Abstract
Free full text

Ubiquitin-independent degradation of cell cycle inhibitors by the REGγ proteasome
Abstract
The cell cycle regulator p21Cip1 is degraded by proteasomes independently of ubiquitination. We now show that degradation of p21 in vivo does not require the 19S proteasome lid, which contains the ubiquitin binding subunit. Instead, the major proteasomal pathway for p21 degradation involves an alternative proteasome lid, the REGγ complex. REGγ binds to p21 in vivo, and deletion of p21’s REGγ binding site greatly extends its half-life. Knock-down of REGγ by RNA interference stabilizes p21, p21 has a significantly extended half life in REGγ −/− murine embryonic fibroblasts, and the p21 abundance is elevated in REGγ −/− mice. The role of REGγ in cell cycle regulation may extend beyond p21 regulation, because p16INK4A and p19Arf also bind to REGγ and are stabilized in REGγ deficient cells.
Introduction
The cell cycle inhibitor p21Cip1 is one member of an unusual group of proteins that can be degraded by proteasomes independently of ubiquitination. Evidence that conjugation to ubiquitin is unnecessary for proteasomal degradation of p21 includes: mutations in p21 that remove all lysine residues prevent p21 ubiquitination in vivo, but have no effect on p21 stability or the proteasome dependence of its degradation; endogenous cellular p21 is fully acetylated at its amino terminus thus eliminating the possibility that proteasomal degradation follows N-terminal ubiquitination; inactivation of either the major cellular E1 enzyme or the Nedd8 activating enzyme, both of which are essential components of the ubiquitination machinery, do not affect p21 degradation by the proteasome (Sheaff et al., 2000; Chen et al., 2004).
The mechanism by which proteasomes recognize p21 independently of ubiquitination is not understood. The most thoroughly analyzed precedent is ornithine decarboxylase (ODC) (Reviewed in Coffino, 2001), which has a specific binding partner, the antizyme protein, that directs it to proteasomes. No such binding partner has been identified for p21. Therefore, there has been speculation that unstructured proteins like p21 may gain direct access to the proteasome core without the need for a specific interaction mechanism. However, only a minor fraction of p21 in vivo exists in a free, unstructured state; almost all p21 is in heterotrimeric complexes with cyclins and cyclin-dependent kinases (Bloom et al., 2003).
The 26S proteasome is comprised of a 20S catalytic barrel-shaped core that is gated on both the top and bottom by 19S lids. The 19S lid is a highly conserved multiprotein complex that binds to polyubiquitin chains and opens the proteasome core in an ATP-dependent process (Reviewed in Hershko and Ciechanover, 1998). However, other proteasome forms utilize different lids to control access to the catalytic core. In particular, the REG complex (also known as 11S or PA28) is an ATP- and ubiquitin-independent proteasome activator that strongly enhances the catalytic activity of proteasomes in vitro (Realini et al., 1997). The REG family of activators has three members: α, β and γ, which share approximately 35% amino acid similarity (Ma et al., 1992; Realini et al., 1997). REG-α and β, which assemble into a heteroheptamer, are highly expressed in immune cells and are believed to function primarily, though perhaps not exclusively, in MHC-1 restricted immune responses (Reviewed in Kloetzel and Ossendorp, 2004). REGγ has a nuclear-restricted expression pattern and can be found independently or associated with 20S proteasomes as a homoheptameric lid.
Two groups independently generated REGγ-deficient mice and demonstrated defects in the regulation of mitosis and apoptosis (Murata et al., 1999; Barton et al., 2004), although the biological pathways mediating those effects have not been elucidated. While previous in vitro data suggested a role for REGγ in the proteasome-dependent degradation of short peptides, recent evidence demonstrated that REGγ is also responsible for the ubiquitin-independent degradation of the steroid receptor co-activator SRC-3/AIB1 in vivo (Li et al., 2006), and may be involved in the degradation of other proteins as well (Moriishi et al., 2003; Moriishi et al., 2007). Thus, the REGγ pathway represents a new, ubiquitin-independent mechanism for protein degradation by proteasomes. We demonstrate that this REGγ-dependent and ubiquitin-independent pathway governs the turnover of three important cell cycle regulators: p21Cip1, p16Ink4a and p19Arf.
Results
Proteasome Degradation of p21, in vitro and in vivo, Does Not Require the 19S Proteasome Lid
The molecular mechanism for the ubiquitin-independent proteasomal degradation of p21Cip1 has not been determined (Sheaff et al., 2000; Touitou et al., 2001; Bendjennat et al., 2003; Bloom et al., 2003; Jin et al., 2003; Chen et al., 2004). One prediction is that this mechanism might be independent of the19S proteasome lid, because this multi-protein complex is necessary for binding to polyubiquitin chains (Reviewed in Hershko and Ciechanover, 1998). We tested this prediction, both in vitro and in vivo, by comparing the requirement of the 19S proteasome lid for the degradation of two related CDK inhibitory proteins: p27Kip1, whose degradation is ubiquitin-dependent, and p21, whose degradation is independent of ubiquitin.
p21 and p27 were translated in vitro using a rabbit reticulocyte lysate system and then incubated with purified 20S proteasomes. p21 was rapidly degraded by 20S proteasomes, with a half-life of approximately 30 minutes (Figure 1A). In contrast, p27, which requires ubiquitination for turnover in vivo (Pagano et al., 1995), was not degraded by 20S proteasomes in vitro (Figure 1B). Because p21 is a small protein and natively unfolded (Kriwacki et al., 1996), it was possible that it could enter the proteasome by diffusion. To address this, we in vitro translated the N- and C- terminal halves of p27. Although both were smaller in molecular size than p21, neither was degraded at a measurable rate by purified 20S proteasomes (data not shown).
