Abstract
Free full text

The GLUT4 Code
Abstract
Despite being one of the first recognized targets of insulin action, the acceleration of glucose transport into muscle and fat tissue remains one of the most enigmatic processes in the insulin action cascade. Glucose transport is accomplished by a shift in the distribution of the insulin-responsive glucose transporter GLUT4 from intracellular compartments to the plasma membrane in the presence of insulin. The complexity in deciphering the molecular blueprint of insulin regulation of glucose transport arises because it represents a convergence of two convoluted biological systems—vesicular transport and signal transduction. Whereas more than 60 molecular players have been implicated in this orchestral performance, it has been difficult to distinguish between mainly passive participants vs. those that are clearly driving the process. The maze-like nature of the endosomal system makes it almost impossible to dissect the anatomical nature of what appears to be a medley of many overlapping and rapidly changing transitions. A major limitation is technology. It is clear that further progress in teasing apart the GLUT4 code will require the development and application of novel and advanced technologies that can discriminate one molecule from another in the living cell and to superimpose this upon a system in which the molecular environment can be carefully manipulated. Many are now taking on this challenge.
IN TYPE II DIABETES, glucose uptake into muscle and fat is impaired, and this is a major consequence of insulin resistance (1). When combined with defective pancreatic insulin secretion, this results in a major dysregulation in blood glucose (1). The transport of glucose into muscle and fat tissue is the rate-limiting step for glucose utilization, and so defining the molecular nature of this process represents an important goal in diabetes research (2). In mammals, the facilitative glucose transporter GLUT4 is of particular relevance to insulin action because its expression is confined to insulin-sensitive cell types such as muscle, fat, and cardiac tissue (3, 4, 5). The regulated entry of glucose into fat and muscle cells in response to insulin or contraction is mediated by the translocation of GLUT4 from intracellular membranes to the plasma membrane (6, 7). To initiate this process, insulin triggers several signaling cascades leading to the physiological effects of insulin. A key component of the insulin signaling cascade is the activation of phosphatidylinositol 3-kinase (PI3-kinase) and its downstream kinases such as Akt (8). Akt is known to mediate many of the physiological effects of insulin including GLUT4 translocation by phosphorylating downstream substrates (9).
To begin to tease apart the molecular regulation of glucose transport by insulin, a more comprehensive description of the GLUT4 trafficking itinerary will be necessary. For simplicity, this can be described in six discrete steps as shown in Fig. 1: 1) Biogenesis of GLUT4 storage vesicles (GSVs)—much data has now shown that the majority of intracellular GLUT4 is found in small 50-nm vesicles referred to as GSVs (10, 11, 12, 13, 14, 15). 2) Transport—elements of the cytoskeleton including microtubules and actin have been implicated in the movement of GSVs to the cell cortex (16, 17, 18, 19). 3) Tethering—a low-affinity interaction between GSVs and the plasma membrane mediated by a tethering complex (20, 21). 4) Docking—assembly of the trans SNARE (soluble N-ethylmalemide-sensitive factor attachment receptor) complex, which is the final committed step before fusion of the GSVs with the plasma membrane (PM) (22). 5) Fusion—rapidly follows where the lipid bi-layers of the GSV and the PM merge. 6) Endocytosis—once incorporated into the surface membrane, GLUT4 can be efficiently retrieved via endocytosis, and this involves at least in part a clathrin-dependent mechanism (23, 24, 25, 26). Many reviews have covered several aspects of GLUT4 trafficking and insulin signaling (2, 27, 28, 29, 30), so here we will first discuss what GSVs are and how they are formed and in a second part how insulin may regulate tethering, docking, and fusion of GSVs with the plasma membrane.
PART I—GSVs: STATIC VS. DYNAMIC SORTING
Where Is GLUT4?
