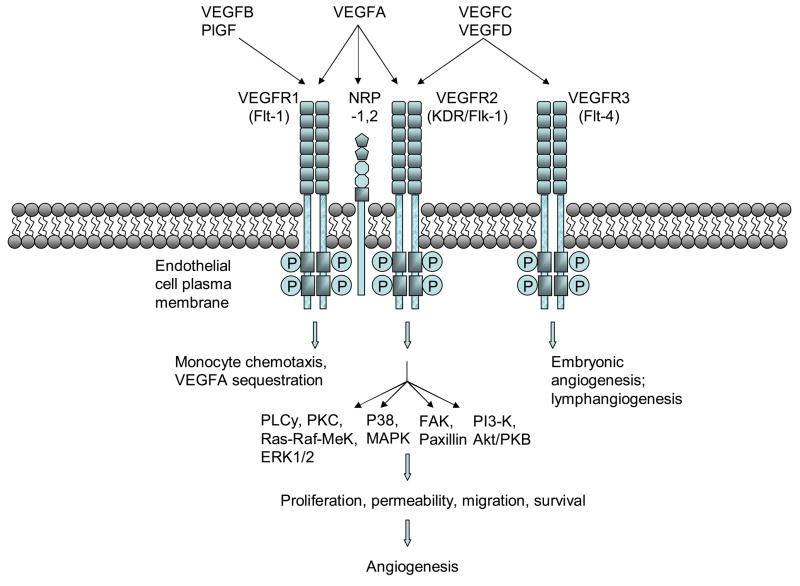
VEGF family members bind to specific VEGF receptors (VEGFR-1,2, or 3), which causes receptor dimerization, autophosphorylation of cytoplasmic tyrosine kinases, and activates intracellular signaling cascades that elicit various angiogenic programs.
Other members of the VEGF gene family also undergo alternative splicing with the exception of VEGF-C. Likewise, VEGF-B, whose biological functions are not entirely clear, and VEGF-D, which is involved in lymphangiogenesis, both have multiple alternatively spliced isoforms (Grimmond et al., 1996; Baldwin et al., 2001).
Expression and regulation
The expression, availability, and activity of VEGF-A are modulated by several mechanisms including hypoxia, oncogene and tumor suppressor dysregulation, transcription factors, inflammatory mediators, and mechanical forces of shear stress and cell stretch (Fig. 1).
The VEGF-A gene is one of numerous genes regulated by hypoxia-inducible factor (HIF)-1α. Under hypoxic conditions, such as within large solid tumors, HIF-1α dimerizes with the constitutively expressed HIF-1β to form a transcription factor that binds to hypoxic response elements (HRE) in the promoter region of the VEGF gene. Further interaction with transcriptional co-activators such as p300 and CBP induces the transcription of HIF target genes including VEGF, VEGFR1, and many other genes regulating angiogenesis, cell proliferation, cell survival, apoptosis, and motility (Lelievre et al., 2001; Semenza, 2003). In contrast, under normoxia, HIF-1α hydroxylation at proline 402 and 564 by three specific oxygen-dependent proline hydroxylases (PHD 1-3) promotes HIF-1α interaction with the von Hippel-Lindau (VHL) protein, a component of an E3 ubiquitin-protein ligase, targeting HIF-1α for proteosomal degradation. The role of HIF signaling in VEGF regulation is further demonstrated in renal clear-cell carcinoma (RCC) (Siemeister et al., 1996), wherein loss-of-function mutations in the VHL tumor suppressor produce impaired ubiquitination and proteosomal degradation of HIF-1α, engendering marked increases in VEGF-A transcription.
Other non-HIF-dependent pathways also regulate VEGF transcription in cancer. The ras oncogene participates in a signaling pathway that ultimately targets a consensus AP-1 sequence existing within the VEGF-A promoter to activate transcription in tumorigenesis (Eferl & Wagner, 2003). The human VEGF gene also contains seven consensus binding sites for β-catenin/TCF within its promoter region, facilitating VEGF expression in colon cancer (Easwaran, V et al., 2003).
Cytokine and growth factor regulation of VEGF expression has been thoroughly described. Inflammatory cytokines, including IL-1α, IL-1β, IL-6, TNFα, and prostaglandin E2, and growth factors such as transforming growth factor (TGF)α, TGFβ, platelet-derived growth factor BB (PDGF-BB), epidermal growth factor (EGF), fibroblast growth factor 4, or keratinocyte growth factor have all been reported to induce VEGF expression (Ben-Av, 1995; Neufeld, 1999). Furthermore, mechanical forces such as stretch and shear stress promote VEGF expression (Egginton et al., 2001).