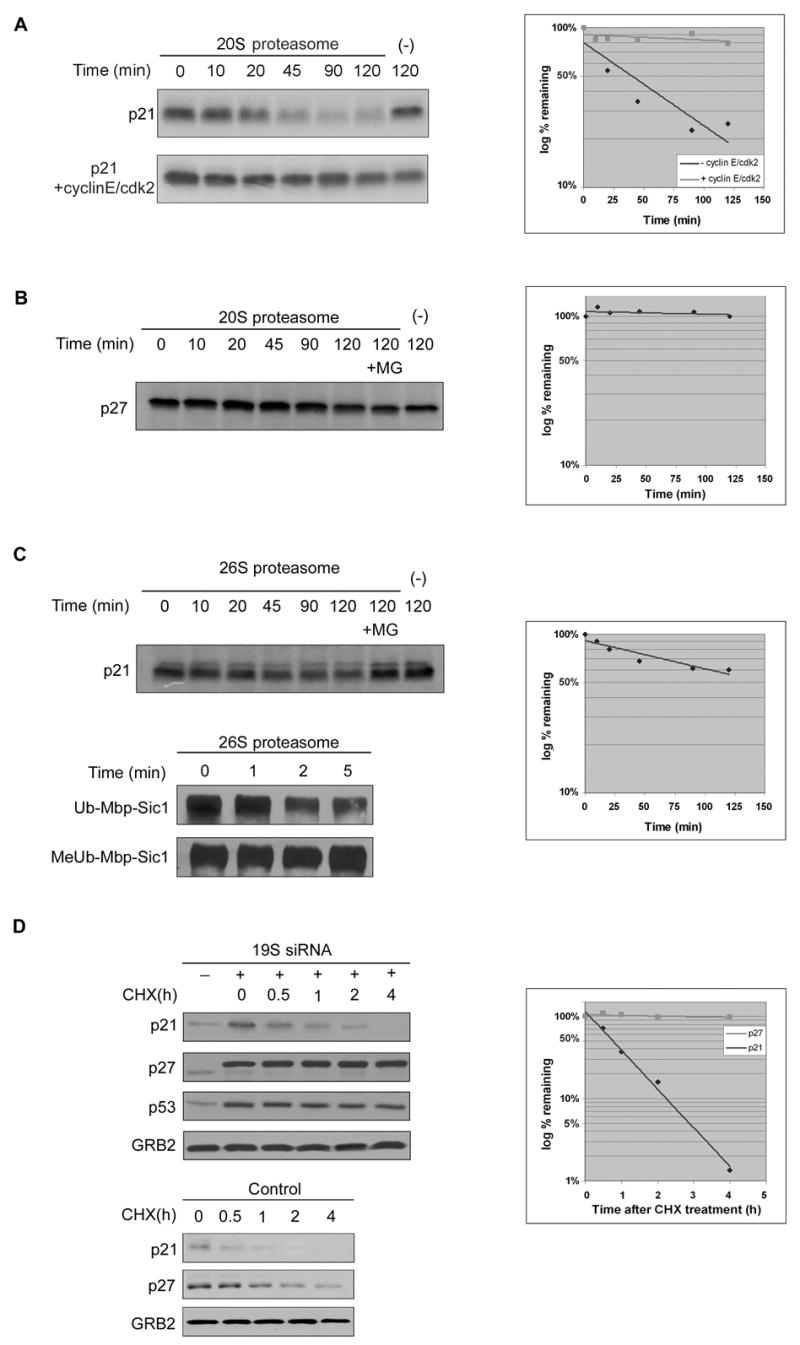
A. Equal amount of in vitro translated and 35S labeled p21 were incubated with purified 20S proteasomes either alone (upper panel) or in the presence of purified cyclin E/cdk2 complexes (lower panel) at 37°C for indicated times. The reaction products were analyzed by SDS-PAGE and detected by autoradiography.
B. Equal amount of in vitro translated and 35S labeled p27 were incubated at 37°C for indicated times in the presence or absence of purified 20S proteasomes.
C. Equal amount of in vitro translated and 35S labeled p21 were incubated at 37°C for indicated times in the presence or absence of purified 26S proteasomes. Ub-Mbp-Sic1 and MeUb-Mbp-Sic1 were used as control for activity of 26S proteasomes.
D. U2OS cells were transfected with siRNA against N2 subunit of the 19S proteasome. Cells were treated with cycloheximide for indicated times. GRB2 was used as a loading control for all experiments.
We further showed that the 19S lid could not facilitate p21’s proteasomal degradation. Thus, when p21 was incubated with 26S proteasomes (the 20S catalytic core plus the 19S lid), it was degraded at a slower rate than when incubated with the 20S core alone (Figure 1C). In contrast, ubiquitinated Mbp-Sic-1 was very rapidly degraded by the 26S proteasome with a half-life of less than 5 minutes (Figure 1C). Sic1 lacking polyubiquitin chains (MeUb-Mbp-Sic1) was not degraded by 26S proteasomes, showing that the contribution of the 19S lid was to allow recognition of ubiquitinated substrates.
We also addressed whether the 19S proteasome lid was required for p21 degradation in vivo. To block 19S function in vivo, we exposed cells to siRNA against its N2 subunit, an approach which has been previously reported to inhibit proteasome activity (Silva et al., 2005). We confirmed that this prevented the degradation of the ubiquitin-dependent proteasome substrates p27 and p53 (Figure 1D; see also Chen et al., 2004). In contrast, inhibition of 19S function had no measurable effect on the half-life of p21 (Figure 1D). The small increase in the steady state level of p21 that occurred after inhibition of the 19S lid was likely due to stabilization of p53 and thus an increase in p21 mRNA abundance (Chen et al., 2004). In summary, our previous results demonstrated that p21 degradation by proteasomes both in vitro and in vivo occurs independently of the 19S proteasome lid.
The REGγ Proteasome Activator Mediates p21 Degradation
In vivo, almost all p21 is assembled into heterotrimeric p21-cyclin-Cdk complexes (Bloom et al., 2003). We therefore tested whether this form of p21 was also a substrate for degradation by 20S proteasomes. p21 was translated in vitro and then incubated with recombinant cyclin E-Cdk2 complexes. Immunodepletion with anti-cyclin E antibodies showed that all of the p21 was bound to cyclin E-Cdk2 complexes (data not shown). In contrast to free p21, which was rapidly degraded when incubated with 20S proteasomes in vitro, p21 that was bound to cyclin E-Cdk2 was very stable (Figure 1A; see also Bloom et al., 2003). Thus, it was unlikely that 20S proteasomes could mediate p21 turnover in vivo. 26S proteasomes were also unlikely to mediate p21 turnover, because they were incapable of degrading p21 in vitro and knock-down of the 19S lid did not alter the rate of p21 degradation in vivo (Figures 1C and 1D).