This has been one of the major questions capturing the imagination of GLUT4 biologists. Early electron microscopy studies were perhaps the most informative showing an enrichment of GLUT4 in tubulo-vesicular elements scattered throughout the cytoplasm of the adipocyte or enriched near t-tubules in muscle with an additional localization in endosomes and the trans-Golgi network (TGN) (10, 31). In addition to increasing surface levels of GLUT4, insulin also increased the amount of GLUT4 in endosomes, providing the first indication that the major effect of insulin is on exocytosis and that in the presence of insulin GLUT4 continuously cycles through the endosomal system (10). The juxtaposition of GLUT4 within the endosomal/TGN system has presented a major obstacle in defining the nature of this intracellular GLUT4 compartment. Firstly, the endosomal/TGN system is complex comprising multiple subcompartments (10, 11). Secondly, the degree of overlap between GLUT4 and endosomal/TGN markers varies considerably in different cell types. For example, in adipose tissue from rodents as little as 13% of GLUT4 is found in the TGN area (10), whereas 50% is found in this region in 3T3-L1 adipocytes (32). Several observations support the existence of a unique population of intracellular insulin-responsive vesicles or GSVs: 1) Chemical ablation of the endosomal compartment with transferrin (Tf)-horseradish peroxidase (HRP) has only a modest effect on insulin-stimulated GLUT4 trafficking to the plasma membrane (33); 2) Electron microscopy studies have shown that peripheral tubulo-vesicular structures are the major insulin-responsive GLUT4 compartment in adipocytes (11, 12); 3) Careful analysis of insulin responsiveness of a variety of proteins found in intracellular GLUT4 compartments has identified only a handful of proteins that behave in an analogous manner to GLUT4 including insulin-responsive aminopeptidase (IRAP) (34, 35) and vesicle-associated membrane protein (VAMP2) (36, 37); and 4) Biochemical fractionation studies showing that the majority of intracellular GLUT4 containing membranes can be separated from those containing endosomal and TGN markers (13, 14, 15). The existence of this separate population of regulatable GLUT4 vesicles or GSVs raises two important issues: how are they formed and how do they traffic in the absence and presence of insulin?
How Are GSVs Formed?
In general, the formation of vesicles is catalyzed by a budding stage where coat proteins are recruited to the donor membrane via interactions with Arf GTPases and adaptor proteins, which in turn interact with cargo on the donor membrane (38, 39, 40). GSVs may form either from endosomes or from parts of the TGN or both. GSV biogenesis has been studied in two ways: by examining either the entry of newly synthesized GLUT4 into this compartment or the re-entry of GLUT4 from the cell surface back into GSVs. One assumes that the biogenesis machinery should be the same in both cases because GSVs are renewable (41). In the case of the former, investigations have examined the time necessary for GLUT4 to acquire insulin responsiveness after electroporation of a green fluorescent protein (GFP)-tagged GLUT4 construct into adipocytes. In 3T3-L1 cells, this process was found to be slow [6–9 h (Ref. 42)], whereas in rat adipocytes it was faster [<3.5 h (Ref. 25)]. This discrepancy warrants further investigation (42). Despite these limitations, a role for Golgi localizing γ adaptin ear homology domain, ARF binding (GGAs) in GLUT4 sorting from the TGN to GSVs has been described (42). This is intriguing because GGAs bind to ubiquitinated cargo, so it will be exciting to determine whether GLUT4 and IRAP are ubiquitinated (43). Curiously, however, GGAs also play a role in sorting membrane proteins such as the mannose 6-phosphate receptor (MPR) to the lysosome (44). Because the MPR is not found in GSVs (36), this requires an additional sorting step in addition to the GGA-dependent step to distinguish between sorting to GSVs vs. lysosomes. As a possible explanation for this additional sorting step, it has been shown that sortillin, an MPR-like protein, may play an important role in GLUT4 trafficking by binding to lumenal domains in GSV cargo proteins such as GLUT4 and IRAP (45). This is surprising, however, because substitution of the IRAP lumenal domain with that of the transferrin receptor, a protein not transported to GSVs, does not perturb the insulin-responsive sorting of IRAP (46). Moreover, a GLUT4/GLUT1 chimera containing the cytosolic domains of GLUT4 and the lumenal domains of GLUT1 traffics like GLUT4 but not GLUT1 (47).
