Abstract
Free full text

Iron metabolism at the host pathogen interface: lipocalin 2 and the pathogen-associated iroA gene cluster
Abstract
The host innate immune defense protein lipocalin 2 binds bacterial enterobactin siderophores to limit bacterial iron acquisition. To counteract this host defense mechanism bacteria have acquired the iroA gene cluster, which encodes enzymatic machinery and transporters that revitalize enterobactin in the form of salmochelin. The iroB enzyme introduces glucosyl residues at the C5 site on 2,3-dihydroxybenzoylserine moieties of enterobactin and thereby prevents lipocalin 2 binding. Additional strategies to evade lipocalin 2 have evolved in other bacteria, such as Mycobacteria tuberculosis and Bacillus anthracis. Targeting these specialized bacterial evasion strategy may provide a mechanism to reinvigorate lipocalin 2 in defense against specific pathogens.
Introduction
Iron is a necessity for most forms of life, but also has chemical and biological properties that require tight regulation. In most living organisms iron is associated with small molecules, proteins or macromolecular complexes. Excess “free iron” can promote hydroxyl radical formation and injury to cells and increase the susceptibility of humans to bacterial infections. Prokaryotes and eukaryotes have evolved a multitude of exquisite biological systems to acquire, transport and store this molecule (Ganz, 2007; Wandersman & Delepelaire, 2004). The apparent redundancy in iron acquisition systems reflects the selective advantage of robust and highly competitive strategies to acquire iron. Iron metabolism is also targeted as a host defense mechanism to prevent the growth of microbial pathogens (Radtke & O’Riordan, 2006), and several host proteins involved in iron metabolism are regulated during inflammatory processes, including transferrin, ferritin, NRAMP1, hepcidin, ferroportin, lactoferrin, and lipocalin 2.
Microbes also compete with one another, and have evolved multiple mechanisms to selectively acquire iron, either directly in the ferrous form, complexed to host molecules, or bound to endogenous or exogenous siderophores. Microbial siderophore transport mechanisms are also targets of a new class of antibiotics, termed siderophore-peptides, which tether anti-microbial peptides of the microcin class to the enterobactin family of siderophores (Thomas et al., 2004). This Trojan horse strategy allows bacteria to eliminate competitors that use any of several different transporters for enterobactin-like siderophores. This review will focus on lipocalin 2, a host strategy to prevent enterobactin-mediated iron utilization, and the bacterial countermeasures employed to evade this host innate immune defense mechanism and preserve enterobactin-based systems of iron acquisition.
Structures of enterobactin, salmochelins and lipochalin 2
(a) Enterobactin
Enterobactin (also known as enterochelin) is a tricyclic 2,3-dihydroxybenzoylserine (DHBS) molecule that binds iron with extraordinarily high affinity, and is made by many Gram-negative enteric bacteria (Raymond, Dertz, & Kim, 2003). Enterobactin, a linearized form of this molecule and the monomeric and dimeric DHBS precursors can all function as siderophores. Although enterobactin is a highly effective means of iron acquisition and it is widely used by microbes, enterobactin’s role in virulence is uncertain.
(b) Salmochelins
Salmochelins were first detected as alternative siderophores that are produced by Salmonella (Hantke, Nicholson, Rabsch, & Winkelmann, 2003). Molecular characterization demonstrated that salmochelins are glucosylated versions of enterobactin (Bister et al., 2004). There are several potential advantages of enterobactin glucosylation. First, enterobactin is a hydrophobic molecule and glucosylation increases its solubility in aqueous solutions, thereby increasing its biological availability and utility in certain environments. Second, glucosylation may affect stability in certain settings. Third, glucosylated enterobactin is not taken up by the enterobactin transporter, FepA, and requires a separate transporter, IroN, preventing competition by IroN-negative bacteria (Figure 1).