In addition to the 26S complex, which is composed of the 20S catalytic core and the 19S lid, there is an alternative form of the proteasome in which an 11S regulatory cap, comprised of subunits from the REG protein family, gates access to the 20S catalytic core (Dubiel et al., 1992; Ma et al., 1992). REGγ is phylogenetically conserved nuclear protein that is present in organisms ranging from worms to human. Moreover, recent studies have shown that REGγ is necessary for the ubiquitin-independent proteasomal degradation of the steroid receptor coactivator-3 (SRC-3) in vivo (Li et al., 2006).
To explore whether REGγ might also be required for the ubiquitin-independent degradation of p21 in vivo, we first used RNA interference to inhibit its expression. Decreased expression of REGγ caused an increase in the steady state level of p21 protein (Figure 2A). The increased abundance of p21 was observed with two independent siRNAs against REGγ, as well as with a pool of 4 siRNAs against REGγ, thus ruling out the possibility of an off-target effect (Figure 2A). The increased steady state abundance of p21 was caused by a four-fold increase in its half-life, from 30 minutes to approximately 2 hours (Figure 2B). The residual slower turnover of p21 remained proteasome dependent (Figure 2B; see Discussion). Thus, knock-down of the 11S proteasome activator stabilized p21, whereas knock-down of the 19S lid did not.
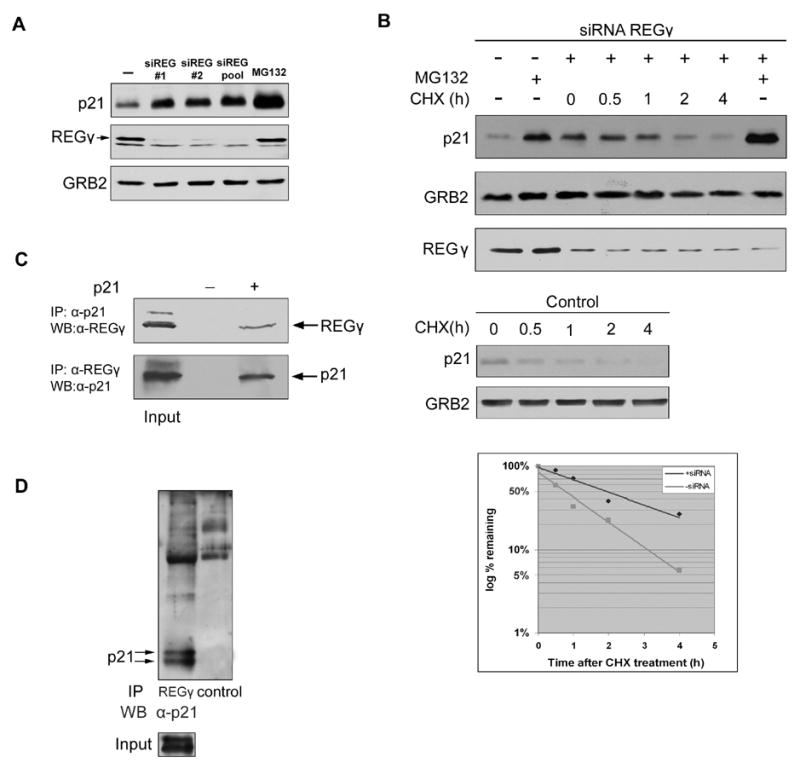
A. Effects of different siRNAs against REGγ on p21 level. U2OS cells were transfected with two individual siRNAs (#1 and #2) or a pool of four siRNAs against REGγ. p21 steady state levels were determined by Western blotting.
B. Effect of RNA interference of REGγ on p21 half life. U2OS cells were transfected with the siRNA pool against REGγ. Cells were treated with cycloheximide for indicated times. p21 half-lives in control U2OS cells were analyzed as well.
C. Interaction between transfected p21 and endogenous REGγ. 293 cells were transfected with p21 and treated with MG-132 for 4 hr. Cell lysates were immunoprecipitated with anti-p21 or anti-REGγ antibodies, and the associated protein was detected by Western blotting.
D. Interaction between endogenous p21 and REGγ. U2OS cells were treated with MG-132 for 6 hr and cell lysates were immunoprecipitated with anti-REGγ antibodies or irrelevant immune serum followed by Western blotting with anti-p21 antibodies.
To explore the mechanism by which REGγ promoted p21 turnover, we asked if the two proteins could bind to each other in vivo. After transfection of 293 cells with a p21 expression vector, the binding of exogenous p21 to endogenous REGγ could be detected by reciprocal co-immunoprecipitations with antibodies to either p21 or REGγ (Figure 2C). In a parallel experiment, exogenously overexpressed p27 did not bind to REGγ (data not shown). Endogenous p21 bound to REGγ was also detected by co-immunoprecipitation with anti-REGγ antibodies (Figure 2D). p21 was not detected in a control immunoprecipitation with an irrelevant immune serum (data not shown). To identify the region of p21 required for its interaction with REGγ, we generated several p21 truncation mutants. We found that the C-terminal half of p21, p21(77–164), bound to REGγ, whereas the amino terminal half of p21, p21(1–76), did not (Figures 3A and 3B). Further mapping showed that the REGγ interaction domain was deleted by truncating p21 at amino acid 140. We then refined its location with the internal deletion of residues 156–161, which completely abrogated the interaction (Figures 3A and 3B). To rule out the possibility that the 156–161 deletion non-specifically altered the overall conformation of p21, and thus had an indirect effect on REGγ binding, we tested its interaction with another p21 binding protein: PCNA. We found that p21Δ156–161 retained the same capacity to bind with PCNA as wild type p21 (Figure 3C; see also Touitou et al., 2001). To confirm that the binding of p21 to REGγ was required for its turnover, we expressed wild type and p21Δ156–161 in 293 cells. Exogenously expressed p21, like endogenous p21, had a half-life of less than one hour, whereas p21Δ156–161 was considerably more stable with a half-life greater than 6 hours (Figure 3D).