McGraw and colleagues (48) have studied the trafficking of GLUT4 from the PM after insulin stimulation. They have carefully mapped the time-course by which GLUT4 escapes ablation with Tf-HRP, thus measuring the sorting of GLUT4 out of endosomes. Using this approach, they find that 40–50% of GLUT4 is localized to endosomes in 3T3-L1 adipocytes under basal conditions. Based on these findings, it has been argued that GSVs are formed from endosomes and that GLUT4 transits from endosomes to GSVs with a t1/2 of 20 min (48). One potential limitation of these studies is that the fate of GLUT4 after its exit from endosomes was not analyzed. This may be important because it has been reported that GLUT4 rapidly transits through endosomes to a subdomain of the TGN also containing the t-SNAREs Syntaxins 6 and 16 (49, 50). Because the Tf receptor does not follow this route (49, 50), this would also register as a non-ablatable compartment in the studies of McGraw and colleagues. It is curious that the TGN is absent from the McGraw model of GLUT4 trafficking because many studies (50, 51, 52) have shown an important role for the TGN in GSV biogenesis. A central feature of the McGraw model as described below is that a large proportion of GLUT4 (~50%) is found in endosomes at steady state. However, this observation is based on the use of the Tf-HRP ablation technique. In view of the enzymatic nature of this technique, if one molecule of Tf-HRP were to be mis-sorted into a postendosomal compartment, a likely outcome based on the nature of endosomal protein sorting, all molecules found in this compartment would be ablated and register as part of the endosomal recycling system. Because GLUT4 is likely concentrated at this step, this may vastly overestimate the amount of GLUT4 in the recycling endosomal system under basal conditions. A further caveat concerns the integrity of the cells used for experimentation. A considerable proportion of GLUT4 is targeted to endosomes in preadipocytes (53). However, as these cells differentiate into mature adipocytes, GLUT4 is withdrawn from endosomes into a more specialized compartment, presumably GSVs (15, 53). It is noteworthy that Karylowski et al. (48) use a model system involving trypsinization of mature adipocytes followed by electroporation, after which the cells are reseeded and studied 2–3 d later. Morphologically, these cells do not represent mature adipocytes due to the conspicuous absence of lipid droplets (48). Hence, whereas this model system may be useful for studying certain aspects of GLUT4 trafficking, we urge caution in using such a system for detailed modeling of GLUT4 sorting between endosomes and postendosomal compartments.
How Do GSVs Traffic in the Absence and Presence of Insulin?
One of the conundrums in the field concerns the fate of GSVs once formed. GLUT4 could be retained in either a static or a dynamic pool (Fig. 2). The static model proposes that GLUT4 may be retained intracellularly by an interaction between GSVs and retention machinery such as TUG (tether, containing a UBX domain, for GLUT4) (54), p115 (55), or by restricting the access of GSVs to the vesicle tethering/docking/fusion apparatus at the cell surface. In the dynamic model, GLUT4 is constantly cycling between two intracellular compartments such as the endosome and the TGN. A dynamic pool could be either endosome derived or comprise a dynamic cycle between TGN and endosomes (Fig. 2, B and C). Distinguishing between these models is important because it will potentially define at which steps acute GLUT4 translocation is regulated. In the static model, regulatory factors likely act at the anchor or the vesicle docking/fusion machinery. In the dynamic model, however, vesicle formation or intracellular vesicle fusion may be important regulatory nodes. McGraw and colleagues (48) have used the model system described above to analyze the kinetics of GLUT4 recycling in adipocytes and report that GLUT4 is rapidly internalized from the cell surface into endosomes from where it exits into GSVs with a t1/2 of 20 min. They also find that the entire cellular cohort of GLUT4 (Fig. 2A) recycles via the adipocyte plasma membrane in the basal state with a t1/2 of 230 min. The only way to rationalize this slow recycling rate in lieu of the high (~50%) proportion of GLUT4 in endosomes at steady state is to invoke an additional sorting step whereby GLUT4 in GSVs continuously cycles back to endosomes in the absence of insulin (otherwise, the endosomal compartment would be rapidly depleted of GLUT4). However, because GLUT4 is known to recycle from endosomes back to the cell surface very rapidly (t1/2 ~ 10 min) (48), this model seems unlikely; otherwise the 50% of GLUT4 in endosomes should freely exchange with the plasma membrane even in the absence of insulin, and this does not appear to be the case even in the studies of Karylowski et al. Hence, again this emphasizes the likelihood that the HRP ablation method overestimates endosomal GLUT4. We do not feel that one can exclude an intracellular cycle for GLUT4, and indeed we have suggested that GLUT4 cycles between GSVs and the TGN (2), the important difference being that this cycle would aid the intracellular sequestration of GLUT4 rather than expose GLUT4 inappropriately to the endosomal recycling system.
