Abstract
Objective
The study of human anxiety disorders has benefited greatly from functional neuroimaging approaches. Individual studies, however, vary greatly in their findings. The authors searched for common and disorder-specific functional neurobiological deficits in several anxiety disorders. The authors also compared these deficits to the neural systems engaged during anticipatory anxiety in healthy subjects.Method
Functional magnetic resonance imaging and positron emission tomography studies of posttraumatic stress disorder (PTSD), social anxiety disorder, specific phobia, and fear conditioning in healthy individuals were compared by quantitative meta-analysis. Included studies compared negative emotional processing to baseline, neutral, or positive emotion conditions.Results
Patients with any of the three disorders consistently showed greater activity than matched comparison subjects in the amygdala and insula, structures linked to negative emotional responses. A similar pattern was observed during fear conditioning in healthy subjects. Hyperactivation in the amygdala and insula were, of interest, more frequently observed in social anxiety disorder and specific phobia than in PTSD. By contrast, only patients with PTSD showed hypoactivation in the dorsal and rostral anterior cingulate cortices and the ventromedial prefrontal cortex-structures linked to the experience and regulation of emotion.Conclusions
This meta-analysis allowed us to synthesize often disparate findings from individual studies and thereby provide neuroimaging evidence for common brain mechanisms in anxiety disorders and normal fear. Effects unique to PTSD furthermore suggested a mechanism for the emotional dysregulation symptoms in PTSD that extend beyond an exaggerated fear response. Therefore, these findings help refine our understanding of anxiety disorders and their interrelationships.Free full text

Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia
Abstract
Objective
The study of human anxiety disorders has benefited greatly from functional neuroimaging approaches. Individual studies, however, vary greatly in their findings. The authors searched for common and disorder-specific functional neurobiological deficits in several anxiety disorders. The authors also compared these deficits to the neural systems engaged during anticipatory anxiety in healthy subjects.
Method
Functional magnetic resonance imaging and positron emission tomography studies of posttraumatic stress disorder (PTSD), social anxiety disorder, specific phobia, and fear conditioning in healthy individuals were compared by quantitative meta-analysis. Included studies compared negative emotional processing to baseline, neutral, or positive emotion conditions.
Results
Patients with any of the three disorders consistently showed greater activity than matched comparison subjects in the amygdala and insula, structures linked to negative emotional responses. A similar pattern was observed during fear conditioning in healthy subjects. Hyperactivation in the amygdala and insula were, of interest, more frequently observed in social anxiety disorder and specific phobia than in PTSD. By contrast, only patients with PTSD showed hypoactivation in the dorsal and rostral anterior cingulate cortices and the ventromedial prefrontal cortex—structures linked to the experience and regulation of emotion.
Conclusions
This meta-analysis allowed us to synthesize often disparate findings from individual studies and thereby provide neuroimaging evidence for common brain mechanisms in anxiety disorders and normal fear. Effects unique to PTSD furthermore suggested a mechanism for the emotional dysregulation symptoms in PTSD that extend beyond an exaggerated fear response. Therefore, these findings help refine our understanding of anxiety disorders and their interrelationships.
Fear and avoidance of trigger cues are common to many anxiety disorders (1) and resemble the arousal and avoidance responses shown by normal subjects to conditioned fear cues (2). Thus, a common element of anxiety disorders may be an abnormally elevated fear response. Based on animal models of fear learning (3, 4), this hypothesis leads to the prediction that amygdalar dysfunction is common to a variety of anxiety disorders. Indeed, amygdalar hyperactivity has been observed during symptom provocation or negative emotional processing in patients with posttraumatic stress disorder (PTSD) (5-8), social anxiety disorder (9-14), specific phobia (15-18), panic disorder (19), and obsessive-compulsive disorder (OCD) (19, 20). However, because of the low statistical power of individual studies and heterogeneity in task design, patient characteristics, imaging modality, and analytic approach, results across these studies have often been inconsistent, and analyses of replicability across studies are needed.
In PTSD, for example, there have been reports of patients having decreased, rather than increased, amygdalar activity (21, 22), as well as reports of no differences between patients and comparison subjects (23-32). Similarly, although many studies have reported increases in amygdalar activity in specific phobia, several studies have reported no patient/comparison subject differences (33-35), and one reported decreased amygdala activation in patients (36). Finally, in panic disorder and OCD, amygdalar hyperactivity appears to be the exception, rather than the rule (37, 38). These inconsistencies have led various authors to argue that the amygdala may play a role in only some fear states, i.e., that it may not play a critical role in symptoms of PTSD (23, 24, 29), specific phobia (34, 37), panic disorder (39), or OCD (37). The roles of several other brain regions are also in controversy, particularly those with a less extensive animal literature (40).