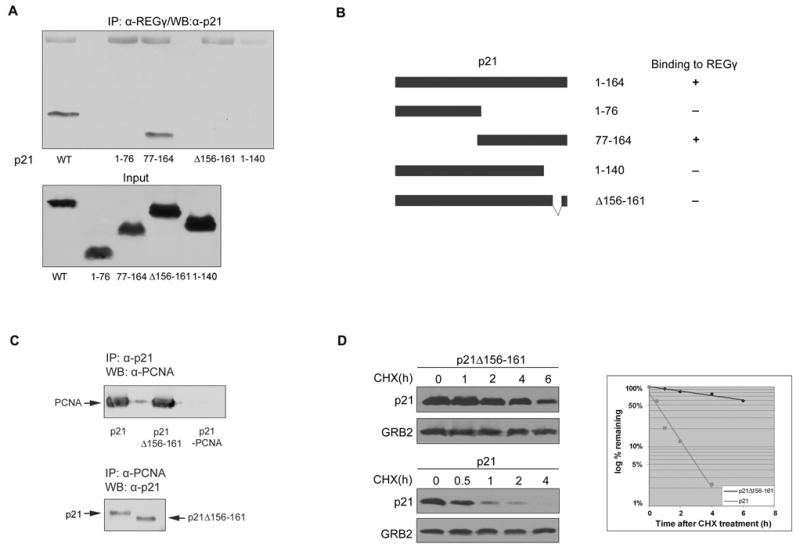
A. Interaction between endogenous REGγ and deletion mutants of p21. 293 cells were transfected with either wild type p21 or a deletion mutant as indicated. Cell lysates were immunoprecipitated with anti-REGγ antibodies followed by Western blotting with anti-p21 antibodies.
B. Schematic diagram of p21 and its binding region to REGγ. (−) indicates no binding and (+) indicates binding with REGγ.
C. p21 Δ156–161 binds to endogenous PCNA. 293 cells were transfected with p21, p21 Δ156–161 or p21 with mutated PCNA binding domain (p21-PCNA) and treated with MG-132 for 4 hr. Cell lysates were immunoprecipitated with anti-p21 or anti-PCNA antibodies, and the associated protein was detected by Western blotting.
D. 293 cells were transfected with either p21 or p21Δ156–161. p21 half-lives were analyzed as in A.
To further characterize the physiological role of REGγ in p21 degradation, we examined p21 stability in REGγ −/− MEFs. As expected, the steady-state level of p21 was higher in REGγ null MEFs than in littermate control REGγ +/− MEFs (Figure 4A). Furthermore, re-expression of exogenous REGγ protein in REGγ null MEFs by retroviral transduction decreased steady-state level of p21 protein, and restored its stability to that observed in control cells (Figures 4A and 4B). Note that p21 was degraded at a slow rate in REGγ −/− MEFs by a proteasome-dependent pathway (Figure 4B), suggesting other pathways for p21 turnover can partially compensate for the absence of REGγ. To determine whether a ubiquitin-dependent pathway was involved in p21 degradation in REGγ −/− cells, we expressed exogenous lysineless p21 (p21K6R) in REGγ −/− MEFs. The half life of p21K6R was similar to that of exogenous or endogenous wild-type p21 (Figure 4C). Therefore, the slow, proteasomal pathway for p21 turnover in REGγ −/− MEFs was also ubiquitin-independent.
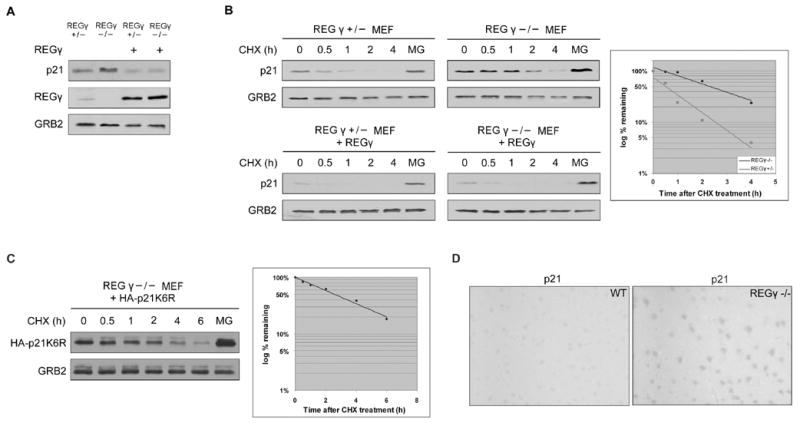
A. REGγ +/− and REGγ −/− MEFs were infected with retrovirus expressing REGγ. Steady-state levels of p21 were determined by Western blotting.
B. p21 half-lives were analyzed as in Figure 2.
C. REGγ −/− MEFs were infected with retrovirus expressing HA-p21K6R. p21 half-lives were analyzed as in Figure 2.
D. p21 immunohistochemistry of brain tissues from wild type and REGγ −/− mice.
We compared the p21 protein levels in tissues from wild type and REGγ −/− mice by immunohistochemistry. In most adult tissues p21 protein was expressed at levels below the limit of detection, in both wild type and null mice (data not shown). However, in the brain p21 protein could be detected in control mice, and its level of expression was substantially increased in REGγ −/− mice (Figure 4D). This observation was consistent with the fact that REGγ is expressed at high levels in the brain compared with REG-α and β (Jiang and Monaco, 1997), and demonstrated a role for REGγ in controlling p21 abundance in vivo.
Lysineless Proteins May Be Additional Substrates of the REGγ Pathway
For the majority of proteins, conjugation of ubiquitin to an internal lysine is the initial event in their degradation by the ubiquitin-proteasome system (Reviewed in Hershko and Ciechanover, 1998). However, a subset of proteins naturally lack lysine residues, and our observations with p21 suggested that turnover by the REGγ pathway might represent an alternative mechanism for the proteasomal degradation of these lysineless proteins.