An essential difference between the static and dynamic models is that the former, although not excluding an intracellular cycle between GSVs and the TGN, predicts that GLUT4 does not readily exchange with the PM in the absence of insulin, whereas in the latter model it will. McGraw and colleagues (48) report that the entire GLUT4 pool exchanges with the PM with t1/2 of 230 min, whereas Govers and colleagues (53) have shown that this is not the case. One possibility is that the high basal recycling reported by Karylowski et al. (48) and Martin et al. (56) reflects the state of the cells used in their system (see above), combined with the fact that in their system they don’t actually measure the amount of GLUT4 that recycles as a percentage of the total GLUT4 expressed in the cell. This could be important because Govers et al. (53) report that there is a silent nonrecycling pool of GLUT4 that, if unrecognized, will lead to a significant over estimate in GLUT4 recycling. These discrepancies emphasize the need to develop further assays to study the formation of GSVs and GLUT4 recycling in insulin-sensitive cells. Such systems would optimally measure the recycling of endogenous GLUT4 molecules expressed in their native environment. In addition, further assays enabling analysis of the dynamic movement of GLUT4 both close to the surface and deep within the cell will be required to resolve the possible existence of intracellular futile cycles.
Regulation of GLUT4 Trafficking by GSV-Associated Proteins
The rab GTPase-activating protein (rabGAP) AS160 (Akt-substrate of 160 kDa or TBC1D4) associates with GLUT4 vesicles via its interaction with the IRAP cytosolic tail (36, 57). AS160 is phosphorylated after insulin stimulation by Akt or other kinases at six sites, with only four of these sites being insulin responsive (58). Expression of an AS160 mutant (AS160–4P), in which these four phosphorylation sites have been mutated, in adipocytes inhibits insulin-stimulated GLUT4 translocation (58, 59). This inhibitory effect was overcome by mutagenizing a critical arginine residue in the putative GAP domain (58, 59). These studies indicate that insulin-regulated phosphorylation of AS160 may somehow modify its GAP activity, and this step plays an important role in GLUT4 trafficking. Insulin-stimulated phosphorylation of AS160 induces 14-3-3 binding, which is critical for the insulin-regulated function of AS160 in GLUT4 trafficking (57). This role may involve either binding of AS160 to GSVs or regulation of AS160’s GAP activity. A model for AS160 function has emerged from these data such that in the unstimulated state AS160 maintains a Rab in its GDP-bound inactive state, thereby preventing GLUT4 translocation. Phosphorylation of AS160 catalyzes 14-3-3 binding and this likely deactivates the GAP activity of AS160 facilitating activation of the Rab protein involved in GSV translocation. Clearly, identification of the Rab controlled by AS160 is a major goal for the future. Rabs 2, 8, 10, and 14 have been identified on immunoisolated GLUT4 vesicles (36, 60), and in vitro GAP assays reveal that the GAP domain of AS160 displays activity toward Rabs 2A, 8A, 10, and 14 (60). A very recent study has implicated a role for Rab10 in GLUT4 trafficking in adipocytes (61), but further study is required to verify where this Rab acts, if insulin indeed increases its GTP loading and if AS160 displays GAP activity toward other Rab GTPases in vivo. Nevertheless, Rab10 is of interest because amino acid sequence alignment of Rabs 10, 11, and 14 indicate that Rabs 14 and 11 are much more closely related to each other than to Rab10, indicating a potentially unique role for Rab10 in GLUT4 trafficking. Intriguingly, Rab10 is one of the closest mammalian homologs of the yeast Sec4 protein (62). Sec4p regulates assembly of the tethering complex known as the exocyst (63), which is of interest because the exocyst has been implicated in insulin-regulated GLUT4 trafficking in adipocytes (21). Hence, Rab10 may function in an analogous manner in mammalian cells to mediate interactions between GSVs and the exocyst complex at the plasma membrane.