Once expressed, a fraction of secreted VEGF protein becomes sequestered to the ECM, an may thereby constitute a reserve of available growth factor that can be released through proteolysis of the VEGF protein by either plasmin or matrix metalloproteinases into truncated freely soluble bioactive peptides (Houk, 1992; Lee, 2005). In contrast, recent reports suggest that plasmin proteolysis generates bioactive VEGF fragments, but with diminished mitogenic activity during wound repair (Eming & Krieg, 2006). Activated VEGF may then bind to two types of receptor tyrosine kinases, VEGF receptor (VEGFR)-1 (Flt-1) or VEGFR2 (Flk-2, KDR). Furthermore, VEGF-A isoform VEGF165 but not VEGF110 or VEGF121 additionally binds to Neuropilin-1 (NRP1) and NRP2. NRP1, which lacks a tyrosine kinase domain, is thought to serve as a co-receptor to VEGFR2 and enhances its signaling by presenting VEGF165 to VEGFR2 (Soker et al., 1998). In contrast, VEGF-B and PlGF specifically binds to VEGFR1 and not VEGFR2, and VEGF-C and –D bind specifically to VEGFR2 and VEGFR3 (Flt-4) and not VEGFR1 (Fig. 2).
Biological function
VEGF is involved in vasculogenesis, angiogenesis, and lymphangiogenesis during embryonic and postnatal development. Both single allele deletion of VEGF and gene knockouts of either VEGFR1 or VEGFR2 produce embryonic lethality from vasculogenic or angiogenic deficits (Ferrara et al., 1996; Shalaby et al., 1995; Fong et al., 1995). VEGF has subsequently been implicated in a variety of functions during adult physiology including ovarian angiogenesis, endochondral bone formation, tissue regeneration, haematopoietic stem cell survival, erythropoietin regulation, and pathological processes such as neoplastic, hematologic, ocular, inflammatory, and ischemic diseases (Ferrara et al., 2003; Zelzer, 2004; Gerber et al., 2002; Jacobi et al., 2004; Tam et al., 2006). Although it was initially suspected that VEGF is mainly involved in the maintenance of nascent vessels and that established vasculature are less dependent on VEGF, recent data suggests that vascular beds in the adult intestine, endocrine pancreas, thyroid and liver actively require VEGF for their maintenance, without which the complexity of the capillary network undergoes partial regression (Kamba et al., 2006; Wei & Kuo, unpublished observations).
In tumor angiogenesis, VEGF is released by tumor cells, as well as, tumor-infiltrating cells, including fibroblasts and monocyte/macrophages (MOs). Fibroblasts and MOs are recruited to tumor stroma following tumor cell-secreted chemotatic factors, PDGF AA and VEGFA, respectively (Dong et al., 2004; Clauss, 1996). VEGF action is directed primarily toward vascular endothelial cells. Other cell types, including neurons, osteoblasts, pancreatic duct cells, retinal progenitor cells, and megakaryocytes express VEGFR2, but at lower levels than do vascular endothelial cells, which may in part explain VEGF specificity for endothelial cells (Matsumoto & Claesson-Welsh, 2001). Moreover, tumor endothelial cells express several-fold higher levels of VEGFR2 than normal vasculature. (Plate et al., 1994). Hence, the expression of VEGF in ischemic areas, combined with the upregulation of VEGFR2 in tumor vascular endothelial cells may contribute to the specificity and relatively benign side-effect profile associated with VEGF antagonists.
VEGF inhibition “normalizes” the tumor vasculature, both reducing the microvessel density as well as reversing the pathophysiologic detachment of tumor pericytes from tumor endothelium (Inai et al., 2004). It has been proposed that such vascular normalization alters tumor insterstitial pressure, allowing improved delivery of chemotherapy to tumors and thereby facilitating the successful addition of anti-angiogenic therapy to chemotherapy observed in clinical trials (reviewed below) (Jain, 2005).
The pro-angiogenic actions of VEGF are thought to primarily occur through activation of VEGFR2 (Fig. 2). Although VEGF binds to VEGFR1 with greater affinity (Kd ~ 10–20pmol/L) than VEGFR2 (Kd ~ 75–125pmol/L), (de Vries et al., 1992; Terman, et al., 1992) VEGFR2 is responsible for most VEGF angiogenic activity including, vascular endothelial cell permeability, proliferation, migration, and survival. VEGF stimulation of VEGFR1 has been reported to result in weaker tyrosine kinase activity in comparison to VEGFR2 (Waltenberger et al., 1994), and genetic deletion of VEGR1 leads to vascular overgrowth and embryonic lethality (Fong et al., 1995); hence VEGFR1 has been proposed to negatively regulate VEGFR2 activity by serving as a decoy receptor to VEGF. Surprisingly, mice bearing genetic deletion of the VEGFR1 tyrosine kinase domain do not exhibit embryonic lethality, which may suggest that the VEGFR1 ecto- and transmembrane domains suffice to sequester VEGF (Hiratsuka et al., 1998). Nonetheless, VEGFR1 has been reported to participate in monocyte/macrophage migration and chemotaxis, matrix metalloproteinase (MMP)-9 expression, and hematopoiesis (Ferrara et al., 2003).