To explore this idea, we first chose to study p16Ink4a, which is an unstable lysineless protein that has been reported previously to be degraded by a proteasome-dependent pathway (Ben-Saadon et al., 2004). p16 was in vitro translated and incubated with 20S proteasomes. Similarly to p21, p16 was rapidly degraded by 20S proteasomes but not 26S proteasomes in vitro (Figure 5A), suggesting ubiquitination and 19S were not required for its turnover. We further showed that exogenously expressed p16 could be co-immunoprecipitated with endogenous cellular REGγ (Figure 5B). Knock-down of REGγ expression with either of two independent siRNAs, as well as with a pool of four siRNAs, all increased the steady state abundance of endogenous p16 (data not shown), and increased the half-life of endogenous p16 from 3 to much longer than 6 hours (Figure 5C). p16 expression was not detected in the REGγ −/− MEFs, precluding its analysis in those cells. Although it has been reported that transfected, exogenous p16 may be ubiquitinated at its amino terminus (Ben-Saadon et al., 2004), our preliminary results showed by mass spectrometry that endogenous cellular p16 was entirely acetylated at its amino terminus, and therefore not a substrate for N-ubiquitination. In sum, our data confirmed that REGγ is involved in p16 degradation in vivo.
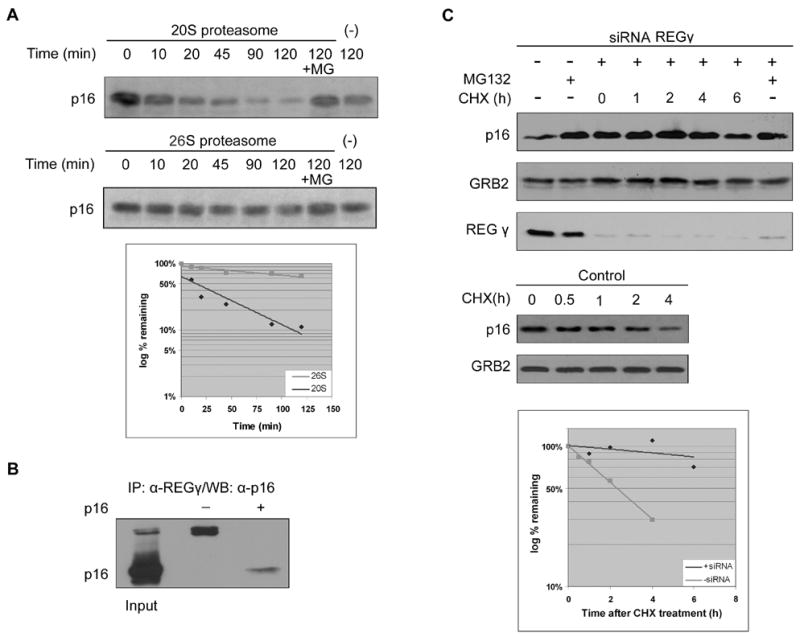
A. Equal amount of in vitro translated and 35S labeled p16 were incubated at 37°C for indicated times in the presence or absence of purified 20S or 26S proteasomes. The reaction products were analyzed by SDS-PAGE and detected by autoradiography.
B. p16 interacts with REGγ. 293 cells were transfected with p16. The interaction with REGγ was identified by immunoprecipitation with anti-REGγ antibodies followed by Western blotting with anti-p16 antibodies.
C. Hela cells were transfected with siRNA against REGγ. p16 half-lives were analyzed as in Figure 2.
The human p14Arf protein is lysineless, and the murine p19Arf protein has a single lysine residue. Previous work has shown that ubiquitination of the internal lysine is not necessary for proteasomal degradation of p19Arf, although ubiquitination of the amino terminus is required for the proteasomal turnover of at least a subset of p19Arf protein (Kuo et al., 2004). We observed by co-immunoprecipitation that p19Arf, like p21 and p16, could bind to REGγ (Figure 6A). p19Arf was not detected in a control immunoprecipitation with an irrelevant immune serum (data not shown). REGγ was also identified by mass spectrometry as a protein that bound to TAP-tagged p19Arf in vivo. Co-immunoprecipitation analyses not only confirmed the association but revealed that an unstable p19Arf mutant lacking residues 2–14 bound a greater amount of REGγ than the wild type protein (Bertwistle and Sherr, personal communication). We detected abundant p19Arf protein in REGγ −/− MEFs, but not in the control REGγ +/− MEFs (Figure 6B). Therefore, in order to investigate whether REGγ promotes p19Arf turnover, the half-lives of p19Arf were compared in REGγ −/− MEFs before and after restoration of REGγ expression by an exogenous expression vector. In REGγ −/− MEFs, p19Arf was very stable, and restoration of REGγ expression in these cells decreased its half-life to less than 6 hours (Figure 6C), which was consistent with previous measurements of stability (Kuo et al., 2004). We were able to compare p19Arf stability in REGγ −/− and REGγ +/− MEFs, by inducing p19Arf expression with exogenous Ras (Figures 6B and 6D). We found that p19Arf was very stable in the Ras-expressing REGγ −/− MEFs, and degraded with a half-life of approximately 4 hours in the Ras-expressing REGγ +/− MEFs, showing that endogenous amounts of REGγ were sufficient to regulate p19Arf stability. Our observations showed that REGγ interacted with p19Arf and promoted its degradation in vivo.
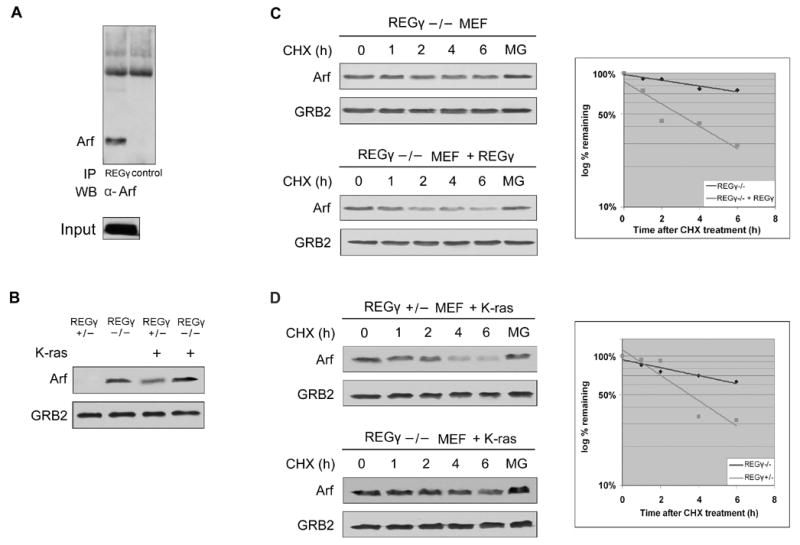
A. p19Arf interacts with REGγ. 293 cells were transfected with p19Arf and interaction with endogenous REGγ was detected by immnoprecipitation with anti-REGγ antibodies or irrelevant immune serum followed by Western blotting with anti-p19Arf antibodies.
