Abstract
Free full text

Hypoxic Switch in Mitochondrial Myeloid Cell Leukemia Factor-1/Mtd Apoptotic Rheostat Contributes to Human Trophoblast Cell Death in Preeclampsia
Abstract
Preeclampsia, a disorder of pregnancy, is characterized by increased trophoblast cell death and altered trophoblast-mediated remodeling of myometrial spiral arteries resulting in reduced uteroplacental perfusion. Mitochondria-associated Bcl-2 family members are important regulators of programed cell death. The mechanism whereby hypoxia alters the mitochondrial apoptotic rheostat is essential to our understanding of placental disease. Herein, myeloid cell leukemia factor-1 (Mcl-1) isoform expression was examined in physiological/pathological models of placental hypoxia. Preeclamptic placentae were characterized by caspase-dependent cleavage of death-suppressing Mcl-1L and switch toward cell death-inducing Mcl-1S. In vitro, Mcl-1L cleavage was induced by hypoxia-reoxygenation in villous explants, whereas Mcl-1L overexpression under hypoxia-reoxygenation rescued trophoblast cells from undergoing apoptosis. Cleavage was mediated by caspase-3/-7 because pharmacological caspase inhibition prevented this process. Altitude-induced chronic hypoxia was characterized by expression of Mcl-1L; resulting in a reduction of apoptotic markers (cleaved caspase-3/-8 and p85 poly-ADP-ribose polymerase). Moreover, in both physiological (explants and high altitude) and pathological (preeclampsia) placental hypoxia, decreased trophoblast syncytin expression was observed. Hence, although both pathological and physiological placental hypoxia are associated with slowed trophoblast differentiation, trophoblast apoptosis is only up-regulated in preeclampsia, because of a hypoxia-reoxygenation-induced switch in generation of proapoptotic Mcl-1 isoforms.
The regulation of Bcl-2 family members is currently the subject of intense investigation because these proteins are important regulators of programed cell death in normal organ development as well as in disease.1,2 Members of this gene family act through a complex network of homo- and heterodimers. The fate of a cell is determined by the ability of cell death suppressors to sequester, and thus neutralize, the actions of cell death inducers.2 Hence, the relative concentration of pro- and anti-apoptotic proteins determines whether a cell lives or dies.3 Many Bcl-2 family members localize permanently or transiently to the outer mitochondrial membrane where they are believed to either regulate or form pores. The opening of these pores results in transitional changes in mitochondrial membrane potential accompanied by release of death-inducing factors from the mitochondrion into the cytoplasm. Several prosurvival Bcl-2 family members can block the induced decrease in mitochondrial membrane potential and inhibit the activation of apoptotic caspases by keeping death-inducing proteins in an inactive state.2
Preeclampsia, a unique human disease of placental origin, affects 5 to 7% of all pregnancies and is clinically manifested by the sudden onset of maternal hypertension and proteinurea.4 Preeclamptic placentae are characterized by altered trophoblast differentiation and increased trophoblast cell death and turnover.5,6,7,8,9,10,11 Failure in trophoblast-mediated maternal vessel remodeling may predispose the preeclamptic placenta to hypoxia/oxidative stress and as such may impact trophoblast cell death and differentiation, possibly via dysregulated expression or function of Bcl-2 family members.12
We recently reported that increased expression of proapoptotic Mtd-L and Mtd-P, a newly identified isoform of the Bcl-2 family member Mtd/Bok (Matador/Bcl-2-related ovarian killer), results in elevated trophoblast cell death in placentae of patients diagnosed with severe early-onset preeclampsia.11 In addition, we demonstrated that both Mtd-L and Mtd-P isoforms induce mitochondrial apoptotic events.11 The death-inducing Mtd-L molecule preferentially interacts with Mcl-1L protein (myeloid cell leukemia factor 1-L: long isoform), which neutralizes its proapoptotic activity.13,14 The Mcl-1 gene was first described as an early induction gene during myeloblastic leukemia cell differentiation15 and subsequently characterized as an important anti-apoptotic molecule.16,17 Mcl-1 is essential for maintenance of hematopoietic stem cells in bone marrow, for early lymphoid development and later in maintenance and survival of mature lymphocytes via selective inhibition of the proapoptotic protein BIM.18
In addition to the prosurvival Mcl-1L molecule, the Mcl-1 gene also gives rise to a death-inducing splicing isoform known as Mcl-1S. This variant is generated via exon II skipping,19,20 resulting in loss of the BH1, BH2, and transmembrane domains, hence generating a truncated proapoptotic BH3-only isoform. Mcl-1L is presently the sole known molecule capable of binding and antagonizing the killing function Mcl-1S.19 Recent studies have demonstrated that activated caspase-3 leads to proteolytic cleavage of conserved aspartate residues (D127 and D157) within the PEST domain of both Mcl-1 variants, hence generating truncated molecules21,22,23,24,25 that exhibit proapoptotic functions.21,25,26
Oxygen plays a key role in the transcriptional regulation of both Mcl-1 and Mtd.11,27,28 We recently reported that Mtd is overexpressed in preeclamptic placentae,11 but the expression status of Mcl-1 in this pathology is currently unknown. Herein, we tested the hypothesis that reduced pO2 as seen in preeclampsia alters the Mtd/Mcl-1 apoptotic rheostat regulating trophoblast turnover. We investigated Mcl-1 expression in human pregnancies complicated by preeclampsia and examined how conditions of in vitro and in vivo placental hypoxia, including altitude-induced chronic hypoxia, affect the Mcl-1/Mtd apoptotic rheostat. Our findings indicate that pathological conditions of oxidative stress compared with physiological low pO2 induces a detrimental switch in the mitochondrial apoptotic balance via caspase-mediated cleavage of prosurvival Mcl-1 into proapoptotic isoforms.
Materials and Methods
Tissue Sampling
Tissue was collected in accordance with participating institutions’ ethics guidelines. Preeclampsia was defined and diagnosed based on the American College of Obstetrics and Gynecology criteria (blood pressure of 140 mmHg systolic or higher and 90 mmHg diastolic or higher occurring after 20 weeks of gestation in a woman with previously normal blood pressure accompanied by urinary excretion of 0.3 g of protein or higher).29 The fifth Korotkoff sound was used for measuring diastolic blood pressure. Preeclamptic placentae (PE; early-onset, n = 49; late-onset/term, n = 10) and preterm normotensive age-matched control placentae (AMC, n = 38) were collected from deliveries at Mount Sinai Hospital. Areas with calcified, necrotic, or visually ischemic tissue were omitted from sampling. Patients suffering from diabetes, kidney disease, or infections were excluded. Early-onset preeclampsia was defined when the patient delivered before 34 weeks of gestation because of preeclampsia. Pregnant patients with essential hypertension (n = 4, at term) and pregnancies affected by intrauterine growth restriction (IUGR) (n = 6, gestational age 35 to 38 weeks with fetal weight less than fifth percentile) without preeclampsia were included as controls. Preterm deliveries were attributable to multiple pregnancies (30%), preterm labor attributable to incompetent cervix (40%), premature preterm rupture of membrane (20%), and spontaneous rupture of membranes (10%). Preterm and term control groups had no clinical or pathological signs of preeclampsia, infections, or other maternal or placental disease. First-trimester human placental tissues (6 to 20 weeks of gestation, n = 16) were obtained from elective terminations of pregnancies by dilatation and curettage. High-altitude (HA; 3100 m, n = 16) and moderate altitude (MA; 1600 m, n = 13) and sea-level/term control (SL, n = 15) placental samples were collected from healthy normal patients with term deliveries. Because of organ heterogeneity and the fact that perfusion can differ depending on location within the placenta,30 multiple specimens (approximately five per region) were randomly sampled from central and peripheral regions of pathological, HA, and control placentae. The SL, MA, and HA groups did not show clinical or pathological signs of preeclampsia, infection, or other placental disease. For summary of clinical data, see Table 1.