Upon VEGF binding, VEGFR2 undergoes dimerization and tyrosine kinase autophosphorylation, which, in turn, activates various signaling cascades. DNA replication and cell proliferation is thought to be mediated by both the Ras-Raf-MEK-ERK and MAPK pathways; cell survival, through PI3-Kinase and Akt/PKB activation; and cell migration through either FAK and Paxillin, PI3Kinase/Akt, or MAPK activation (Byrne et al., 2005).
VEGF and disease
VEGF is a key effector of many post-natal pathological processes and diseases in the adult, and hence represents an important target of currently available and developing pharmacologic therapies. These conditions include neoplastic (solid tumors and hematological malignancies), ocular, inflammatory, vascular, and ischemic diseases (Ferrara et al., 2003).
The central role of VEGF in tumor angiogenesis and neoplastic diseases has been well-established. Although VEGF is one of many pro-angiogenic factors that drive tumor angiogenesis, simply targeting VEGF alone, by soluble VEGFR or monoclonal anti-VEGF antibodies, suffices to significantly impair tumor angiogenesis and growth (Kuo et al., 2001; Hurwitz et al. 2004). This pivotal role of VEGF in tumor angiogenesis may be attributable in part to its mitogenic potency and participation in multiple angiogenic processes (including endothelial cell proliferation, migration, survival, and chemotaxis), which may synergistically decouple with VEGF inhibition. Consistent with this notion of tumor dependence on angiogenesis, and thus, VEGF, are the observations that a vast array of cancers are associated with VEGF mRNA expression, including melanoma, colorectal, gastric, breast, lung, and renal cell carcinomas (RCC), and that patients with tumor types that expressed high levels of VEGF mRNA (e.g. RCC) statistically had lower 5-year survival rates (Berger et al., 1995). As discussed below, the efficacy of monoclonal antibodies and small molecules inhibitors targeting VEGF in treating RCC, breast, lung, and metastatic colorectal cancers, further underscore the role of VEGF in human neoplastic pathophysiology. In addition to solid tumors, many hematological malignancies, including T cell lymphoma, acute lymphoblastic leukemia, Burkitt’s lymphoma, acute lymphocytic leukemia, and chronic myelocytic leukemia are also associated with increased VEGF expression (Gerber & Ferrara, 2003).
Similarly, VEGF has been implicated in the pathogenic neovascularization of many human ocular diseases, including age-related macular degeneration (AMD), diabetic retinopathy (DR), retinopathy of prematurity, retinal vein occlusion, and iris and corneal neovascularization (Aiello et al., 1994; Kliffen et al., 1997; Lashkari et al., 2000; Philipp et al., 2000). Though differences in the complex pathogenesis of these disorders clearly exist, they generally all entail ischemia-induced upregulation of VEGF, subsequent neovascularization and vascular leakage, and may ultimately lead to associated morbidities such as vitreous hemorrhages, retinal detachment, neovascular glaucoma, and blindness (Sivak-Callcott et al., 2001). As with neoplastic diseases, anti-VEGF therapy (ranibizumab and pegaptanib sodium are discussed below) against neovascular ‘wet’ AMD has proven to be efficacious treatment. Other conditions involving VEGF, include inflammatory disorders such as rheumatoid arthritis, psoriasis, and atherosclerosis; vascular diseases, such as infantile hemangiomas; and ischemic conditions, such as myocardial, limb, and focal cerebral ischemia (Folkman, 1995; Celletti et al., 2001; Greenberg & Jin, 2005).
Clinical trials of VEGF Inhibitors
The recent success of a growing number of VEGF antagonists in clinical trials underscores the critical role of VEGF in neovascular diseases and the efficacy of targeting VEGF as a therapeutic strategy. While the strategies for VEGF inhibition include diverse approaches such as soluble receptors, anti-receptor antibodies and aptamers (Holash, J. et al, 2002; Zhang, Miao, 2006; Gragoudas, 2004), we focus below on clinical experience with monoclonal antibody and small molecule inhibitor approaches for which phase III data are available (Table 1).