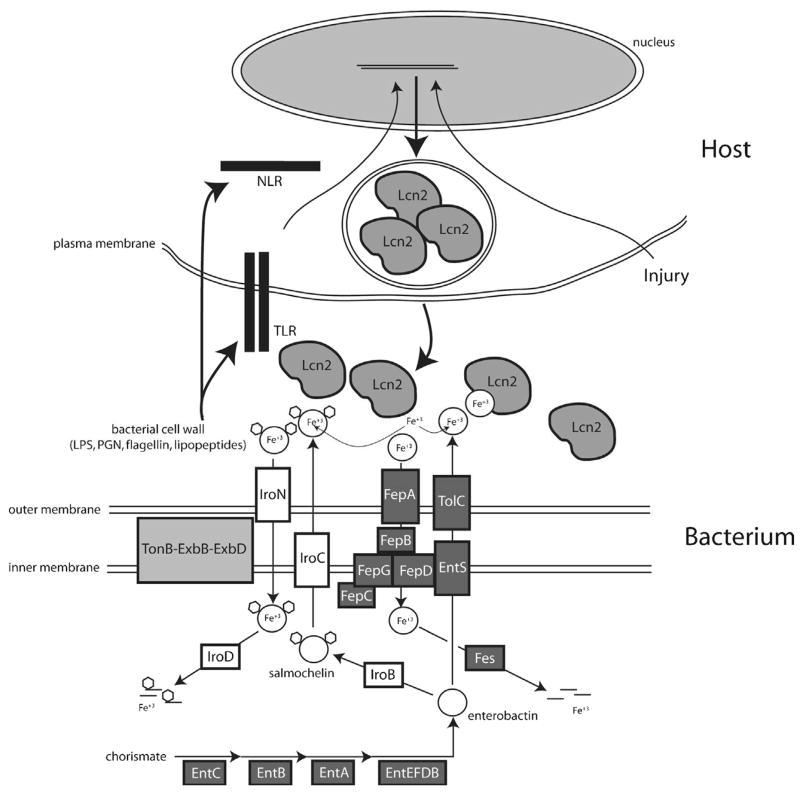
Bacteria synthesize enterobactin siderophores from chorismate using genes encoded by the ent operon. Enterobactin is transported out of the inner membrane via EntS, shuttled through the periplasmic space via an unknown mechanism, and secreted through the outer membrane protein TolC. Enterobactin can be glucosylated by IroB to generate salmochelin. The transport of salmochelin is still under investigation, and the functional role of IroC is disputed. The IroC protein has been implicated as a inner membrane exporter (Baumler et al., 1996) and an inner membrane importer (Zhu, Valdebenito, Winkelmann, & Hantke, 2005). We have illustrated the former model where salmochelin is exported through the inner membrane protein IroC, and then shuttled across the periplasmic space and secreted through the outer membrane via an unknown mechanism. Both siderophores bind iron and are transported into the cells by their respective transporters (FepA for enterobactin and IroN for salmochelin) in a TonB complex-dependent manner. An uncharacterized mechanism (possibly involving IroC) shuttles the salmochelin when bound to iron through the periplasm and across the inner membrane. FepB shuttles enterobactin through the periplasm and the FepD/FepG/FepC complex transports enterobactin across the inner membrane into the cell cytoplasm. Once in the bacterial cell cytoplasm, the siderophores are degraded to release iron. Host cells, such as macrophages, sense the presence of bacteria via their innate immune receptors (Toll-like receptors, TLR, and NOD- like receptors, NLR), and induce the expression and secretion of lipocalin 2 (Lcn2). Neutrophils constitutively express lipocalin 2 in their granules and release their granule contents upon activation. The secreted lipocalin 2 binds enterobactin and efficiently competes with FepA to prevent enterobactin-mediated iron acquisition. Salmochelin does not bind lipocalin 2 and circumvents this host defense mechanism. Other signals, such as ischemic or toxic injury, can induce lipocalin 2 expression via unknown mechanisms, presumably to fortify host defense in this state of increased vulnerability.
Further insight into the functional significance of enterobactin glucosylation came from studies that identified the iroA gene cluster as the locus that controls salmochelin biogenesis and utilization (Hantke et al., 2003). Interestingly, the iroA locus is present in a wide variety of pathogenic Gram-negative bacteria, suggesting that this locus contributes to virulence during infection. Indeed, animal studies confirmed that this locus contributes to virulence in mouse models of urinary tract and avian infections caused by E. coli (Russo et al., 2002), whereas evidence supporting the independent contribution of enterobactin receptors in bacterial virulence is lacking (Rabsch et al., 2003).
(c) Lipocalin 2
Lipocalin 2 is a protein with many different names (siderocalin, neutrophil gelatinase associated lipocalin or NGAL, uterocalin, neu-related lipocalin and oncoprotein 24p3) and a variety of putative functions (Devireddy, Teodoro, Richard, & Green, 2001; Goetz et al., 2002; Nielsen et al., 1996; Yang et al., 2002). Clarity to the biological role of lipocalin 2 came from structural studies of Roland Strong’s group, who definitively demonstrated that lipocalin 2 binds enterobactin type bacterial siderophores (Goetz et al., 2002). Additional studies demonstrated that lipocalin 2 has a broad specificity for siderophore binding, including carboxymycobactin and bacillibactin (Abergel et al., 2006; Holmes, Paulsene, Jide, Ratledge, & Strong, 2005). In addition, a putative lipocalin 2-binding mammalian siderophore has also been detected but not yet identified (Devireddy, Gazin, Zhu, & Green, 2005; Mori et al., 2005). Thus it is now apparent that lipocalin 2 binds several structurally diverse siderophores, and utilizes unique molecular contacts to affect ligand binding.