PART II—HOW DOES INSULIN REGULATE GLUT4 TRANSLOCATION AT THE CELL SURFACE?
Tethering
The initial encounter between a vesicle and a target membrane is thought to involve tethering. Tethering is mediated by multisubunit tethering complexes such as the exocyst complex at the cell surface (64). The purpose of the tethering stage is possibly to allow a degree of regulation of vesicle transport before the commitment of a vesicle to fusion and to ensure the specificity of the fusion reaction. The exocyst complex was first identified in yeast and consists of Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p (65). In yeast, overexpression of the t-SNARE Sso1p and Sso2p can overcome the effects of mutations in the exocyst complex (66). The Rab GTPase Sec4p associates with the exocyst (63) and to the t-SNARE Sec9p [synaptosomal-associated protein (SNAP) 25 homolog] (67), thus providing a possible link between tethering and docking. Other GTPases such as Rho1, Rho3, Cdc42, and RalA have also been linked to the exocyst complex (68). Mammalian homologs of all exocyst subunits have been identified, and the complex has been implicated in various cell surface trafficking pathways (69), including insulin-stimulated GLUT4 translocation (20, 21). Moreover, components of the exocyst complex associate with the adipocyte plasma membrane in an insulin-responsive manner (21). Regulation of the exocyst components in response to insulin-stimulation was proposed to occur via activation of the Rho-like GTPase, TC10 (20, 21). By analogy with studies in yeast, it will also be important to determine the contribution of other GTPases, such as CDC42 and Ral, to exocyst function and insulin action.
Docking and Fusion
In the docking stage, SNARE proteins on both the GSV and the plasma membrane interact to generate a ternary complex of coiled-coil domains, which is extremely stable (70). VAMP2 has been shown to take part in a number of exocytic fusion events including GLUT4 vesicle fusion (11, 22, 37), aquaporin vesicle fusion (71), and synaptic vesicle fusion with the plasma membrane (72). VAMP2 has been shown to bind the t-SNAREs Syntaxin 1 and Syntaxin 4 in cooperation with the corresponding SNAP proteins SNAP-25 and SNAP-23, respectively (72). To mediate ternary complex formation and fusion, the VAMP2 sequence is dominated by a SNARE motif that can interact with t-SNAREs at the plasma membrane (72). Inhibition of VAMP2 function using either botulinum toxin (B, D, F, and G) or tetanus toxin inhibits GLUT4 vesicle fusion (73, 74, 75). In muscle and fat tissue, insulin stimulation induces the movement of VAMP2 to the plasma membrane with similar kinetics to GLUT4 (36). Regulation of the fusion step at the plasma membrane is further modified by interactions between Syntaxin-4 and the Sec1-family member Munc18c (76, 77).