Table 1
Pivotal anti-VEGF phase III clinical trials (acronyms are elaborated below table)
| Anti-VEGF drug | Indication | Phase III Trials | Results (control vs. anti-VEGF drug/combination) | Status of trial/drug | |||
|---|---|---|---|---|---|---|---|
| median overall survival (OS) | progression- free survival (PFS) | duration of response (DR) | objective response (OR) | √ = completed;√√ FDA- approved; X= on-going | |||
| Monoclonal Antibody | (months) | (months) | (months) | % | |||
| IFL −/+ bevacizumab | first-line MCC | AV2107g Hurwitz et al., ’04 | 15.6 vs. 20.3 | 6.2 vs. 10.6 | 7.1 vs. 10.4 | 34.8 vs. 44.8 | √, √√, improved OS, PFS, DR, OR |
| FOLFOX4 −/+ bevacizumab | 2nd-line MCC | ECOG 3200 Giantonio et al., ’05 | 10.8 vs. 12.9 | 4.8 vs. 7.2 | 9.2 vs. 21.8 | √, √√, improved OS, avastin-only arm discont’d | |
| paclitaxel & carboplatin −/+ bevacizumab | first-line NSCLC | ECOG 4599 Sandler et al., ’06 | 10.3 vs. 12.3 | 4.5 vs. 6.2 | 15 vs. 35 | √, √√, improved OS, PFS, OR | |
| paclitaxel −/+ bevacizumab | first-line MBC | ECOG 2100 Miller et al., ’05b | 25.2 vs. 28.4 p=0.1 | 6.11 vs. 11.4 | 13.8 vs. 29.9 | X, FDA review, improved PFS, OR; OS data immature | |
| Small molecule antagonists | |||||||
| placebo vs. sorafenib | 2nd-line mRCC | TARGETs Escudier et al., ’07 | 15.9 vs. 19.3*(*48% crossover) | 2.8 vs. 5.5* | 0, 2, 53 vs. <1, 10, 74* * complete/partial-response, stable disease | X, √√, improved PFS study unblinded | |
| IFN-alfa vs. sunitinib malate | first-line mRCC | NCT000-98657/-83889 Motzer et al., ’07 | median survival data not mature | 5 vs. 11 | 6 vs. 31 | X, √√, study unblinded, improved PFS & OR; OS data pending | |
| placebo vs. sunitinib malate | imatinib-resistant GIST | NCT00075218 Demetri et al. ’06 | 56.9% vs. 79.4%**6 month survival ASCO ’06 | 1.6 vs. 6.8 | 0 & 48 vs. 7 & 58* *partial response, stable disease, >22wk | √, √√, improved PFS, OS; study unblinded | |
| Humanized anti-VEGF Fab fragment | Loss of visual acuity % who lost <15 letters | Severe vision loss %who lost ≥30 letters | % Legally blind ≤20/200 | Gain in visual acuity (% of subjects) gain of ≥ 15 letters | |||
| 0.3&0.5mg ranibizumab (Rz) vs. sham injection | wet AMD | MARINA trial RosenfeldRosenfeld et al., ’06 | 1 year: 95 & 95 vs. 62% 2 years: 92 & 90 vs. 53% | 0.8 & 1.2 vs. 14.3% 3.4 & 2.5 vs. 22.7% | 12.2 &11.7 vs 43 14.7 &15 vs 47.9 | √, √√ improved visual acuity | |
| 0.3 & 0.5mg Rz + sham PDT vs. sham ocular injections + PDT | wet AMD | ANCHOR trial Brown et al., ’06 | 94 & 96 vs. 64% | 0 & 0 vs. 13.3% | 22.1 & 16.4% vs. 60.1% | 35.7 & 40.3% vs. 5.6 % | √, 2-year data reported at Retina ’07; study extended as HORIZON |
| Pegylated oligonucleotide aptamer | |||||||
| 0.3, 1, & 3mg pegaptanib sodium vs. sham | wet AMD | VISION trials Gragoudas et al.,’04 (Chakravarthy et al., ’06)* | 70, 71, & 65 vs. 55% 0.3, 1, 3mg vs sham, 13.5mo (59 vs. 45%)* (0.3mg & 25.5 months)* | 10, 8 & 14 vs. 22% (13.5 months) | 38, 43 & 44% vs. 56% (13.5 months) | 6, 7, 4 vs. 2% (13.5 months) | √, √√ improved visual acuity |
irinotecan/5-FU/leucovorin (IFL); oxaliplatin/5-FU/leucovorin (FOLFOX4); verteporfin photodynamic therapy (PDT); metastatic colorectal cancer (MCC); non-squamous, non-small cell lung cancer (NSCLC); metastatic breast cancer (MBC); metastatic renal cell carcinoma (mRCC); gastrointestinal stromal tumors (GIST); neovascular age-related macular degeneration (wet AMD)