Table 1
Clinical Parameters of Participants
| Preterm control, n = 38 | Term control, n = 16 | Moderate altitude, n = 13 | Early-onset preeclamptic, n = 49 | Late-onset preeclamptic, n = 10 | High altitude, n = 16 | |
|---|---|---|---|---|---|---|
| Mean maternal age (years) | 30 ± 5.0 | 33 ± 4.5 | 29.0 ± 2.5 | 29 ± 6.1 | 32.0 ± 1.7 | 29.0 ± 6.7 |
| Mean gestational age (range in weeks) | 29.4 ± 4.5 (23 to 35) | 39.6 ± 0.9 (38 to 41) | 39.4 ± 1.4 (37 to 41) | 29.8 ± 3.1 (25 to 34) | 39.5 ± 0.7 (39 to 41) | 39.1 ± 1.3 (37 to 41) |
| Blood pressure | S: 115 ± 4.0 | S: 111 ± 6.0 | S: 113 ± 5.0 | S: 182 ± 11 | S: 158 ± 18.2 | S: 116 ± 8.0 |
| D: 70 ± 6.5 | D: 67 ± 6.3 | D: 72 ± 5.0 | D: 110 ± 7.0 | D: 95 ± 9.7 | D: 72 ± 5.0 | |
| Proteinuria | Absent | Absent | Absent | 3.4 ± 1.4 | 1 ± 1.5 | Absent |
| Edema | Absent | Absent | Absent | Present: 85% | Present: 50% | Absent |
| Absent: 15% | Absent: 50% | |||||
| Fetal weight (g) | A.G.A.: 1300 ± 730 | A.G.A.: 3328 ± 421 | A.G.A.: 3553 ± 312 | A.G.A.: 1160 ± 352 | A.G.A.: 3387 ± 486 | A.G.A.: 3060 ± 190 |
| Mode of delivery | CS: 19 | CS: 8 | CS: 2 | CS: 44 | CS: 4 | CS: 2 |
| VD: 19 | VD: 8 | VD: 14 | VD: 5 | VD: 6 | VD: 14 |
Data are represented as mean ± SD. Maternal age of participants ranged from 18 to 41 years. S, systolic; D, diastolic; A.G.A., appropriate for gestational age; VD, vaginal delivery; CS, caesarian section delivery.
Human Chorionic First Trimester Villous Explant Culture and z-VAD-fmk and z-DEVD-fmk Treatments
Explant cultures were performed as previously described.31 In brief, placental tissues (5 to 8 weeks of gestation) were placed in ice-cold phosphate-buffered saline (PBS) and processed within 2 hours of collection. Tissues were aseptically dissected to remove decidual tissue and fetal membranes. Small fragments of placental villi (25 to 45 mg wet weight) were teased apart, placed on Millicell-CM culture dish inserts (Millipore Corp., Bedford, MA) precoated with 0.15 ml of undiluted Matrigel (Collaborative Biomedical Products, Bedford, MA), and transferred to a 24-well culture dish. Explants were cultured in serum-free Dulbecco’s modified Eagle’s medium/F12 (Life Technologies, Inc., Grand Island, NY) supplemented with 100 μg/ml streptomycin, 100 U/ml penicillin, and incubated overnight at 37°C in 5% CO2 in air to allow attachment. Explants were maintained in standard condition (5% CO2 in 95% air) or in an atmosphere of 3% O2/92% N2/5% CO2 for 48 hours at 37°C. The morphological integrity and viability of villous explants under the various oxygenation conditions were monitored daily as previously reported.11,31 Explants from more than 18 different placentae in more than 15 separate experiments were used. A minimum of three explants per experimental condition was used at all times. Explants were exposed to hypoxia-reoxygenation (HR) as previously described11,32 in the presence of 100 μmol/L of the pan-caspase inhibitor z-VAD-fmk (BioMol, Plymouth Meeting, PA) or caspase-3-specific inhibitor z-DEVD-fmk (R&D Systems, Minneapolis, MN) both dissolved in dimethyl sulfoxide (equivalent volume of dimethyl sulfoxide in the absence of inhibitors was used in control conditions).
RNA Analysis
RNA extraction was performed using a RNeasy mini kit (Qiagen, Valencia, CA), reverse-transcribed using a random hexamer approach, and amplified by 40 cycles of quantitative polymerase chain reaction (PCR) (15 minutes at 95°C, cycle: 30 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C). Southern blot analysis was performed as previously described,11 using a 32P-labeled full-length Mcl-1L probe. Primer sequences used in reverse transcriptase (RT)-PCR/Southern blot analysis were: Mcl-1 (NM_021960): forward 5′-ATGTTTGGCCTCAAAAGAAACGCG-3′ and reverse 5′-GGCTATCTTATTAGATATGCCAA-3′ (Mcl-1L: predicted size = 1054 bp and Mcl-1S: predicted size = 806 bp) and β-actin (NM_001101): forward 5′-CTTCTACAATGAGCTGCGTG-3′, reverse 5′-TCATGAGGTAGTCAGTCAGG-3′ (predicted size = 304 bp). All amplified and cloned products were confirmed by sequencing. Quantitative PCR was performed using the SYBR Green I dye DyNamo HS kit (MJ Research, Waltham, MA) based on the manufacturer’s protocol using isoform-specific primers for Mtd-L and Mtd-P (Mtd-L: forward 5′-GCCTGGCTGAGGTGTGC-3′, Mtd-P: forward 5′-GCGGGAGAGGCGATGA-3′, reverse (both L and P) 5′-TGCAGAGAAGATGTGGCCA-3′, Mcl-1L: forward 5′-ATGCTTCGGAAACTGGACAT-3′, Mcl-1S: forward 5′-CCTTCCAAGGATGGGTTTG-3′, Mcl-1: reverse (both L and S) 5′-CTAGGTTGCTAGGGTGCAA-3′). For syncytin and cytokeratin 7 analyses, qRT-PCR was performed using Assays-on-Demand TaqMan primers and probe (Applied Biosystems, Foster City, CA). Analysis was done using the DNA Engine Opticon2 System (MJ Research). Data for all qPCR analyses were normalized against expression of 18S ribosomal RNA as previously described.33
Western Blot Analysis
Western blotting was performed as previously described.34 Fifty μg of total protein from placental tissue was subjected to 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Membranes were probed at 4°C overnight with a 1:1000 dilution of primary antibodies: rabbit polyclonal Mtd antibody capable of recognizing all isoforms as previously reported11; Mcl-1-specific rabbit polyclonal antibody (SC-819 clone S-19 from Santa Cruz Biotechnology, Santa Cruz, CA); specific cleaved caspase-3 (Asp175) (5A1); cleaved caspase-8 (Asp384) rabbit polyclonal antibodies (Cell Signaling, Beverly, MA), and anti-PARP p85 (active PARP) fragment rabbit polyclonal antibodies (1:300 dilution; Promega, Madison, WI). Rabbit polyclonal antisera against N-terminal amino acids (28 to 41) of syncytin (NM_014590) was generated by injecting rabbits first with 400 μg of KHL-conjugated peptide in Freund’s complete adjuvant followed by three boosts of 100 μg in incomplete adjuvant. Titer was checked by enzyme-linked immunosorbent assay using bovine serum albumin-conjugated peptide as antigen. For anti-Mtd, Mcl-1, and syncytin antibodies, preimmune serum and competing peptides were used as controls. After overnight incubation, membranes were washed with TBS/T and incubated for 60 minutes at room temperature with 1:5000 diluted horseradish peroxidase-conjugated anti-rabbit (Santa Cruz Biotechnology). Blots were exposed to chemiluminescent ECL-plus reagent (Amersham, Piscataway, NJ). All blots were confirmed for equal protein loading and transfer using Ponceau staining.35
Immunohistochemistry
Immunohistochemical analyses were performed using an avidin-biotin-based immunoperoxidase approach, as previously described.32 Nonspecific binding sites were blocked using 5% (v/v) normal goat serum and 1% (w/v) bovine serum albumin in Tris-buffer. Slides were incubated overnight at 4°C with a 1:200 dilution of rabbit polyclonal anti-Mtd or anti-Mcl-1 antibodies. After washing, slides were probed with a 300-fold dilution of biotinylated goat anti-rabbit or goat anti-mouse IgG (Vector Laboratories, Burlingame, CA) for 1 hour at 4°C. Avidin-biotin complex was applied for 1 hour. Slides were developed in 0.075% (w/v) 3,3-diaminobenzidine in PBS (pH 7.6) containing 0.002% (v/v) H2O2, counterstained with hematoxylin, dehydrated in an ascending ethanol series, cleared in xylene, and mounted. In control experiments, primary antibodies were replaced with blocking solution [5% (v/v) normal goat serum and 1% (w/v) bovine serum albumin].