A picture is now emerging whereby a major effect of insulin is to modify one of the steps in GLUT4 trafficking either at or very close to the plasma membrane. To address this issue, it was essential to develop techniques that could resolve discrete steps of GLUT4 trafficking in more detail. Total internal reflection fluorescence (TIRF) microscopy is a tool that allows fluorescent events occurring within 150 nm of the plasma membrane to be examined at high resolution, such as the fusion of a vesicle containing GLUT4-GFP (78, 79, 80). TIRF microscopy relies on generation of an evanescent wave that forms at a point where the excitation laser beam hits the interface between the glass coverslip and the aquatic phase of the sample at an angle that is greater than the critical angle at which all the light is reflected (81). By applying this technology to GFP-tagged GLUT4 expressed in adipocytes and using image analysis software capable of single particle analysis, it has been possible for the first time in two recent studies to visualize the movement of individual GLUT4 vesicles into the TIRF zone, tethering/docking of the vesicles, and their subsequent fusion (78, 80). These studies have shown that the GSV dwell time for the tethering/docking step was moderately decreased by insulin (78). In concert with a major effect of insulin distal to the tethering/docking step, the rate of GSV fusion frequency was increased in insulin-treated cells (78, 80). Intriguingly, the study by Bai et al. (78) showed that the majority of tethered/docked vesicles subsequently undocked from the membrane and only a minority of vesicles subsequently fused. In addition, this study also showed that tethering/docking was regulated by PI3-kinase and AS160 (78). The importance of these data is that they open the way for detailed molecular studies in the future. For example, the consequences of disrupting exocyst vs. SNARE function on the various parameters described above will provide new insights into this process and help to sharpen the focus of future efforts aimed at dissecting the major point of action of insulin and possibly other agonists such as exercise. In addition, by combining this approach with even further advances such as FRET, it should be feasible to monitor the assembly of polypeptide complexes in living cells so that we can begin to dissect, for example, which aspect of SNARE function might be regulated by insulin.
Signaling Events at the Cell Surface
Akt is often regarded as the most important substrate in the insulin signaling pathway because many physiological effects of insulin stimulation are mediated by Akt kinase activity (8). There are three Akt isoforms, but only the Akt2 isoform has been shown to be necessary for insulin-stimulated GLUT4 translocation and glucose uptake in muscle and fat tissue by small interfering RNA-mediated knock-down (82, 83). In addition, expression of constitutively active Akt is sufficient to induce GLUT4 translocation to the same extent as insulin in adipocytes (59). Furthermore, Akt2 knockout mice have impaired muscle and fat glucose uptake (84). Although these studies provide compelling arguments that Akt is both necessary and sufficient for insulin-stimulated GLUT4 translocation, they are all limited by their reliance upon long-term ablation or exposure to the Akt signal. Such an environment may lead to numerous adaptive or indirect effects, hence generating a problem in the interpretation of the results.
There are a number of studies indicating that the key Akt-regulated step in glucose transport is at the plasma membrane (see above). Treatment of insulin-stimulated 3T3-L1 adipocytes with the PI3-kinase inhibitor LY294002 or wortmannin causes the accumulation of GLUT4-containing vesicles just underneath the plasma membrane (85, 86). In adipocytes derived from Munc18c−/− mice (85), translocation of GLUT4 was no longer wortmannin sensitive, indicating that somehow Munc18c is involved in the PI3-kinase-dependent fusion step. A cold temperature block likewise blocked Akt activation and GLUT4 docking and fusion, but not movement of GSVs to the cell surface (87). An in vitro fusion assay developed by Koumanov et al. (88) has shown that GSV fusion with the PM was Akt dependent. Recently, a paper by Gonzales and McGraw (83) argued that fusion was Akt independent; however, this study did not measure docking and fusion directly, making the conclusion of these studies less substantial. TIRF microscopy studies have also shown that at least the tethering/docking step is PI3-kinase dependent and AS160 dependent but did not exclude a role for each of these molecules in GSV fusion (78). These studies suggest that there are other Akt substrates at the plasma membrane that play a role in GLUT4 vesicle fusion at steps distal to vesicle tethering. The list of Akt substrates is continuing to grow, and the known substrates of Akt clearly indicate the diversity of processes that are controlled by Akt and that could contribute to GLUT4 fusion at the PM. Substrates that are likely candidates to confer the full GLUT4 translocation response should localize to the plasma membrane at some point. Further studies looking at insulin-regulated Akt substrates at the plasma membrane using techniques such as quantitative mass spectrometry-based phosphoprotein analysis will be important for identifying the key molecules involved in GLUT4 fusion at the plasma membrane.