Bevacizumab (Avastin) is a recombinant humanized monoclonal antibody targeting VEGF-A and all of its isoforms. In the randomized double-blind placebo-controlled phase III trial that ultimately led to FDA approval in February 2004, bevacizumab was administered in combination with irinotecan and bolus 5-FU/leucovorin (IFL) chemotherapy versus IFL alone as first-line therapy for metastatic colorectal cancer. The bevacizumab-IFL regimen improved median survival from 15.6 to 20.3 (p < 0.001), progression free survival (6.2 to 10.6 months), and time to progression (6.7 to 8.8 months) in parallel (Hurwitz, 2004). Improvements in overall survival (10.8 vs 12.9 months) and time to progression (4.6 vs 7.2 months) were also noted in a separate phase III study examining oxaliplatin, leucovorin, and 5-fluorouricil (FOLFOX 4) with and without bevacizumab as second-line therapy for previously-treated advanced colorectal cancer. Notably, the single-agent bevacizumab arm of the study was discontinued due to efficacy that was clearly inferior to the FOLFOX 4-only arm, suggesting the importance of concomitant chemotherapy (Giantonio, 2005). The addition of bevacizumab to chemotherapy also improved survival in phase III studies of advanced non-squamous, non-small cell lung cancer (NSCLC) (overall survival 10.3 vs 12.3 months, p = 0.0075), and potentially in locally recurrent or metastatic breast cancer (MBC) (Sandler, 2006). Although a phase III trial demonstrated that the addition of bevacizumab to capecitabine improved objective response rates (9.1% vs 19.8% vs., p=0.001) in previously-treated MBC patients, significant improvements were not observed for either progression free survival or overall survival (Miller, 2005a). This improvement in response rates prompted a separate phase III trial, testing the efficacy of bevacizumab in combination with paclitaxel against previously untreated MBC. In this setting, bevacizumab improved objective response rates and progression free survival, while overall survival data is still pending (Miller et al., 2005b). Similar data is expected in renal cell carcinoma. In general bevacizumab has been well tolerated with known toxicities including hypertension, proteinuria, bleeding and thrombosis.
Small molecule antagonists of VEGF receptors have also recently demonstrated efficacy in randomized trials. These agents, including sorafenib (BAY 43-9006) and sunitinib malate (Sutent) are orally bioavailable and exhibit broad-spectrum activity against numerous kinases including VEGF receptors. Sorafenib received FDA approval for advanced/metastatic RCC following phase III data indicating improved progression-free survival (2.8 vs 5.5 weeks, p <0.001) and overall survival (15.9 vs 19.3 months, p = 0.02, not significant by O’Brien–Fleming boundaries for statistical significance) (Escudier, 2007). This was achieved despite crossover of nearly half of the patients from placebo to sorafenib after interim analysis demonstrated a significant sorafenib benefit, leading to unblinding of the study. Similarly, sunitinib received initial FDA approval in January 2006 for imatinib-resistant gastrointestinal stromal tumors (GIST) and for advanced renal cell carcinoma (RCC), the latter based on phase II response rates. Subsequent phase III data in metastatic RCC confirmed both improved progression-free survival for sunitinib versus IFN-α (11 vs 5 months), as well as objective response rate (31 vs 6%), with survival analysis pending (Motzer, 2007). While the broad specificity of sorafenib and sunitinib likely contribute to their efficacy, this also precludes definitive attribution of activity to VEGF antagonism.
Finally, VEGF inhibition has also been successfully used for treatment of neovascular ‘wet’ age-related macular degeneration (AMD). Pegaptanib sodium (Macugen), a pegylated oligonucleotide aptamer selectively targeting VEGF165, and ranibizumab (Lucenis), a recombinant, humanized anti-VEGF Fab fragment, have both received FDA approval for treatment of neovascular AMD. Notably, ranibizumab both retarded vision loss and improved vision in a signification fraction of patients and was superior to photodynamic therapy (Rosenfeld, 2006). An ongoing NIH study for wet AMD is comparing ranibizumab vs Avastin, as the latter may be significantly less costly (Steinbrook, 2006).