Transfection Experiments
Human choriocarcinoma JEG-3 cells (75% confluence) were transfected with 1.5 μg/35-mm dish of either empty pcDNA3.1 vector or pcDNA-3-Mcl-1L vector (kindly provided by Dr. Ruth Craig, Dartmouth Medical School, Hanover, NH) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, cells were treated with 10 mmol/L of the NO donor sodium nitroprusside (SNP; Sigma, St. Louis, MO) or exposed to HR for 5 hours (3 hours at 3% O2 followed by 2 hours at 20% O2) as previously described.11 Cells were then harvested, and the percentage of dead cells was assessed by trypan blue staining.
Statistical Analysis
Data are represented as mean ± SEM of at least three separate experiments performed in triplicate. For comparison of data between multiple groups, we used Kruskal-Wallis one-way analysis of variance with posthoc Dunnett’s test. For comparison between two groups, we used a paired and unpaired Student’s t-test as appropriate. Significance defined as P < 0.05.
Results
Mcl-1 Transcript and Protein Expression in Preeclampsia
Placental transcript and protein expression of Mcl-1 was first assessed in placentae of severe early-onset preeclamptic patients (PE) relative to AMCs. Quantitative real-time PCR (qRT-PCR) using isoform-specific primers for anti-apoptotic Mcl-1L and proapoptotic Mcl-1S demonstrated that Mcl-1L mRNA expression was unchanged between PE and AMC, whereas Mcl-1S expression was significantly increased in PE (fourfold, P = 0.001) relative to AMCs (Figure 1a). Sequence analysis of the PCR products confirmed identity of Mcl-1L and Mcl-1S (data not shown).
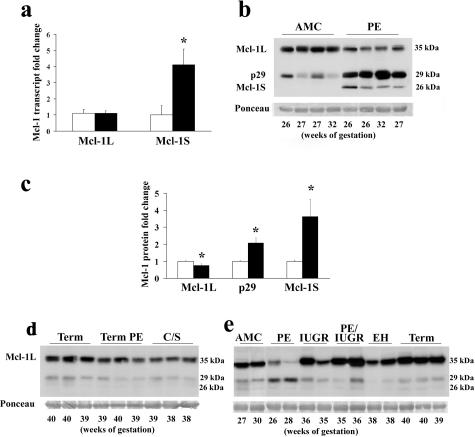
Mcl-1 expression in preeclamptic pregnancies. a: qRT-PCR analysis of Mcl-1L and Mcl-1S transcripts in placental tissues from severe early-onset preeclampsia (PE, black bar) and normotensive AMC tissues (open bar). Fold changes are relative to AMC Mcl-1 and Mcl-1S control levels. b: Representative Mcl-1 immunoblot performed on AMC and PE total protein lysates. c: Densitometric analysis of Mcl-1-specific protein isoform bands between AMC (open bar, n = 22) and PE (black bar, n = 25). d: Representative Mcl-1 immunoblot of normal term placentae (term), term preeclamptic placentae (term PE), and normal term elective caesarian section placentae in the absence of labor (C/S). e: Representative Mcl-1 immunoblot of placentae from severe early-onset preeclampsia, IUGR pregnancies, 35 to 37 weeks IUGR + PE pregnancies, pregnant patients with essential hypertension (EH), and normal term placentae (term). All immunoblots were confirmed for equal protein loading using Ponceau staining. *P < 0.05.
Western blot analyses revealed that Mcl-1L protein expression (Mr ~37 kd) was decreased in early-onset PE relative to AMC (Figure 1b) despite no change in transcript levels (Figure 1a). In addition, two shorter Mcl-1-immunoreactive bands migrating at relative molecular weights of 29 and 26 kd, respectively, were observed, both of which seemed to be more abundant in PE tissues (Figure 1b). Preabsorption of anti-Mcl-1 antibody with corresponding peptide abolished the appearance of all Mcl-1 immunoreactive bands (data not shown). Densitometric analysis confirmed that in early PE Mcl-1L, protein expression was decreased by 25% (P = 0.01), whereas Mcl-1 p29 and Mcl-1 p26 expression increased by twofold (P = 0.003) and 3.6-fold (P = 0.01), respectively (Figure 1c). No notable differences were observed in Mcl-1L and Mcl-1 p29 expression between late preeclampsia relative to normal term deliveries and nonlabor C/S deliveries (Figure 1d). Furthermore, the controls age-matched to the severe early-onset preeclampsia (Figure 1b), despite clinical heterogeneity in the cause of preterm delivery, did not differ in Mcl-1 isoform profile expression relative to the term deliveries with and without labor as shown in Figure 1d. When Mcl-1 protein expression was compared between early-onset PE placentae and other placental pathologies (IUGR pregnancies, late-onset preeclampsia associated with IUGR, essential hypertension) and normal term tissues, the switch in Mcl-1 protein profile was predominantly once again observed only in placentae of patients with the early severe form of PE (Figure 1e).
Mcl-1 Cleavage in Human Villous Explants Is Regulated by Caspase Activation
The appearance of several specific Mcl-1-immunoreactive bands suggested the presence of various Mcl-1 isoforms. Taking into account our transcript data, the band migrating at 26 kd is consistent with the predicted molecular weight of Mcl-1S, originally cloned from a placental cDNA library.19,20 To investigate whether the p29 Mcl-1-immunoreactive band can be a result of caspase-mediated cleavage, we exposed first trimester placental explants to conditions of HR known to induce caspase activation and trophoblast apoptosis in vitro.11,36 Explants were exposed to HR in the presence or absence of z-VAD-fmk, a broad-based inhibitor of caspase activity and caspase-mediated Mcl-1L cleavage.21,22,23,26 Relative to untreated controls, tissues exposed to HR demonstrated a notable Mcl-1 isoform switch; ie, Mcl-1L protein decreased with a concomitant increase of p29 immunoreactive Mcl-1 protein (Figure 2a). Exposure of HR-treated explants to z-VAD-fmk abrogated the appearance of the immunoreactive p29 Mcl-1 band, restored Mcl-1L content, and increased Mcl-1S amount relative to HR and untreated tissues (Figure 2, a and c). To investigate whether Mcl-1 cleavage under HR was mediated by executioner caspases, explants under HR were incubated in the presence and absence of a caspase-3/7-specific inhibitor. Similar to z-VAD-fmk treatment, z-DEVD-fmk exposure under HR conditions prevented caspase-mediated Mcl-1 p29 formation and increased both Mcl-1L and Mcl-1S levels (Figure 2b). Thus, both Mcl-1L and Mcl-1S seem to be regulated by caspase cleavage under HR stress and p29 Mcl-1 is most likely the previously described proapoptotic cleavage product of Mcl-1L.21,22,23,24,25
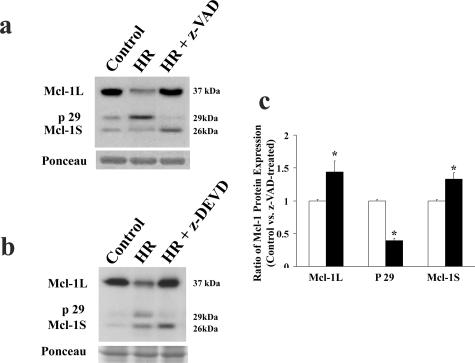
Inhibition of caspase activity and its effect on Mcl-1 cleavage. a: Representative immunoblot of Mcl-1 isoforms in control (untreated explant) and explants exposed to HR in the presence of 100 μmol/L concentration of pan-caspase inhibitor z-VAD-fmk relative to vehicle treatment (control). b: Representative Mcl-1 immunoblot of untreated control explants and explants exposed to HR (3 hours at 3% O2 followed by 2 hours at 20% O2) in the presence or absence of caspase-3 inhibitor z-DEVD-fmk. c: Densitometric analysis of Mcl-1-specific protein isoforms between HR (open bar)- and HR + z-VAD-fmk (black bar)-treated explants. *P < 0.05. n = 5 separate experiments performed in triplicate.