B. Steady-state levels of p19Arf in REGγ +/− and REGγ −/− MEFs before and after infection with retrovirus expressing K-ras.
C. REGγ −/− MEFs were infected with retrovirus expressing REGγ. Half-lives of p19Arf were measured as in Figure 2.
D. REGγ +/− and REGγ −/− MEFs were infected with retrovirus expressing K-ras. Half-lives of p19Arf were measured as in Figure 2.
Discussion
Alternative activator complexes increase the diversity of proteasome substrates beyond the ubiquitinated proteins recognized by the 26S proteasome. These alternative activator complexes include proteins from the REG family, which can bind to substrates independently of ubiquitination. The REGα/β heteroheptameric complex functions in the proteasomal processing of antigenic epitopes in the immune system (Reviewed in Rechsteiner and Hill, 2005), but it has not been shown to have other functions in somatic cells. In contrast, the REGγ homoheptameric complex appears to have roles that extend beyond antigen processing. REGγ null mice have phenotypes that include decreased cell proliferation and increased apoptosis, implying that the REGγ complex may regulate the stability of specific proteins that act in these pathways. Indeed, previous work has shown that REGγ can be essential for the ubiquitin-independent degradation of proteins by the proteasome outside of the immune system (Li et al., 2006), although the specific protein targets identified thus far would not account for the role of REGγ in controlling cell division or apoptosis.
We now show that REGγ controls the abundance of three important cell cycle regulatory proteins: p21Cip1, p16INK4a, and p19Arf, all of which modulate both cell proliferation and cell death. All of these proteins bound to REGγ in vivo, and each was stabilized in REGγ deficient cells. The stabilization of these cell cycle inhibitors in REGγ-deficient cells is consistent with previous reports showing that REGγ null MEFs proliferate slowly (Murata et al., 1999; Barton et al., 2004). Knock-down of REGγ in Drosophila cells also inhibits cell proliferation (Masson et al., 2003), suggesting that this might be a conserved function of this pathway. It is interesting that the same 6 amino acids in p21 that are necessary for binding to REGγ have previously been reported to interact with the C8 subunit of the proteasome core (Touitou et al., 2001). Although the relationship between the binding of p21 to both C8 and REGγ remains to be defined, it is possible that these reflect concerted steps in the pathway of p21 degradation.
p19Arf, p16 and p21 had slow, but measurable rates of turnover after knock-down or knock-out of REGγ that remained sensitive to inhibition of proteasomes. In the case of p21 we showed that the residual proteasomal turnover in REGγ-deficient cells did not require lysine residues in p21 and was therefore ubiquitin-independent. The most likely explanation was that REG-α and β could partially compensate for the absence of REGγ, since they are expressed at low levels in almost every tissue. The increased abundance of p21 specifically in the brains of REGγ-deficient mice was consistent with this, because the relative levels of REG-α and β are particularly low levels in the central nervous system.
At present, it is still unclear whether there might be any rules or generalizations that describe the choice of proteins that are degraded by the REGγ pathway. One possibility is that this pathway is specialized for the proteasomal degradation of unstructured proteins. p19Arf, p21 and p16 are all unstructured when not associated with specific binding partners (such as cyclins and Cdks, for p21 and p16, and nucleophosmin in the case of p19Arf).
Our data would not seem to support this hypothesis. p27 is unstructured when not associated with cyclins and Cdks, but its turnover is strictly ubiquitin-dependent and is not regulated by REGγ. Moreover, although monomeric p21 is unstructured, the great majority of p21 in vivo is highly structured within p21-cyclin-Cdk complexes. We have also shown that certain truncation mutants of p21, which would be unstructured like full-length p21, are not substrates of the REGγ pathway. Therefore, our experiments suggest that specific motifs, as opposed to a generally unfolded structure, are necessary for substrates to be recognized by REGγ.
Another special class of proteins that may be targeted by the REGγ pathway are proteins that lack lysine residues. Both p16 and human p14Arf are naturally lysineless proteins (murine p19Arf has a single lysine that is not required for its proteasomal degradation), and there are more than one hundred other examples of lysineless proteins in the eukaryotic protein databases (Ben-Saadon et al., 2004). Thus the REGγ pathway may provide a ubiquitin-independent mechanism that allows the degradation of lysineless proteins by the proteasome. Viral proteins constitute a substantial subset of naturally lysineless proteins, which raises the possibility that the REGγ pathway might be particularly important in controlling viral pathogenesis.