Structural modeling of salmochelin binding to lipocalin 2 provided an alternative explanation for the virulence trait of salmochelins relative to enterobactin. The introduction of a glucose residue at any 5 position of the DHBS molecules in enterobactin was predicted to sterically clash with residues in the siderophore binding pocket of lipocalin 2 (Fischbach et al., 2006). This analysis lead to the hypothesis that iroA gene cluster permits evasion of an important host defense mechanism – lipocalin 2-mediated sequestration of enterobactin. Biochemical studies, in vitro studies and in vivo infection models confirmed that salmochelins do not bind lipocalin 2, and that evasion of lipocalin 2 completely explained the increased virulence of iroA gene cluster-harboring E. coli during intraperitoneal infection (Fischbach et al., 2006).
This strategy of siderophore modification to evade lipocalin 2 binding is not unique to enterobactin. In fact the other known lipocalin 2 binding siderophores also have modified versions that are synthesized by human pathogens. Pathogenic Mycobacteria synthesize carboxymycobactins molecules that have long fatty acid moieties, which are predicted to interfere with lipocalin 2 binding (Holmes et al., 2005). Bacillus anthracis makes petrobactin, which also does not bind lipocalin 2 (Abergel et al., 2006). The requirement for pathogens to evade lipocalin 2 corroborates the effectiveness of lipocalin 2 in thwarting iron acquisition through binding to enterobactin, short-chain carboxymycobactins, and bacillibactin. It should also be noted that bacteria have evolved mechanisms to utilize host iron binding molecules, and have transporters for transferrin, lactoferrin, ferritin, hemes and heme-containing proteins. It is presently not known whether lipocalin 2 can also be utilized by bacteria for iron acquisition.
Functions of lipocalin 2
Given the complexity of iron acquisition mechanisms and the ability of many pathogens to evade lipocalin 2, what is its present day biological role? Is it just a remnant of the past or does lipocalin 2 prevent bacterial infections? As mentioned above the main testament to the biological significance of lipocalin 2 is the apparent necessity for pathogens to possess some means to evade it. Because lipocalin 2 exerts strong selective pressure on pathogenic bacteria, lipocalin 2 resistance is required for establishing a parasitic relationship with the host. Thus lipocalin 2 represents a barrier that keeps at bay other bacteria, some of which are our own commensals or flora. One would predict that some commensal bacteria may attain pathogenic qualities in the absence of lipocalin 2, and that its innate immune function is the selective force that has preserved lipocalin 2 in our genome (as well as the genomes of chimps, monkeys, cows, mice, rats, opossums and dogs).
Lipocalin 2 has also been attributed specific functions in several other biological processes, including neoplasia (Devireddy et al., 2001; Lin et al., 2005), kidney development and repair (Mori et al., 2005; Yang et al., 2002), implantation (Suire, Stewart, Beauchamp, & Kennedy, 2001), and involution of the uterus and mammary gland (Ryon, Bendickson, & Nilsen-Hamilton, 2002), which may also be relevant to lipocalin 2 biology. A role for lipocalin 2 in the induction of apoptosis was postulated based on in vitro studies (Devireddy et al., 2001). However, these results could not be replicated with lipocalin 2-deficient mice that were independently derived by two separate groups, indicating that lipocalin 2 is unlikely to have an obligate role in the induction of apoptosis (Berger et al., 2006; Flo et al., 2004). Similarly, lipocalin 2-deficient mice raised in specific pathogen-free facilities show no defects in reproduction, kidney development or iron metabolism (Flo et al., 2004), arguing against an obligate role for lipocalin 2 in these biological processes. However, these observations were made with mice that are raised in iron replete conditions, and redundant pathways may mask some of lipocalin 2’s biological potential. Additional studies in iron stressed states may provide alternative views and further insight into these putative functions of lipocalin 2.