Oxygen Regulation of Mcl-1 in First Trimester Human Placental Tissues
Because preeclampsia is associated with placental hypoxia/oxidative stress, the effects of varying oxygenation on Mcl-1 expression were assessed in vitro using villous explants exposed to 20% oxygen, 3% oxygen, and HR. In 3% oxygen (normal oxygen tension for <8 weeks) relative to standard 20% O2 conditions, mRNA expression of Mcl-1L significantly increased (more than 2.5-fold, P = 0.0095), whereas that of Mcl-1S decreased (0.2-fold, P = 0.01) (Figure 3a). The opposite expression profile was observed under HR conditions in which Mcl-1L decreased (twofold, P = 0.01) and Mcl-1S increased (2.5-fold, P = 0.01) relative to 20% control (Figure 3a). The protein profile of Mcl-1 under 3% oxygen showed increased Mcl-1L expression relative to the 20% O2 controls (Figure 3b). Importantly, HR resulted in markedly decreased levels of anti-apoptotic Mcl-1L and a concomitant increased formation of proapoptotic Mcl-1c (p29 Mcl-1) relative to 20% O2 (Figure 3b). Although the expression of Mcl-1S at the transcript level was markedly increased in HR, this pattern was not evident at the protein level.
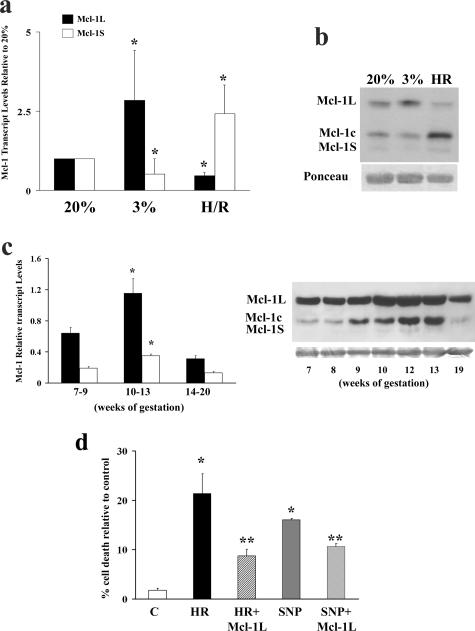
Effect of varying oxygenation on Mcl-1 expression. a: qRT-PCR analyses of Mcl-1L (black bars) and Mcl-1S (open bars) in first trimester villous explants exposed to 20% O2, 3% O2, and HR. b: Representative immunoblot of Mcl-1 isoforms in first trimester villous explants exposed to 20% O2, 3% O2, and HR. c: Left: qRT-PCR analyses of Mcl-1L (black bars) and Mcl-1S (open bars) in placental tissue from 7 to 20 weeks of gestation; right: Mcl-1 immunoblot of normal first and second trimester placental tissues. d: Percentage of cell death measured by trypan blue exclusion in JEG-3 cells transfected with Mcl-1L and subsequently subjected to HR (3 hours at 3% O2 followed by 2 hours at 20% O2) and SNP treatments. All immunoblots were confirmed for equal protein loading using Ponceau staining. *P < 0.05 versus C; **P < 0.05 versus HR and SNP.
During first trimester, the human placenta experiences a surge in oxygenation ~10 to 12 weeks of gestation when the intervillous space opens to maternal circulation. Rapid placental oxygenation, similar to a reperfusion injury, may hence result in a transient state of oxidative stress.37 Therefore, we hypothesized that Mcl-1 cleavage would occur at that period. Indeed, there was increased mRNA expression of both Mcl-1L and Mcl-1S (Figure 3c, left) and formation of Mcl-1c (p29 Mcl-1) at 10 to 13 weeks (coinciding with the placental surge of oxidative stress), which declined at later gestational ages (Figure 3c, right).
Next, we assessed the function of Mcl-1L under conditions of oxidative stress (HR) because this condition has been shown to be the strongest inducer of Mtd-L and -P expression.11 Because SNP, a nitric oxide donor, is a powerful inducer of cell death,38 SNP treatment was used as positive control. Both HR and SNP treatments of JEG-3 cells significantly increased cell death when compared with cells maintained at 20% O2 (Figure 3d). Mcl-1L overexpression in JEG-3 cells significantly decreased HR or SNP-induced cell death when compared with cells transfected with empty vector (Figure 3d).
Mcl-1 Expression in in Vivo Chronic Placental Hypoxia
As previously demonstrated, preeclamptic placentae have a global gene expression similarity to placental tissues obtained from HA pregnancies because both conditions may be affected by aberrant placental oxygenation.39 As such, Mcl-1 expression was next examined under physiologically reduced placental oxygenation using HA placentae from normal pregnancies. Placental Mcl-1 isoform mRNA expression was different between HA and MA relative to SL control placentae (SL). Although Mcl-1L transcript was significantly increased in HA (~2.3-fold, P = 0.005) and MA (~2-fold, P = 0.022) relative to SL, Mcl-1S transcript levels were significantly decreased in HA and MA relative to SL samples (HA versus SL, ~0.4-fold, P < 0.05; MA versus SL, ~0.5-fold, P = 0.016) (Figure 4, a and b).
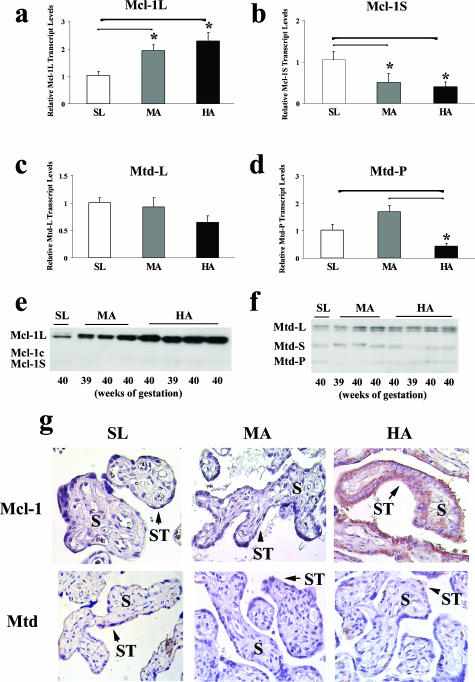
Transcript expression of Mcl-1 and Mtd isoforms in placental tissue from SL, MA, and HA pregnancies. a–d: qRT-PCR analysis of Mcl-1L (anti-apoptotic), Mcl-1S (proapoptotic), Mtd-L (proapoptotic), and Mtd-P (proapoptotic) transcript expression in SL (n = 10), MA (n = 10), and HA (n = 16) placentae. *P < 0.05. e and f: Mcl-1 and Mtd immunoblots of protein lysates obtained from SL, MA, and HA placentae. All immunoblots were confirmed for equal protein loading using Ponceau staining (not depicted). g: Immunohistochemical localization of Mcl-1 (top) and Mtd (bottom) in SL, MA, and HA placentae. S, stroma; ST, syncytium.