Materials and Methods
Reagents, Plasmids and Cell Culture
Plasmids were kindly provided as follows: pSRα-TKneo-HA-p19Arf (C.J. Sherr, Memphis, TN) (Kuo et al., 2004); pCDNA3.1-REGγ (B.W. O’Malley, Houston, TX) (Li et al., 2006); pCMV-hp16, pCMV-HA-p21(1–76), and pCMV-HA-p21(77–164) (M. Welcker, Seattle, WA). pCS2-hp21 and pCS2-K6R were described previously (Clurman et al., 1996; Sheaff et al., 2000). p21(1–140), p21Δ156–161, p19Arf, p19Arf(1–120), and p19Arf(1–150) were cloned into the pCS2 vector. Reagents were purchased from the following sources: polyclonal antibodies to p21 (C19), p21 (N20), p27 (C19), p16 (H-156), GRB2 (C-23), HA (Y11) and monoclonal antibodies to p19Arf (5-C3-1), p53 (Pab240), and PCNA (PC10) (Santa Cruz Biotechnology); monoclonal antibodies to p21 (SXM30) and p16 (G175–405) (BD-Pharmingen), and GRB2 (BD-Transduction Laboratories); polyclonal antibodies to REGγ (38–3800, Zymed); HRP-conjugated anti-mouse, anti-rabbit, and anti-rat IgG (Amersham); pQCXIP retroviral vectors (BD-Clontech); cycloheximide and MG-132 (Sigma); 20S and 26S proteasome (Biomol); TnT Coupled Reticulocyte Lysate System (Promega); ImmunoCruz Staining System Kit (Santa Cruz Technology). Ub-Mbp-Sic1, MeUb-Mbp-Sic1, and rabbit polyclonal anti-Sic serum were kindly provided by R.J. Deshaies (La Jolla, CA). MEFs were isolated from 13.5-day-PC REGγ +/+, REGγ +/−, and REGγ −/− embryos (Nakayama et al., 1996). Primary MEFs were immortalized using the 3T3 protocol (Todaro and Green, 1963) and immortal MEFs (3–5 passages after immortalization or around 20 passages from the beginning) were used in this study. All cells lines were cultured in DMEM supplemented with 10% bovine growth serum (HyClone).
Transient transfection, Retroviral infection and RNA interference
For transient gene expression, 293T cells were transfected with 5 μg/60mm dish of each expression vector by the calcium-phosphate method. Cells were collected 48 hr after transfection and subjected to half life analysis. Cycloheximide (Sigma) was used at 10 μM for half life analysis and MG-132 (Sigma) at 50 μg/ml for indicated times. For retroviral infections, viruses were produced from Phoenix ecotropic cells by transient transfection of REGγ or HA-p21K6R cloned into pQCXIP. MEFs were subjected to one round of infection and selected 48 hr after infection using 4 μg/ml puromycin. All siRNA duplexes were purchased from Dharmacon. The sequences for siRNAs are as follows: siRNA #1 (J-012133-05, sense sequence: GAAUCAAUAUGUCACUCUAUU; siRNA #2 (J-012133-06, sense sequence: UCUGAAGGAACCAAUCUUAUU); SMARTpool (L-012133-00). 50 nM quantities of each siRNA were transfected into cells using SilentFect following manufacture’s instruction (BioRad). Cells were harvested 48 hr after transfection and half life analysis was performed as previously described. Gels were quantified using Image J software.
Western Blotting and Immunoprecipitation
Cells were lysed in NETN buffer (20mM Tris-HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 10 μg/ml each of aprotonin, leupeptin, and pepstatin, 10 mM NaF, 10 mM Na vanadate), followed by scraping and sonication. For each immunoprecipitation, 7 μg of the indicated antibodies and 500 μg lysate protein were incubated overnight at 4°C followed by incubation with 20 μl protein A beads (Amersham) for 2 hr at 4°C. Immunoprecipitates were subjected to Western blotting as described (Clurman et al., 1996).
In vitro Translation, Binding and Degradation Assay
p21, p27, and p16 were synthesized using in vitro transcription/translation TnT Coupled Reticulocyte Lysate System (Promega) following manufacture’s protocol. Each in vitro translated protein (5 μl/time point) was incubated with 20S proteasomes (1 μg/time point, BioMol) in 20S assay buffer (20 mM Tris pH 7.2, 1mM EDTA, 1 mM DTT) or with 26S proteasomes (1 μg/time point, BioMol) in 26S assay buffer (20 mM Tris pH 7.2, 25 mM KCl, 10 mM NaCl, 1 mM MgCl2, 1 mM DTT, 4 mM ATP, 50 mM phosphocreatine, and 17.5 U/ml creatine phosphokinase) respectively at 37 °C for indicated times, and the reaction was stopped by addition of 4X SDS loading buffer. For in vitro binding assay, in vitro translated p21 or p16 were preincubated with purified cyclin E/cdk2 complexes in 20 S assay buffer for 1 hr at 4 °C prior to addition of proteasomes. Cyclin E/cdk2 complexes were prepared as previously described (Sheaff et al., 1997). Samples were resolved by SDS-PAGE and visualized by autoradiography. For Ub-Mbp-Sic1 and MeUb-Mbp-Sic1, 200 ng protein was used in each reaction and degradation assay was performed as previously described. Samples were analyzed by Western blotting.
Immunohistochemistry
Tissues from wild type and REGγ −/− mice were fixed in 10% formalin in PBS and processed as paraffin sections. p21 immunostaining was performed according to the protocol provided in the Immuno-Cruz Staining System Kit (Santa Cruz Biotechnology). Briefly, deparaffinized tissue sections were steamed for 30 min in 0.1M citric acid and blocked in serum for 30 min at room temperature. Samples were subsequently incubated with anti-p21 antibodies (p21 C19, 1:100 dilution) for 1 hr and biotinylated secondary antibodies for 1 hr, and visualized using the chromogen 3′3′-diaminobenzidine (DAB).