CONCLUSIONS
Despite considerable progress in our understanding of regulated GLUT4 trafficking the search for the Holy Grail continues. Novel technologies such as TIRF microscopy, in vitro fusion assays, mass spectrometry, and small interfering RNA have provided important clues. These clues point to an important role for the Ser/Thr-kinase Akt and its downstream targets. Each clue, however, presents new mysteries. AS160 is clearly one key symbol, but how does it act and what does it do? TIRF microscopy suggests that the search should now re-focus on the PM at the point beyond where GSVs encounter this structure in a flirtatious manner but at the stage preceding the fusion reaction itself. Indeed, this is perhaps the most nebulous aspect of the GLUT4 code because there are many clues yet to be found. In neurons and other cells, the docking reaction is managed by a host of auxiliary factors including synaptotagmin, Munc13, motor proteins, and rab effectors. We suggest that identification of such molecules involved in GLUT4 trafficking will bring us one step closer to breaking the code.
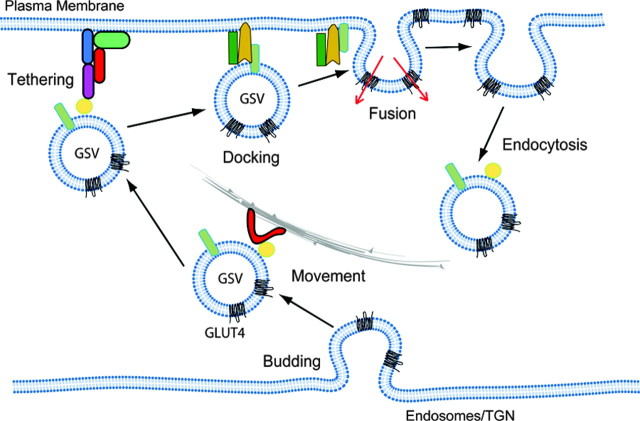
The GLUT4 Trafficking Itinerary
GLUT4 trafficking can be dissected into six discreet steps as shown: budding [biogenesis of GLUT4 storage vesicles (GSVs)], transport, tethering, docking, fusion and endocytosis.
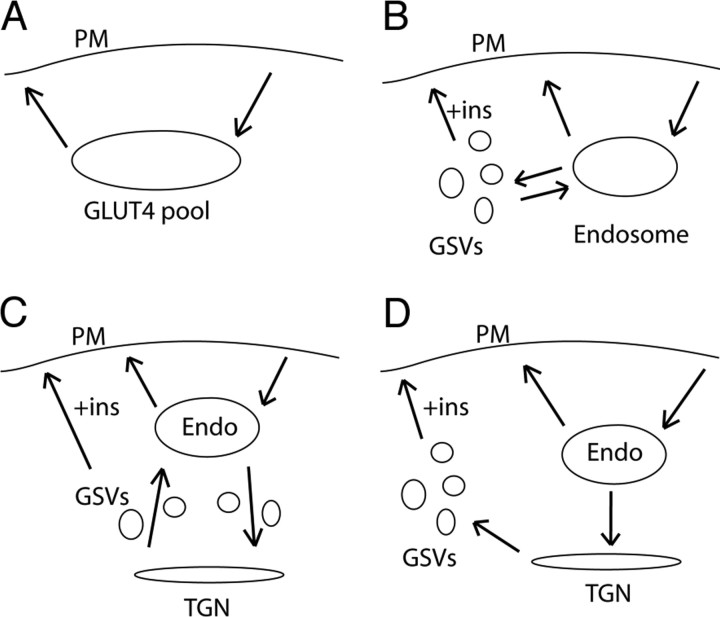
Dynamic vs. Static Models of Intracellular GLUT4 Retention
A, In GLUT4 recycling experiments the intracellular GLUT4 is seen as one pool. B, McGraw and colleagues (48 ) favor a model in which GLUT4 recycles between endosomes and GSVs in the basal state. C, An alternative dynamic model in which GSVs are an intermediate of GLUT4 cycling between TGN and endosomes (Endo). D, Static model in which GSVs form a stable pool for GLUT4 that is only released into the plasma membrane/endosome cycle in the presence of insulin (ins).