Because Mtd is one of the interacting partners of Mcl-113,14 and Mtd-L and Mtd-P expressions have been shown to increase in preeclamptic placentae as well as under conditions of oxidative stress,11 their expression was herein examined in conditions of chronic hypoxia. Although Mtd-L mRNA expression was not significantly different between the various groups, the number of Mtd-P transcripts was significantly decreased in HA samples relative to MA (4-fold, P < 0.0001) and SL tissues (0.5-fold, P = 0.04) (Figure 4d).
Similar to Mcl-1 mRNA expression, Mcl-1L protein expression was increased in HA and to a lesser extent in MA when compared with SL samples (Figure 4e). Both Mcl-1Lc and Mcl-1S molecules were hardly detectable at the protein level in SL, MA, and HA samples with no apparent change in expression (Figure 4e). Although expression of all known Mtd proteins (L: ~28 kd; S: ~18 kd; and P: ~15 kd) could be observed, no apparent difference in Mtd expression was noted between the conditions tested (Figure 4f).
Mcl-1 immunoreactivity in placental sections from SL, MA, and HA samples was predominantly observed in trophoblast cell layers (Figure 4g, top) with low to absent staining in stromal cells. Mtd immunoreactivity was generally low and also restricted to trophoblast cells. Positive immunoreactivity for Mtd was greater in SL tissue compared with HA and MA tissues (Figure 4g, bottom). No positive staining was observed in control sections in which Mtd and McL-1 antibodies were replaced with nonimmune IgG (data not shown).
Caspase Activation in in Vivo Chronic and Pathological Placental Hypoxia
Excessive cell death via death receptor is a well-known phenomenon occurring frequently in trophoblast cells of preeclamptic placentae.3,40,41 Although this death pathway was described previously, downstream events related to caspase-8 activation have not been explored in PE tissues. Relative to AMC tissues, cleaved caspase-8 was increased in severe early-onset preeclampsia (Figure 5a, left), consistent with previous reports showing increased activation of caspase-3.42
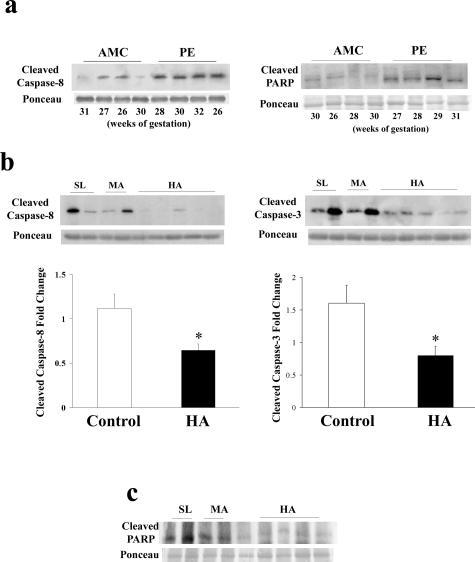
Markers of cell death in conditions of chronic and pathological placental hypoxia. a: Representative cleaved caspase-8 and p85 PARP immunoblots in AMC and PE tissues. b: Top: Representative immunoblots of cleaved caspase-3 and -8 in HA and control tissues (SL and MA); bottom: densitometric analysis of cleaved caspase-3 and -8 protein in HA (n = 12) relative to control tissues (SL and MA, n = 18). SL samples were set at 1 and all other (MA and HA) samples were normalized to the SL samples. Values of MA and SL samples were combined together as controls to which HA values were compared. c: Representative immunoblot of p85 PARP in HA and control tissues (SL and MA). All immunoblots were confirmed for equal protein loading using Ponceau staining. *P < 0.05.
Because we observed a shift in the Mtd/Mcl-1 apoptotic rheostat toward increased prosurvival and decreased death-inducing isoforms in HA, we also measured markers of trophoblast cell death in HA and control placentae. Expression of cleaved caspase-3, a known marker of trophoblast apoptosis,11,32 was reduced in HA by 50% (P = 0.0297) relative to controls (MA and SL) (Figure 5b, right). Similar to cleaved caspase-3, expression of activated caspase-8 in HA was decreased by 40% relative to controls (MA and SL) (P = 0.0335) (Figure 5b, left). We validated our apoptosis findings by measuring the p85 fragment of cleaved poly-ADP-ribose polymerase (PARP), another classical marker of apoptosis. As anticipated, p85 PARP was increased in preeclamptic tissue relative to age-matched normotensive controls (Figure 5a, right), whereas a marked decrease in p85 PARP content was observed in HA samples relative to SL and MA tissues (Figure 5c).
Trophoblast Cell Fusion in Pathological and in Vivo Chronic Placental Hypoxia
Because placentae from HA pregnancies exhibit thinning of trophoblast membranes possibly because of reduced trophoblast cell death/turnover,43 we next investigated the expression of syncytin,44,45 a typical marker for cytotrophoblast fusion into syncytium. HA samples had significantly reduced syncytin mRNA expression relative to MA or SL tissues (Figure 6a). The decreased syncytin expression at HA was not because of a shift in trophoblast cell population because expression of trophoblast-specific cytokeratin 7 was similar in HA and SL placentae (Figure 6b).
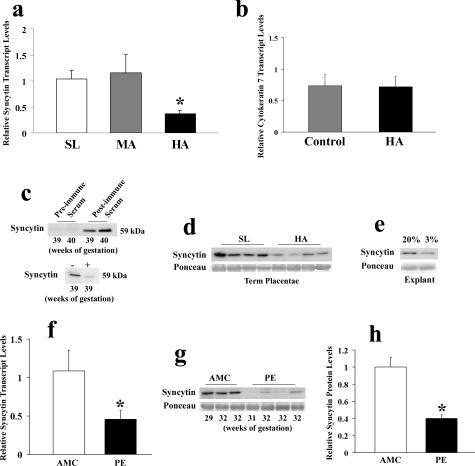
Syncytin protein and transcript expression in conditions of chronic placental hypoxia (HA placentae). a: qRT-PCR analysis of syncytin in SL (n = 8), MA (n = 10), and HA (n = 10) placental tissues. b: qRT-PCR analysis of cytokeratin 7 in HA (n = 9) and control tissues (SL and MA, n = 9). c: Representative immunoblot performed on total protein lysates from third trimester normal tissues with preimmune control serum, serum of rabbits immunized with syncytin peptide (top) and serum preadsorbed with peptide (bottom). d: Representative syncytin immunoblot performed on total protein lysate from HA and control (SL) placental tissues. e: Representative syncytin immunoblot performed on total protein lysate from explants maintained under 20% and 3% oxygen. f: qRT-PCR analysis of syncytin in AMC (n = 10) and PE (n = 8) placental tissues. g: Representative syncytin immunoblot performed on total placental protein lysates from AMC and PE tissues. h: Densitometric syncytin protein analysis between AMC (n = 13) and early-onset preeclamptic tissues (PE, n = 13). All immunoblots were confirmed for equal protein loading using Ponceau staining. *P < 0.05.