Perspective
The concept of employing angiogenesis inhibition as a therapeutic strategy against neovascular diseases originated several decades ago. However, the breathtaking pace of both basic and clinical progress in this area has now transformed this elegant hypothesis into clinical reality confirmed by numerous randomized clinical trials. These successes have established anti-angiogenic therapy in general, and VEGF inhibition in specific, as a new treatment modality and in fact a new standard of care for numerous solid tumor subtypes. In the near future, other approaches to VEGF inhibition such as soluble receptor and anti-receptor strategies should complement continued development of the current repertoire of monoclonal antibody and small molecule inhibitors. Further, the development of therapeutics directed against entirely different non-VEGF based angiogenic processes, and combination thereof with VEGF inhibition, should continue to mark basic science, translational and clinical investigations into angiogenesis inhibition as a vibrant and exciting area for the foreseeable future.
Acknowledgments
We thank members of the Kuo laboratory for helpful comments and Kevin Wei for permission to cite unpublished observations. Q.T.H. was supported by the Stanford Medical Scholars program. This review was supported by grants from the Brain Tumor Society, Prostate Cancer Foundation and NIH grants 1 R01 CA95654-01, 1 R01 NS052830-01 and 1 R01 HL074267-01 to C. J. K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England Journal of Medicine. 1994;331:1480–1487. [Abstract] [Google Scholar]
- Baldwin ME, Roufail S, Halford MM, Alitalo K, Stacker SA, Achen MG. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. The Journal of Biological Chemistry. 2001;276:44307–44314. [Abstract] [Google Scholar]
- Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Letters. 1995;372:83–87. [Abstract] [Google Scholar]
- Berger DP, Herbstritt L, Dengler WA, Marme D, Mertelsmann R, Fiebig HH. Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Annals of Oncology. 1995;6:817–825. [Abstract] [Google Scholar]
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. The New England Journal of Medicine. 2006;355:1432–1444. [Abstract] [Google Scholar]
- Byrne AM, Bouchier-Hayes DJ, Harmey JH. Angiogenic and cell survival functions of Vascular Endothelial Growth Factor (VEGF) Journal of Cellular and Molecular Medicine. 2005;9:777–794. [Europe PMC free article] [Abstract] [Google Scholar]
- Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nature Medicine. 2001;7:425–429. [Abstract] [Google Scholar]
- Chakravarthy U, Adamis AP, Cunningham ET, Jr, Goldbaum M, Guyer DR, Katz B, Patel M VISION Clinical Trial Group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Opthalmology. 2006;113:1508–1521. [Abstract] [Google Scholar]
- Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. The Journal of Biological Chemistry. 1996;271:17629–17634. [Abstract] [Google Scholar]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. The Lancet. 2006;368:1329–1338. [Abstract] [Google Scholar]
- DeVries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. [Abstract] [Google Scholar]
- Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, Fuh G, Gerber HP, Ferrara N. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. The European Molecular Biology Organization Journal. 2004;23:2800–2810. [Europe PMC free article] [Abstract] [Google Scholar]
- Easwaran V, Lee SH, Inge L, Fantl WJ, et al. β-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Research. 2003;63:3145–3153. [Abstract] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double edged sword in tumorigenesis. Nature Review Cancer. 2003;3:859–868. [Abstract] [Google Scholar]
- Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovascular Research. 2003;49:634–646. [Abstract] [Google Scholar]
- Eming SA, Krieg T. Molecular mechanisms of VEGF-A action during tissue repair. The Journal of Investigative Dermatology. 2006;11:79–86. [Abstract] [Google Scholar]
- Escudier B, Szczylik C, Eisen T, Stadler WM, Schwartz B, Shan M, Bukowski RM. Journal of Clinical Oncology; 2005 ASCO Annual Meeting Proceedings; 2005. p. 4510. Part I of II (June 1 Supplement) [Google Scholar]
- Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. The New England Journal of Medicine. 2007;356:125–134. [Abstract] [Google Scholar]
- Ferrara N, Henzel JH. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochemical and Biophysical Research Communications. 1989;161:851–858. [Abstract] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–342. [Abstract] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003;9:669–676. [Abstract] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. The New England Journal of Medicine. 1971;285:1182–86. [Abstract] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature. 1995;1:27–31. [Abstract] [Google Scholar]
- Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. [Abstract] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. The role of Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. [Abstract] [Google Scholar]
- Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. The Journal of Molecular Medicine. 2003;81:20–31. [Abstract] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. [Abstract] [Google Scholar]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. High-dose bevacizumab in combination with FOLFOX-4 improves survival in patients with previously treated advanced colorectal cancer. Eastern Cooperative Oncology Group (ECOG), study. 2005:E3200. abstract 169a. [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptinib for neovascular age-related macular degeneration. The New England Journal of Medicine. 2004;351:2805–2816. [Abstract] [Google Scholar]
- Greenberg DA, Kunlin J. From angiogenesis to neuropathology. Nature. 2005;438:954–959. [Abstract] [Google Scholar]
- Grimmond S, Lagercrantz J, Drinkwater C, Silins G, Townson S, Pollock P, et al. Cloning and characterization of a novel human gene related to vascular endothelial growth factor . Genome Research. 6:124–131. [Abstract] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9349–9354. [Europe PMC free article] [Abstract] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Yancopoulos GD, Rudge JS, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11393–11398. [Europe PMC free article] [Abstract] [Google Scholar]
- Houk KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. The Journal of Biological Chemistry. 1992;267:26031–26037. [Abstract] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Kabbinavar F, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. The New England Journal of Medicine. 2004;350:2335–2342. [Abstract] [Google Scholar]
- Inai T, Mancuso MR, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. American Journal of Pathology. 2004;165:35–52. [Europe PMC free article] [Abstract] [Google Scholar]
- Jacobi J, Tam BY, Wu G, Hoffman J, Cooke JP, Kuo CJ. Adenoviral gene transfer with soluble vascular endothelial growth factor receptors impairs angiogenesis and perfusion in a murine model of hindlimb ischemia. Circulation. 2004;110:2424–2429. [Abstract] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. [Abstract] [Google Scholar]
- Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. American Journal of Physiology-Heart and Circulatory Physiology. 2006;290:H560–576. [Abstract] [Google Scholar]
- Kuo CJ, LaMontagne KR, Jr, Folkman J, Mulligan RC, Javaherian K, et al. Comparative evaluation of the antitumor activity of antitumor activity of antiangiogenic proteins delivered by gene transfer. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4605–4610. [Europe PMC free article] [Abstract] [Google Scholar]
- Kliffen M, Sharma HS, Mooy CM, Kerkvliet S, de Jong PT. Increased expression of angiogenic growth factors in age-related maculopathy. British Journal of Ophthalmology. 1997;81:154–162. [Europe PMC free article] [Abstract] [Google Scholar]
- Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. American Journal of Pathology. 2000;156:1337–1344. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. Journal of Cell Biology. 2005;169:681–691. [Europe PMC free article] [Abstract] [Google Scholar]
- Lelievre E, Lionneton F, Soncin F, Vandenbunder B. The Ets family contains transcriptional activators and repressors involved in angiogenesis. The International Journal of Biochemistry and Cell Biology. 2001;33:391–407. [Abstract] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Science’s Signal Transduction Knowledge Environment. 2001;112:21. [Abstract] [Google Scholar]
- Miao HQ, Hu K, Jimenez X, Navarro E, Zhang H, Lu D, Ludwig DL, Balderes P, Zhu Z. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochemical and Biophysical Research Communications. 2006;345:438–445. [Abstract] [Google Scholar]
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacer L, Dicler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. Journal of Clinical Oncology. 2005a;23 (4):792–799. [Abstract] [Google Scholar]
- Miller KD, Wang M, Gralow J, et al. E2100 study: A randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. Presentation at December, 2005 San Antonio Breast Cancer Symposium; San Antonio. 2005b. Texas (streaming webcast available on-line at http://www.sabcs.org/SymposiumOnline/index.asp#webcast).
- Motzer RJ, Hutson E, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Kim ST, Baum CM, Figlin RA. Phase III randomized trial of sunitinib malate (SU11248) versus interferon-alfa (IFN-α) as first-line systemic therapy for patients with metastic renal cell carcinoma (mRCC) Journal of Clinical Oncology 2006 ASCO Annual Meeting Proceedings Part I. 2006;24(18S June 20 Supplement):LBA3. [Google Scholar]
- Motzer RJ, Hutson E, Tomczak, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England Journal of Medicine. 2007;356:115–124. [Abstract] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltrok Z. Vascular endothelial growth factor (VEGF) and its receptors. The Federation of American Societies for Experimental Biology Journal. 1999;13:9–22. [Abstract] [Google Scholar]
- Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. International Journal of Cancer. 1994;59:520–529. [Abstract] [Google Scholar]
- Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Investigative Ophthalmology & Visual Science. 2000;41:2514–2522. [Abstract] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. The New England Journal of Medicine. 2006;355:1419–1431. [Abstract] [Google Scholar]
- Sandler AB, Gray R, Brahmer J, Dowlati A, Schiller JH, Perry MC, Johnson DH. Randomized phase II/III Trial of paclitaxel (P) plus carboplatin with or without bevacizumab (NSC #704865) in patients with advanced non-squamous non-small cell lung cancer (NSCLC): An Eastern Cooperative Oncology Group (ECOG) Trial – E4599. Journal of Clinical Oncology; 2005 ASCO Annual Proceedings; 2005. abstract 4. [Google Scholar]
- Sandler AB, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Rogerio L, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. The New England Journal of Medicine. 2006;355:2542–2550. [Abstract] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. [Abstract] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Review Cancer. 2003;3:721–732. [Abstract] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breiman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. [Abstract] [Google Scholar]
- Siemeister G, Weindel K, Mohrs K, Barleon B, Martiny-Baron G, Marme D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau protein. Cancer Research. 1996;56:2299–2301. [Abstract] [Google Scholar]
- Sivak-Callcott, O’Day DM, Gass JDM, Tsai JC. Evidenced-based recommendations for the diagnosis and treatment of neovascular glaucoma. Opthalmology. 2001;108:1767–1776. [Abstract] [Google Scholar]
- Soker S, Fidder H, Neufeld G, Klagsburn M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumour cells that bind VEGF165 via its exon 7-encoded domain. The Journal of Biological Chemistry. 1996;271:5761–5767. [Abstract] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelal and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. [Abstract] [Google Scholar]
- Steinbrook R. The price of sight—ranibizumab, bevacizumab, and the treatment of macular degeneration. The New England Journal of Medicine. 2006;355:1409–1412. [Abstract] [Google Scholar]
- Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Niyak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nature Medicine. 2006;12:793–800. [Abstract] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochemical and Biophysical Research Communications. 1992;187:1579–1586. [Abstract] [Google Scholar]
- Waltenberger J, Claesson-Welsh LC, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. The Journal of Biological Chemistry. 1994;269:26988–26995. [Abstract] [Google Scholar]
- Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biocel.2007.04.010
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2752727?pdf=render
Citations & impact
Impact metrics
Article citations
ALK5/VEGFR2 dual inhibitor TU2218 alone or in combination with immune checkpoint inhibitors enhances immune-mediated antitumor effects.
Cancer Immunol Immunother, 73(10):190, 06 Aug 2024
Cited by: 0 articles | PMID: 39105882 | PMCID: PMC11303640
Impaired Testicular Function without Altering Testosterone Concentration Using an Anti-Follicular-Stimulating Hormone Receptor (Anti-FSHr) Single-Chain Variable Fragment (scFv) in Long-Tailed Macaques (Macaca fascicularis).
Animals (Basel), 13(14):2282, 12 Jul 2023
Cited by: 0 articles | PMID: 37508065 | PMCID: PMC10376863
The splicing factor YBX1 promotes the progression of osteosarcoma by upregulating VEGF165 and downregulating VEGF165b.
Heliyon, 9(8):e18706, 26 Jul 2023
Cited by: 2 articles | PMID: 37554848 | PMCID: PMC10405001
Profiling and targeting cancer stem cell signaling pathways for cancer therapeutics.
Front Cell Dev Biol, 11:1125174, 25 May 2023
Cited by: 5 articles | PMID: 37305676 | PMCID: PMC10247984
Review Free full text in Europe PMC
A systematic review, meta-analysis, and network analysis of diagnostic microRNAs in glaucoma.
Eur J Med Res, 28(1):137, 27 Mar 2023
Cited by: 2 articles | PMID: 36973823 | PMCID: PMC10041737
Review Free full text in Europe PMC
Go to all (99) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00075218
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Anti-VEGF and beyond: shaping a new generation of anti-angiogenic therapies for cancer.
Drug Discov Today, 16(23-24):1052-1060, 22 Aug 2011
Cited by: 22 articles | PMID: 21875682
Review
Therapeutic anti-VEGF antibodies.
Handb Exp Pharmacol, (181):131-150, 01 Jan 2008
Cited by: 39 articles | PMID: 18071944
Review
Antiangiogenesis beyond VEGF inhibition: a journey from antiangiogenic single-target to broad-spectrum agents.
Cancer Treat Rev, 40(4):548-557, 06 Dec 2013
Cited by: 45 articles | PMID: 24360358
Review
[VEGF, anti-vEGF and diseases].
Bull Acad Natl Med, 192(2):289-300; discussion 300-2, 01 Feb 2008
Cited by: 2 articles | PMID: 18819684
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R01 CA095654
Grant ID: 1R01 CA 95654-01
Grant ID: R01 CA095654-05
NHLBI NIH HHS (3)
Grant ID: R01 HL074267-04
Grant ID: 1R01 HL 074267-01
Grant ID: R01 HL074267
NINDS NIH HHS (3)
Grant ID: 1R01 NS 052830-01
Grant ID: R01 NS052830
Grant ID: R01 NS052830-04