Clinical applications
Multiple studies have now demonstrated a potential role for measuring serum or urine lipocalin 2 in patients. In the setting of acute renal failure lipocalin 2 is increased early and then normalizes, and is an early marker of injury to renal tubular epithelial cells. The ability to detect lipocalin 2 in the urine may allow detection of kidney injury earlier than traditional markers, such as serum creatinine and blood urea nitrogen. This along with the identification of other markers, such as KIM-1 (Vaidya, Ramirez, Ichimura, Bobadilla, & Bonventre, 2006), has fueled interest to develop diagnostic markers that detect and characterize kidney injury (Nguyen & Devarajan, 2007), analogous to the protein and enzyme markers that are currently used to diagnose myocardial infections. However, lipocalin 2 expression is not limited to the kidney. Lipocalin 2 is constitutively expressed in neutrophils and upregulated in the setting of sepsis and inflammation. During sepsis in mice, there is massive upregulation of lipocalin 2 expression in the liver and spleen, suggesting that lipocalin 2 functions as an acute phase protein (Flo et al., 2004). In addition, lipocalin 2 is expressed in many human tissues and upregulated in some tumors (Friedl, Stoesz, Buckley, & Gould, 1999; Nielsen et al., 1996). Thus lipocalin 2 may prove to be a useful diagnostic marker, but due to its pleiotropic expression, additional clinical and diagnostic information may be necessary to interpret abnormal lipocalin 2 levels in urine or serum.
The iroA gene cluster as a therapeutic target
The prevalence of the iroA gene cluster in pathogenic Gram-negative bacteria and its role in virulence support its use as a drug target. The evasion of lipocalin 2 appears to be critical for pathogenic bacteria, and especially for establishing systemic infections. Thus targeting this pathway of iron acquisition may render certain bacteria susceptible to lipocalin 2 and other host derived mechanisms that limit iron acquisition during infection. The advantage of targeting the iroA gene cluster lies in its strong association with pathogens. Thus targeting a pathogen-specific virulence factor limits the spectrum of bacteria that are affected by the antibiotic, thereby reducing the incidence of adverse effects associated with the use of broader range antibiotics such as gastrointestinal illnesses and secondary infections (eg Clostridium difficile).
Conclusions
The experimental analysis of lipocalin 2 has lead to an in depth molecular level understanding of host-pathogen interactions. To counteract this host defense mechanism designed to inhibit bacterial iron acquisition, bacteria have acquired enzymatic machinery and transporters that revitalize enterobactin in the form of salmochelins. This same counterattack has been employed in principle by other siderophores produced by Mycobacteria tuberculosis and Bacillus anthracis. The diversity of such mechanism to avoid lipocalin 2 suggests this gene has had strong selective pressure on bacterial pathogens. Targeting these specialized bacterial evasion strategies may provide a mechanism to reinvigorate lipocalin 2 in defense against specific pathogens, without disturbing our normal protective flora. In addition, we do not completely understand the role of lipocalin 2 in infectious, inflammatory and injury processes, and whether lipocalin 2 has physiological functions in iron metabolism in addition to its role in host defense. Understanding the regulation of lipocalin 2 and its function in infection, inflammation and tissue injury deserves further attention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abergel RJ, Wilson MK, Arceneaux JE, Hoette TM, Strong RK, Byers BR, et al. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci U S A. 2006;103(49):18499–18503. [Europe PMC free article] [Abstract] [Google Scholar]
- Baumler AJ, Tsolis RM, van der Velden AW, Stojiljkovic I, Anic S, Heffron F. Identification of a new iron regulated locus of Salmonella typhi. Gene. 1996;183(1–2):207–213. [Abstract] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103(6):1834–1839. [Europe PMC free article] [Abstract] [Google Scholar]
- Bister B, Bischoff D, Nicholson GJ, Valdebenito M, Schneider K, Winkelmann G, et al. The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals. 2004;17(4):471–481. [Abstract] [Google Scholar]
- Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123(7):1293–1305. [Abstract] [Google Scholar]
- Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293(5531):829–834. [Abstract] [Google Scholar]
- Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 2006;103(44):16502–16507. [Europe PMC free article] [Abstract] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921. [Abstract] [Google Scholar]
- Friedl A, Stoesz SP, Buckley P, Gould MN. Neutrophil gelatinase-associated lipocalin in normal and neoplastic human tissues. Cell type-specific pattern of expression. Histochem J. 1999;31(7):433–441. [Abstract] [Google Scholar]
- Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18(2):394–400. [Abstract] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043. [Abstract] [Google Scholar]
- Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100(7):3677–3682. [Europe PMC free article] [Abstract] [Google Scholar]
- Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13(1):29–41. [Abstract] [Google Scholar]
- Lin H, Monaco G, Sun T, Ling X, Stephens C, Xie S, et al. Bcr-Abl-mediated suppression of normal hematopoiesis in leukemia. Oncogene. 2005;24(20):3246–3256. [Abstract] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–621. [Europe PMC free article] [Abstract] [Google Scholar]
- Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2007 published online March 30, 2007. [Europe PMC free article] [Abstract] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38(3):414–420. [Europe PMC free article] [Abstract] [Google Scholar]
- Rabsch W, Methner U, Voigt W, Tschape H, Reissbrodt R, Williams PH. Role of receptor proteins for enterobactin and 2,3-dihydroxybenzoylserine in virulence of Salmonella enterica. Infect Immun. 2003;71(12):6953–6961. [Europe PMC free article] [Abstract] [Google Scholar]
- Radtke AL, O’Riordan MX. Intracellular innate resistance to bacterial pathogens. Cell Microbiol. 2006;8(11):1720–1729. [Abstract] [Google Scholar]
- Raymond KN, Dertz EA, Kim SS. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A. 2003;100(7):3584–3588. [Europe PMC free article] [Abstract] [Google Scholar]
- Russo TA, McFadden CD, Carlino-MacDonald UB, Beanan JM, Barnard TJ, Johnson JR. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun. 2002;70(12):7156–7160. [Europe PMC free article] [Abstract] [Google Scholar]
- Ryon J, Bendickson L, Nilsen-Hamilton M. High expression in involuting reproductive tissues of uterocalin/24p3, a lipocalin and acute phase protein. Biochem J. 2002;367(Pt 1):271–277. [Europe PMC free article] [Abstract] [Google Scholar]
- Suire S, Stewart F, Beauchamp J, Kennedy MW. Uterocalin, a lipocalin provisioning the preattachment equine conceptus: fatty acid and retinol binding properties, and structural characterization. Biochem J. 2001;356(Pt 2):369–376. [Europe PMC free article] [Abstract] [Google Scholar]
- Thomas X, Destoumieux-Garzon D, Peduzzi J, Afonso C, Blond A, Birlirakis N, et al. Siderophore peptide, a new type of post-translationally modified antibacterial peptide with potent activity. J Biol Chem. 2004;279(27):28233–28242. [Abstract] [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–529. [Abstract] [Google Scholar]
- Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. [Abstract] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. [Abstract] [Google Scholar]
- Zhu M, Valdebenito M, Winkelmann G, Hantke K. Functions of the siderophore esterases IroD and IroE in iron-salmochelin utilization. Microbiology. 2005;151(Pt 7):2363–2372. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.biocel.2007.07.003
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2695446?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.biocel.2007.07.003
Article citations
Exploring the Potential of Exosomes as Biomarkers in Tuberculosis and Other Diseases.
Int J Mol Sci, 25(5):2885, 01 Mar 2024
Cited by: 0 articles | PMID: 38474139 | PMCID: PMC10931916
Review Free full text in Europe PMC
Iron deficiency and supplementation in heart failure.
Nat Rev Cardiol, 21(7):463-486, 07 Feb 2024
Cited by: 3 articles | PMID: 38326440
Review
Understanding How Minerals Contribute to Optimal Immune Function.
J Immunol Res, 2023:3355733, 01 Nov 2023
Cited by: 5 articles | PMID: 37946846 | PMCID: PMC10632063
Review Free full text in Europe PMC
Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options.
Int J Mol Sci, 24(13):10537, 23 Jun 2023
Cited by: 10 articles | PMID: 37445714 | PMCID: PMC10341809
Review Free full text in Europe PMC
Immunomodulation therapy offers new molecular strategies to treat UTI.
Nat Rev Urol, 19(7):419-437, 22 Jun 2022
Cited by: 17 articles | PMID: 35732832 | PMCID: PMC9214477
Review Free full text in Europe PMC
Go to all (34) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2.
Proc Natl Acad Sci U S A, 103(44):16502-16507, 23 Oct 2006
Cited by: 193 articles | PMID: 17060628 | PMCID: PMC1637611
Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin.
PLoS Pathog, 5(10):e1000622, 16 Oct 2009
Cited by: 122 articles | PMID: 19834550 | PMCID: PMC2757716
Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia.
mBio, 3(6):e00224-11, 20 Nov 2012
Cited by: 81 articles | PMID: 23169997 | PMCID: PMC3509427
[Potential of novel antimycobacterial immune factors, SLPI and lipocalin 2].
Kekkaku, 85(6):523-530, 01 Jun 2010
Cited by: 1 article | PMID: 20662248
Review
Funding
Funders who supported this work.
NIAID NIH HHS (3)
Grant ID: R01 AI062859
Grant ID: R01 AI062859-02
Grant ID: AI062859