To characterize further the expression of the fusogenic syncytin protein, a polyclonal anti-syncytin antibody was generated. Antibody specificity was tested on placental protein lysates from the same third trimester samples with either preimmune or postimmune serum. A specific band at ~59 kd (the theoretical molecular weight of syncytin) was recognized only when the postimmune serum of rabbits was used (Figure 6c). This band was significantly reduced when competed with the peptide used for immunization (Figure 6c). Similar to transcript levels, syncytin protein expression was decreased in conditions of chronic placental hypoxia as determined by expression in HA placentae relative to control samples (Figure 6d). Exposing first trimester explants to 3% O2 also resulted in decreased syncytin expression (Figure 6e). We also tested syncytin expression in pathological conditions of placental hypoxia and found that syncytin mRNA and protein expression levels were reduced in preeclamptic placentae relative to controls (Figure 6, f–h), in agreement with previous reports.46,47,48 Last, we examined the spatial localization of syncytin (Figure 7). In AMC SL placentae, syncytin immunostaining localized to the trophoblast layers. Positive immunoreactivity for syncytin was markedly decreased in the trophoblast layers of both HA and PE placentae.
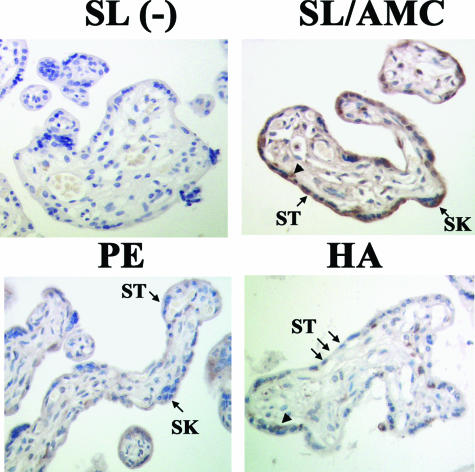
Spatial localization of syncytin in placental tissue from SL, HA, and PE pregnancies. Immunohistochemical localization of syncytin in SL (top right), PE (bottom left), and HA placentae (bottom right). AMC control placentae showed a similar staining pattern as SL placentae. Negative control (−) (top right). ST, syncytium; SK, syncytial knots; arrowheads, cytotrophoblasts.
Discussion
During normal placentation, a balance between proliferation, differentiation, and apoptosis is required to regulate cellular homeostasis and maintenance of proper placental function. Although it is now established that apoptosis is a key physiological event in placental tissue morphogenesis, the underlying mechanisms coordinating cell death in normal and abnormal placentation remain to be elucidated. Data presented herein demonstrate that pathological oxygenation versus physiological low pO2 induces a detrimental switch in the trophoblast Mcl-1/Mtd apoptotic rheostat, likely contributing to dysregulated cell death and leading to placental pathology. In particular, we demonstrate that 1) Mcl-1 isoform expression as well as its caspase-3-mediated cleavage are oxygen-dependent events in the human placenta; 2) Mcl-1 protein profile is tilted toward expression of cell death promoting isoforms in severe early-onset preeclampsia and toward protective isoforms in altitude-induced chronic placental hypoxia; and finally, 3) although trophoblast cell fusion is reduced in both physiological and pathological placental hypoxia, molecular markers of trophoblast cell death are decreased in HA pregnancies and increased in preeclampsia.
Among Bcl-2 family members, Mcl-1 function is uniquely regulated via complex transcriptional, posttranscriptional, and post-translational processes. The PEST domain found in both Mcl-1 splicing isoforms is recognized as a substrate for caspase-3-mediated cleavage.24 This cleavage is a unique regulatory mechanism conferring differential Mcl-1 protein function.21,22,23,24,25,26 Mcl-1L is a potent anti-apoptotic molecule believed to sequester other members of the proapoptotic channel forming Bcl-2 subfamily such as Bak.49 Although it has been established that Mcl-1L neutralizes the killing ability of Mtd/Bok,13 the precise molecular events leading to suppression of cell death in this pathway remain unclear.13
Oxygen is a potent regulator of apoptotic cell death. Several Bcl-2 family members, including BH3-only ligands such as Nix and Nip as well as Mcl-1,28 have been shown to be directly regulated by oxygen via HIF-1.50,51 Our observation of increased Mcl-1L expression under in vitro or in vivo reduced oxygenation is consistent with HIF-1 mediated Mcl-1L regulation.28 Furthermore, we found increased processing of proapoptotic Mcl-1c and Mcl-1S in vivo at 10 to 13 weeks, when trophoblast cells experiences a rapid surge in oxygenation, and in vitro in villous explants undergoing HR injury. Thus, transcriptional regulation of Mcl-1 and differential expression of its isoforms are likely attributable to specific oxygen conditions experienced by the placental tissue. Although the expression of Mcl-1S at the transcript level was markedly increased in HR, this pattern was not evident at the protein level. This could be explained by the fact that Mcl-1S, being a low abundant isoform (relative to the L), is rapidly degraded/cleaved at the protein level because of caspase activation under HR conditions or could also reflect alteration of splicing without a concomitant elevation in translation or increased McL-1S stabilization. Thus, to our knowledge this is the first evidence of regulation of Mcl-1 expression and cleavage by oxygen in a human organ and importantly dysregulation of these events in a human disorder.
In severe early-onset preeclampsia, excess trophoblast cell death, likely induced by hypoxia (~10 mmHg or <1 to 2% O2)52,53 or intermittent oxygenation36 increases trophoblast shedding, which is believed to generate a Th1-type maternal inflammatory response and generalized maternal endothelial cell injury.54,55 A tilt in Mcl-1 expression toward generation of death-inducing molecules in severe preeclamptic placentae combined with increased expression of killer Mtd-P isoform in this disease,11 initiates a detrimental pathological switch toward trophoblast demise accompanied by increased shedding. Our transfection studies showing that anti-apoptotic Mcl-1L overexpression can rescue trophoblast (JEG-3) cells from undergoing apoptosis under detrimental oxygen conditions supports the idea that pathological reduced oxygen tilts the Mcl-1/Mtd balance toward cell death. In contrast to preeclamptic placentae, placental tissues from HA experience chronically reduced oxygenation, estimated at 20% below the intervillous pO2 of ~60 mmHg (8% O2) observed in near term SL pregnancy or the equivalent of 5 to 6% O2. Interestingly, normal placentae from HA conditions display decreased markers of apoptosis and increased Mcl-1L expression. In support of our molecular findings, previous studies have reported fewer areas of syncytial damage or denudations in HA placentae relative to lower altitudes,56 again suggesting slowed apoptosis-mediated trophoblast turnover. Hence, conditions of chronic hypoxia may actually stimulate an adaptive molecular response by minimizing the burden of apoptotic-mediated trophoblast shedding in these pregnancies. Such speculation may be controverted since an association between HA residence and an increased incidence of preeclampsia has been reported.57 Although the altitude pattern of increased placental expression of anti-apoptotic molecules in HA tissues may to a certain extent protect against trophoblast cell death, other factors may contribute to the increased incidence of preeclampsia under chronic hypoxia.
Similar to increased Mtd-L and Mtd-P expression in early-onset severe preeclampsia,11 cleavage of Mcl-1L in this pathology may be another unique placental feature of the early severe form of this disease, which does not occur in control aged-matched patients, in late-onset preeclampsia even with IUGR or in other placental pathologies, including idiopathic IUGR and essential hypertension. This molecular evidence once again reiterates the need and importance for proper pathological classification of preeclampsia, conceivably because of different underlying etiologies involved in the pathogenesis of this multifaceted and often misconstrued disorder.