Acknowledgments
We thank C. Sherr for discussions of unpublished work, and members of the Roberts and Clurman labs for advice and support. We thank R. Deshaies for the gift of polyubiquitinated Sic1, and methyl-ubiquitinated Sic1. This work was supported by grants from the NIH to JMR and an R.L. Richardson Foundation grant to Austin College.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton LF, Runnels HA, Schell TD, Cho Y, Gibbons R, Tevethia SS, Deepe GS, Jr, Monaco JJ. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172:3948–3954. [Abstract] [Google Scholar]
- Ben-Saadon R, Fajerman I, Ziv T, Hellman U, Schwartz AL, Ciechanover A. The tumor suppressor protein p16 (INK4a) and the human papillomavirus oncoprotein-58 E7 are naturally occurring lysine-less proteins that are degraded by the ubiquitin system. Direct evidence for ubiquitination at the N-terminal residue. J Biol Chem. 2004;279:41414–41421. [Abstract] [Google Scholar]
- Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A, Fotedar R. UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell. 2003;114:599–610. [Abstract] [Google Scholar]
- Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M. Proteasome-mediated degradation of p21 via N-terminal ubiquitination. Cell. 2003;115:71–82. [Abstract] [Google Scholar]
- Chen X, Chi Y, Bloecher A, Aebersold R, Clurman BE, Roberts JM. N-acetylation and ubiquitin-independent proteasomal degradation of p21(Cip1) Mol Cell. 2004;16:839–847. [Abstract] [Google Scholar]
- Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. [Abstract] [Google Scholar]
- Coffino P. Regulation of cellular polyamines by antizyme. Nat Rev Mol Cell Biol. 2001;2:188–194. [Abstract] [Google Scholar]
- Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [Abstract] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. [Abstract] [Google Scholar]
- Jiang H, Monaco JJ. Sequence and expression of mouse proteasome activator PA28 and the related autoantigen Ki. Immunogenetics. 1997;46:93–98. [Abstract] [Google Scholar]
- Jin Y, Lee H, Zeng SX, Dai MS, Lu H. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitination. Embo J. 2003;22:6365–6377. [Europe PMC free article] [Abstract] [Google Scholar]
- Kriwacki RW, Hengst L, Tennant L, Reed SI, Wright PE. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: conformational disorder mediates binding diversity. Proc Natl Acad Sci U S A. 1996;93:11504–9. [Europe PMC free article] [Abstract] [Google Scholar]
- Kuo ML, den Besten W, Bertwistle D, Roussel MF, Sherr CJ. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 2004;18:1862–1874. [Europe PMC free article] [Abstract] [Google Scholar]
- Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, Tsai SY, Tsai MJ, O’Malley BW. The SRC-3/AIB1 coactivator is degraded in a ubiquitin-and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–392. [Abstract] [Google Scholar]
- Ma CP, Slaughter CA, DeMartino GN. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J Biol Chem. 1992;267:10515–10523. [Abstract] [Google Scholar]
- Masson P, Lundgren J, Young P. Drosophila proteasome regulator REGgamma: transcriptional activation by DNA replication-related factor DREF and evidence for a role in cell cycle progression. J Mol Biol. 2003;327:1001–1012. [Abstract] [Google Scholar]
- Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Miyamura T, Suzuki T, Koike K, Matsuura Y. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci U S A. 2007;104:1661–1666. [Abstract] [Google Scholar]
- Moriishi K, Okabayashi T, Nakai K, Moriya K, Koike K, Murata S, Chiba T, Tanaka K, Suzuki R, Suzuki T, Miyamura T, Matsuura Y. Proteasome activator PA28gamma-dependent nuclear retention and degradation of hepatitis C virus core protein. J Virol. 2003;77:10237–10249. [Europe PMC free article] [Abstract] [Google Scholar]
- Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, Tanaka K, Chiba T. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. [Abstract] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. [Abstract] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. [Abstract] [Google Scholar]
- Realini C, Jensen CC, Zhang Z, Johnston SC, Knowlton JR, Hill CP, Rechsteiner M. Characterization of recombinant REGalpha, REGbeta, and REGgamma proteasome activators. J Biol Chem. 1997;272:25483–25492. [Abstract] [Google Scholar]
- Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. [Abstract] [Google Scholar]
- Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. CyclinE-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. [Abstract] [Google Scholar]
- Sheaff RJ, Singer JD, Swanger J, Smitherman M, Roberts JM, Clurman BE. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol Cell. 2000;5:403–410. [Abstract] [Google Scholar]
- Silva JM, Li MZ, Chang K, Ge W, Golding MC, Rickles RJ, Siolas D, Hu G, Paddison PJ, Schlabach MR, Sheth N, Bradshaw J, Burchard J, Kulkarni A, Cavet G, Sachidanandam R, McCombie WR, Cleary MA, Elledge SJ, Hannon GJ. Second-generation shRNA libraries covering the mouse and human genomes. Nat Genet. 2005;37:1281–1288. [Abstract] [Google Scholar]
- Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. [Europe PMC free article] [Abstract] [Google Scholar]
- Touitou R, Richardson J, Bose S, Nakanishi M, Rivett J, Allday MJ. A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 alpha-subunit of the 20S proteasome. Embo J. 2001;20:2367–2375. [Europe PMC free article] [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.molcel.2007.05.022
Read article for free, from open access legal sources, via Unpaywall:
http://www.cell.com/article/S1097276507003231/pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Investigating the p21 Ubiquitin-Independent Degron Reveals a Dual Degron Module Regulating p21 Degradation and Function.
Cells, 13(19):1670, 09 Oct 2024
Cited by: 0 articles | PMID: 39404432 | PMCID: PMC11476297
Ubiquitin-independent degradation of Bim blocks macrophage pyroptosis in sepsis-related tissue injury.
Cell Death Dis, 15(9):703, 30 Sep 2024
Cited by: 0 articles | PMID: 39349939 | PMCID: PMC11442472
PSME3 promotes lung adenocarcinoma development by regulating the TGF-β/SMAD signaling pathway.
Transl Lung Cancer Res, 13(6):1331-1345, 26 Jun 2024
Cited by: 1 article | PMID: 38973962
PA200-Mediated Proteasomal Protein Degradation and Regulation of Cellular Senescence.
Int J Mol Sci, 25(11):5637, 22 May 2024
Cited by: 0 articles | PMID: 38891826 | PMCID: PMC11171664
Review Free full text in Europe PMC
Proteasome-dependent degradation of histone H1 subtypes is mediated by its C-terminal domain.
Protein Sci, 33(5):e4970, 01 May 2024
Cited by: 1 article | PMID: 38591484 | PMCID: PMC11002908
Go to all (190) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway.
Mol Cell, 26(6):831-842, 01 Jun 2007
Cited by: 179 articles | PMID: 17588518
Oxidative challenge enhances REGγ-proteasome-dependent protein degradation.
Free Radic Biol Med, 82:42-49, 02 Feb 2015
Cited by: 5 articles | PMID: 25656993
REGgamma proteasome mediates degradation of the ubiquitin ligase Smurf1.
FEBS Lett, 584(14):3021-3027, 24 May 2010
Cited by: 23 articles | PMID: 20580715
REGgamma, a proteasome activator and beyond?
Cell Mol Life Sci, 65(24):3971-3980, 01 Dec 2008
Cited by: 92 articles | PMID: 18679578 | PMCID: PMC11131756
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA118043
Grant ID: R01 CA118043-01