Acknowledgments
We thank all members of the James lab for useful discussions regarding the manuscript. D.E.J. is a senior principal research fellow of the National Health and Medical Research Council. We have attempted to acknowledge the contribution of colleagues where appropriate, but due to space limitations this was not always possible.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
First Published Online August 23, 2007
Abbreviations: GAP, GTPase-activating protein; GGA, Golgi localizing γ adaptin ear homology domain, ARF binding; GLUT4, insulin-responsive glucose transporter; GFP, green fluorescent protein; GSV, GLUT4 storage vesicle; HRP, horseradish peroxidase; IRAP, insulin-responsive aminopeptidase; MPR, mannose 6-phosphate receptor; PI3-kinase, phosphatidylinositol 3-kinase; PM, plasma membrane; SNAP, synaptosomal-associated protein; SNARE, soluble N-ethylmalemide-sensitive factor attachment receptor; Tf, transferrin; TGN, trans-Golgi network; TIRF, total internal reflection fluorescence; VAMP, vesicle-associated membrane protein.
References
Articles from Molecular Endocrinology are provided here courtesy of The Endocrine Society
Full text links
Read article at publisher's site: https://doi.org/10.1210/me.2007-0282
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/mend/article-pdf/22/2/226/10719679/mend0226.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Alpha-lipoic Acid Prevents Bone Loss in Type 2 Diabetes and Postmenopausal Osteoporosis Coexisting Conditions by Modulating the YAP/Glut4 Pathway.
Cell Biochem Biophys, 82(2):669-685, 23 Jan 2024
Cited by: 0 articles | PMID: 38261247
Inhibitors of RNA and protein synthesis cause Glut4 translocation and increase glucose uptake in adipocytes.
Sci Rep, 12(1):15640, 19 Sep 2022
Cited by: 3 articles | PMID: 36123369 | PMCID: PMC9485115
Integrated Analysis of the ceRNA Network and M-7474 Function in Testosterone-Mediated Fat Deposition in Pigs.
Genes (Basel), 13(4):668, 10 Apr 2022
Cited by: 3 articles | PMID: 35456474 | PMCID: PMC9032878
Lactobacillus plantarum TWK10 Attenuates Aging-Associated Muscle Weakness, Bone Loss, and Cognitive Impairment by Modulating the Gut Microbiome in Mice.
Front Nutr, 8:708096, 13 Oct 2021
Cited by: 24 articles | PMID: 34722603 | PMCID: PMC8548577
Maternal Lead Exposure Impairs Offspring Learning and Memory via Decreased GLUT4 Membrane Translocation.
Front Cell Dev Biol, 9:648261, 25 Feb 2021
Cited by: 5 articles | PMID: 33718391 | PMCID: PMC7947239
Go to all (58) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bridging the GAP between insulin signaling and GLUT4 translocation.
Trends Biochem Sci, 31(4):215-222, 15 Mar 2006
Cited by: 116 articles | PMID: 16540333
Review
GLUT4's itinerary in health & disease.
Indian J Med Res, 125(3):373-388, 01 Mar 2007
Cited by: 6 articles | PMID: 17496362
Review
GLUT4 translocation: the last 200 nanometers.
Cell Signal, 19(11):2209-2217, 21 Jun 2007
Cited by: 60 articles | PMID: 17629673
Review
Mapping insulin/GLUT4 circuitry.
Traffic, 12(6):672-681, 15 Mar 2011
Cited by: 83 articles | PMID: 21401839
Review