Normal physiological regulation of trophoblast cell fusion utilizes a molecular apoptotic cascade in which mononucleated cytotrophoblast cells fuse to maintain a multinucleated syncytiotrophoblast cell layer.58 Normal SL term placentae have a well-defined syncytiotrophoblast layer, which is in contrast to HA placentae, which show thinning of the syncytiotrophoblast layer and a hyperproliferative cytotrophoblast phenotype59 suggesting decreased syncytial renewal under conditions of reduced oxygenation.56,60,61 Decreased expression of active caspase-8, a promoter of trophoblast cell fusion,62 in conjunction with reduction of other cell death markers in HA placentae, as demonstrated by decreased cleaved p85 PARP formation, suggests a slowdown of syncytial formation and consequently trophoblast shedding during chronic placental hypoxia. Interestingly, in pathological placental hypoxia (preeclampsia), increase in caspase-336,42 and caspase-8 activation together with cleavage of PARP indicate increased apoptosis. Activation of caspase-8 in PE could also be secondary because of aberrant expression of its upstream activators such as activation of death receptors by elevated FasL and tumor necrosis factor-α as previously reported.6,7,9,10,11,40,41 Decreased syncytin expression, a key regulator of trophoblast cell fusion,44,45 may also negatively impact normal trophoblast differentiation resulting in thinning of the placental syncytium at HA. Studies have shown that in conditions of reduced oxygenation, syncytin as well as its binding receptor (ASCT2) are down-regulated relative to standard oxygenation conditions.63 Decreased syncytin expression in pregnancies complicated by preeclampsia has also been reported.46,48 These findings support our observations of reduced syncytin expression in conditions of altitude-induced placental hypoxia or pathological hypoxia (PE) relative to control. Impaired regulation of trophoblast fusion in HA placentae may limit de novo syncytial synthesis and maintenance at the expense of normal shedding/deportation of syncytial debris. Hence, decrease in the dynamic rate of syncytial renewal and shedding may provide a molecular explanation with respect to the thinned syncytial phenotype observed in HA placentae.
In conclusion, this study provides evidence that the Mcl-1/Mtd rheostat regulates trophoblast apoptosis in a different manner under physiological and pathological conditions of placental hypoxia. Although both preeclampsia and HA placentation are characterized by aberrant villous trophoblast differentiation, possibly because of down-regulation of syncytin expression; only preeclamptic placentae experience an apoptotic insult, believed to be responsible for accelerated trophoblast turnover. In HA pregnancies, decreased trophoblast cell death in conjunction with decreased trophoblast turnover/differentiation may present an important adaptive response to chronic placental hypoxia. The impact of aberrant oxygenation on Mcl-1 isoform expression and stability in preeclamptic placental tissues may influence events leading to the clinical manifestations of this disease.
Footnotes
Address reprint requests to Isabella Caniggia, M.D., Ph.D., Mount Sinai Hospital, Samuel Lunenfeld Research Institute, 600 University Ave., Room 871C, Toronto, ON, Canada M5G 1X5. E-mail: ac.no.irhsm@aigginac.
Supported by the Canadian Institutes of Health Research (grant MOP-62845, a new investigator award to A.J., and a mid-career award from the Ontario Women’s Health Council to I.C.), the National Institutes of Health (grant HD042737), the Hospital for Sick Children (Restracomp training program grant to N.S.), and the University of Toronto (Ontario graduate scholarship program grant to N.S.).
M.P. is the holder of a Canadian research chair (tier 1) in respiration.
References
- Hsu SY, Hsueh AJ. Tissue-specific Bcl-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev. 2000;80:593–614. [Abstract] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. [Abstract] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. [Abstract] [Google Scholar]
- Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med. 1992;326:927–932. [Abstract] [Google Scholar]
- Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67. [Abstract] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. [Abstract] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. [Abstract] [Google Scholar]
- Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. [Abstract] [Google Scholar]
- Leung DN, Smith SC, To KF, Sahota DS, Baker PN. Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2001;184:1249–1250. [Abstract] [Google Scholar]
- Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. Am J Pathol. 2003;162:637–643. [Europe PMC free article] [Abstract] [Google Scholar]
- Soleymanlou N, Wu Y, Wang JX, Todros T, Ietta F, Jurisicova A, Post M, Caniggia I. A novel Mtd splice isoform is responsible for trophoblast cell death in pre-eclampsia. Cell Death Differ. 2005;12:441–452. [Abstract] [Google Scholar]
- Jurisicova A, Detmar J, Caniggia I. Molecular mechanisms of trophoblast survival: from implantation to birth. Birth Defects Res C Embryo Today. 2005;75:262–280. [Abstract] [Google Scholar]
- Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA. 1997;94:12401–12406. [Europe PMC free article] [Abstract] [Google Scholar]
- Inohara N, Ekhterae D, Garcia I, Carrio R, Merino J, Merry A, Chen S, Nunex G. Mtd, a novel Bcl-2 family member activates apoptosis in the absence of heterodimerization with Bcl-2 and Bcl-XL. J Biol Chem. 1998;273:8705–8710. [Abstract] [Google Scholar]
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA. 1993;90:3516–3520. [Europe PMC free article] [Abstract] [Google Scholar]
- Yang T, Kozopas KM, Craig RW. The intracellular distribution and pattern of expression of Mcl-1 overlap with, but are not identical to, those of Bcl-2. J Cell Biol. 1995;128:1173–1184. [Europe PMC free article] [Abstract] [Google Scholar]
- Zhou P, Qian L, Bieszczad CK, Noelle R, Binder M, Levy NB, Graig RW. Mcl-1 in transgenic mice promotes survival in a spectrum of hematopoietic cell types and immortalization in the myeloid lineage. Blood. 1998;92:3226–3239. [Abstract] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. [Abstract] [Google Scholar]
- Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–25261. [Abstract] [Google Scholar]
- Bingle CD, Craig RW, Swales BM, Singleton V, Zhou P, Whyte MK. Exon skipping in Mcl-1 results in a bcl-2 homology domain 3 only gene product that promotes cell death. J Biol Chem. 2000;275:22136–22146. [Abstract] [Google Scholar]
- Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–10500. [Abstract] [Google Scholar]
- Snowden RT, Sun XM, Dyer MJ, Cohen GM. Bisindolylmaleimide IX is a potent inducer of apoptosis in chronic lymphocytic leukaemic cells and activates cleavage of Mcl-1. Leukemia. 2003;17:1981–1989. [Abstract] [Google Scholar]
- Han J, Goldstein LA, Gastman BR, Froelich CJ, Yin XM, Rabinowich H. Degradation of Mcl-1 by granzyme B: implications for Bim-mediated mitochondrial apoptotic events. J Biol Chem. 2004;279:22020–22029. [Abstract] [Google Scholar]
- Han J, Goldstein LA, Gastman BR, Rabinovitz A, Rabinowich H. Disruption of Mcl-1.Bim complex in granzyme B-mediated mitochondrial apoptosis. J Biol Chem. 2005;280:16383–16392. [Abstract] [Google Scholar]
- Michels J, O’Neill JW, Dallman CL, Mouzakiti A, Habens F, Brimmell M, Zhang KY, Craig RW, Marcusson EG, Johnson PW, Packham G. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene. 2004;23:4818–4827. [Abstract] [Google Scholar]
- Herrant M, Jacquel A, Marchetti S, Belhacene N, Colosetti P, Luciano F, Auberger P. Cleavage of Mcl-1 by caspases impaired its ability to counteract Bim-induced apoptosis. Oncogene. 2004;23:7863–7873. [Abstract] [Google Scholar]
- Gao S, Fu W, Durrenberger M, De Geyter C, Zhang H. Membrane translocation and oligomerization of hBok are triggered in response to apoptotic stimuli and Bnip3. Cell Mol Life Sci. 2005;62:1015–1024. [Abstract] [Google Scholar]
- Piret JP, Minet E, Cosse JP, Ninane N, Debacq C, Raes M, Michiels C. Hypoxia-inducible factor-1-dependent overexpression of Mcl-1 protects hypoxic cells against ter-butyl hydroperoxide-induced apoptosis. J Biol Chem. 2005;280:9336–9344. [Abstract] [Google Scholar]
- American College of Obstetricians and Gynecologists ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Int J Gynaecol Obstet. 2002;77:67–75. [Abstract] [Google Scholar]
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. [Europe PMC free article] [Abstract] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFβ3. J Clin Invest. 2000;105:577–587. [Europe PMC free article] [Abstract] [Google Scholar]
- Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-β3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–1650. [Europe PMC free article] [Abstract] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods. 2001;25:402–408. [Abstract] [Google Scholar]
- MacPhee DJ, Mostachfi H, Han R, Lye SJ, Post M, Caniggia I. Focal adhesion kinase is a key mediator of human trophoblast development. Lab Invest. 2001;81:1469–1483. [Abstract] [Google Scholar]
- Moore NK, Viselli SM. Staining and quantification of proteins transferred to polyvinylidene fluoride membranes. Anal Biochem. 2000;279:241–242. [Abstract] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. [Abstract] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. [Europe PMC free article] [Abstract] [Google Scholar]
- Yoshioka Y, Yamamuro A, Maeda S. Nitric oxide/cGMP signaling pathway protects RAW264 cells against nitric oxide-induced apoptosis by inhibiting the activation of p38 mitogen-activated protein kinase. J Pharmacol Sci. 2006;101:126–134. [Abstract] [Google Scholar]
- Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–4308. [Europe PMC free article] [Abstract] [Google Scholar]
- Hsu CD, Harirah H, Basherra H, Mor G. Serum soluble Fas levels in preeclampsia. Obstet Gynecol. 2001;97:530–532. [Abstract] [Google Scholar]
- Black S, Kadyrov M, Kaufmann P, Ugele B, Emans N, Huppertz B. Syncytial fusion of human trophoblast depends on caspase 8. Cell Death Differ. 2004;11:90–98. [Abstract] [Google Scholar]
- Aban M, Cinel L, Arslan M, Dilek U, Kaplanoglu M, Arpaci R, Dilek S. Expression of nuclear factor-κB and placental apoptosis in pregnancies complicated with intrauterine growth restriction and preeclampsia: an immunohistochemical study. Tohoku J Exp Med. 2004;204:195–202. [Abstract] [Google Scholar]
- Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–191. [Abstract] [Google Scholar]
- Frendo JL, Olivier D, Cheynet V, Blond JL, Bouton O, Vidaud M, Evain-Brion D, Mallet F. Direct involvement of HERV-W Env glyco-protein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23:3566–3574. [Europe PMC free article] [Abstract] [Google Scholar]
- Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, Keith JC, Jr, McCoy JM. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. [Abstract] [Google Scholar]
- Knerr I, Beinder E, Rascher W. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am J Obstet Gynecol. 2002;186:210–213. [Abstract] [Google Scholar]
- Keith JC, Jr, Pijnenborg R, Van Assche FA. Placental syncytin expression in normal and preeclamptic pregnancies. Am J Obstet Gynecol. 2002;187:1122–1124. [Abstract] [Google Scholar]
- Lee X, Keith JC, Jr, Stumm N, Moutsatsos I, McCoy JM, Crum CP, Genest D, Chin D, Ehrenfels C, Pijnenborg R, van Assche FA, Mi S. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22:808–812. [Abstract] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97:9082–9087. [Europe PMC free article] [Abstract] [Google Scholar]
- Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res. 2001;61:6669–6673. [Abstract] [Google Scholar]
- Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. Am J Physiol. 2000;278:C982–C988. [Abstract] [Google Scholar]
- Kilani RT, Mackova M, Davidge ST, Guilbert LJ. Effect of oxygen levels in villous trophoblast apoptosis. Placenta. 2003;24:826–834. [Abstract] [Google Scholar]
- Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. [Abstract] [Google Scholar]
- Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–160. [Abstract] [Google Scholar]
- Mayhew TM, Bowles C, Yucel F. Hypobaric hypoxia and villous trophoblast: evidence that human pregnancy at high altitude (3600 m) perturbs epithelial turnover and coagulation-fibrinolysis in the intervillous space. Placenta. 2002;23:154–162. [Abstract] [Google Scholar]
- Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–1168. [Abstract] [Google Scholar]
- Huppertz B, Frank HG, Reister F, Kingdom J, Korr H, Kaufmann P. Apoptosis cascade progresses during turnover of human trophoblast: analysis of villous cytotrophoblast and syncytial fragments in vitro. Lab Invest. 1999;79:1687–1702. [Abstract] [Google Scholar]
- Ali KZ. Stereological study of the effect of altitude on the trophoblast cell populations of human term placental villi. Placenta. 1997;18:447–450. [Abstract] [Google Scholar]
- Jackson MR, Mayhew TM, Haas JD. Morphometric studies on villi in human term placentae and the effects of altitude, ethnic grouping and sex of newborn. Placenta. 1987;8:487–495. [Abstract] [Google Scholar]
- Alsat E, Wyplosz P, Malassine A, Guibourdenche J, Porquet D, Nessmann C, Evain-Brion D. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168:346–353. [Abstract] [Google Scholar]
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. [Abstract] [Google Scholar]
- Kudo Y, Boyd CA, Sargent IL, Redman CW. Hypoxia alters expression and function of syncytin and its receptor during trophoblast cell fusion of human placental BeWo cells: implications for impaired trophoblast syncytialization in pre-eclampsia. Biochim Biophys Acta. 2003;1638:63–71. [Abstract] [Google Scholar]
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Read article at publisher's site: https://doi.org/10.2353/ajpath.2007.070094
Read article for free, from open access legal sources, via Unpaywall:
http://ajp.amjpathol.org/article/S0002944010619839/pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2353/ajpath.2007.070094
Article citations
Understanding the intersection between placental development and cancer: Lessons from the tumor suppressor BAP1.
Commun Biol, 7(1):1053, 27 Aug 2024
Cited by: 0 articles | PMID: 39191942 | PMCID: PMC11349880
Review Free full text in Europe PMC
Diagnosis, Prevention, and Management of Fetal Growth Restriction (FGR).
J Pers Med, 14(7):698, 28 Jun 2024
Cited by: 1 article | PMID: 39063953 | PMCID: PMC11278205
Review Free full text in Europe PMC
Ancestry dependent balancing selection of placental dysferlin at high-altitude.
Front Cell Dev Biol, 11:1125972, 21 Mar 2023
Cited by: 0 articles | PMID: 37025168 | PMCID: PMC10070852
CLDN1 regulates trophoblast apoptosis and proliferation in preeclampsia.
Reproduction, 161(6):623-632, 05 May 2021
Cited by: 3 articles | PMID: 33784242 | PMCID: PMC8111329
The Multifaceted Roles of the BCL-2 Family Member BOK.
Front Cell Dev Biol, 8:574338, 15 Sep 2020
Cited by: 21 articles | PMID: 33043006 | PMCID: PMC7523462
Review Free full text in Europe PMC
Go to all (36) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
RefSeq - NCBI Reference Sequence Database
- (1 citation) RefSeq - NM_001101
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Mtd/Bok takes a swing: proapoptotic Mtd/Bok regulates trophoblast cell proliferation during human placental development and in preeclampsia.
Cell Death Differ, 17(5):846-859, 27 Nov 2009
Cited by: 31 articles | PMID: 19942931
Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor.
Hum Reprod, 32(6):1218-1229, 01 Jun 2017
Cited by: 16 articles | PMID: 28402449 | PMCID: PMC6075585
Aberrant TGFβ Signaling Contributes to Altered Trophoblast Differentiation in Preeclampsia.
Endocrinology, 157(2):883-899, 14 Dec 2015
Cited by: 27 articles | PMID: 26653761
Epigenetic and non-epigenetic regulation of syncytin-1 expression in human placenta and cancer tissues.
Cell Signal, 26(3):648-656, 09 Nov 2013
Cited by: 28 articles | PMID: 24216608
Review
Funding
Funders who supported this work.
NICHD NIH HHS (3)
Grant ID: R01 HD042737-04
Grant ID: HD042737
Grant ID: R01 HD042737