Despite some shared key features, anxiety disorders also differ in a number of fundamental ways. Symptoms of hypervigilance and hyperarousal, dissociation, emotional numbing, and reexperiencing phenomena (nightmares and flashbacks) are particularly characteristic of PTSD (1). These symptoms are not observed during normal fear conditioning, suggesting that PTSD involves either different or more profound emotional dysregulation than other anxiety disorders. Direct comparisons of functional abnormalities between disorders within the context of a single study are, however, rare. Quantitative meta-analysis can help resolve these ambiguities by permitting formal assessment of the evidence for regional brain dysfunction and quantitative comparisons across disorders.
In this article, we used a voxelwise meta-analysis to compare activation patterns from positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) studies across three of the most well-studied anxiety disorders—PTSD, social anxiety disorder, and specific phobia. First, we tested for evidence that the three disorders involve common neural alterations, which reflect abnormally elevated fear. To do so, we compared neuroimaging results from each disorder to results from studies of fear conditioning in healthy subjects. Second, we identified regions in which disorder-specific abnormalities may be related to disorder-specific symptoms. Finally, we performed novel tests of coactivation across regions to test whether, across individual studies, limbic dysregulation is reliably associated with medial prefrontal dysfunction (41, 42).
This meta-analysis offers a unique window onto the neural mechanisms of clinical anxiety that may guide further hypothesis-driven investigations into the neural basis of psychiatric disorders. Comparing brain activity between disorders in this way also holds promise for providing insight into the relationships between clinical conditions and may inform current efforts to classify psychiatric disorders (43).
Method
Study Selection
Studies were selected by searching PubMed and reference lists for PET or fMRI studies of anxiety disorders (PTSD, social anxiety disorder, specific phobia, OCD, generalized anxiety disorder, and panic disorder) or fear conditioning in healthy volunteers available until September 2006. To be eligible, studies must have reported coordinates of peak activations for comparisons between patient and matched comparison groups across multiple brain regions. We included the studies that contrasted a negative emotional condition with neutral or positive emotional conditions or a resting baseline. Only PTSD (6-8, 21-32), social anxiety disorder (9-14, 44, 45), specific phobia (15-18, 33, 36), and fear conditioning (46-55) had the required 10 comparisons meeting inclusion criteria to allow for a viable meta-analysis. Examples of negative stimuli include trauma-related stimuli or reminder scripts, emotional words, or faces in PTSD; emotional faces and public speaking or its anticipation in social anxiety disorder; and feared objects (i.e., spiders) and emotional faces in specific phobia. Fear-conditioning studies compared brain responses to a conditioned stimulus presented alone (without an associated unconditioned aversive stimulus) with responses to nonconditioned neutral stimuli. This contrast avoids confounding fear responses related to a conditioned stimulus with those to unconditioned aversive stimuli.
Data Analysis
As in previous meta-analytic work, we analyzed the locations of reported peak activation coordinates across the three-dimensional space of the brain (56-61). Studies reported a set of coordinates for each discrete negative versus comparison relation within each study, separately for the patients > comparison subjects (hyperactivation) and comparison subjects > patients (hypoactivation) directions. We note that hyperactivations may occur either because patients activated a region more than comparison subjects or deactivated it less than comparison subjects (relative to a third, arbitrary, baseline condition) and vice versa for hypoactivations. Nonetheless, in both circumstances, hyperactivation in patients reflects greater overall regional activity in patients (patients > comparison subjects) and the inverse for hypoactivation. As shown in Table 1, studies reported coordinates from between one and three comparisons.
TABLE 1
Summary of the Included Studies for the Meta-Analysis of Functional Neuroimaging Studies in PTSD, Social Anxiety Disorder, and Specific Phobiaa
| Disorder | Reference | Comparison Subjects | Patients | Emotional Stimulation | Included Contrasts | Symptom Provocation? | Method |
|---|---|---|---|---|---|---|---|
| Posttraumatic stress disorder (PTSD) | Bremner et al. (1999) (23) | 12 | 10 | Neutral and traumatic scripts | Trauma-neutral | Yes | Positron emission tomography (PET) |
| PTSD | Bremner et al. (1999) (24) | 10 | 10 | Neutral and traumatic sounds/pictures | Trauma-neutral | Yes | PET |
| PTSD | Bremner et al. (2004) (25) | 9 | 12 | Color naming color and emotional words | Emotional-neutral | No | PET |
| PTSD | Bremner et al. (2003) (26) | 11 | 10 | Neutral and emotional words | Deep emotional-neutral | No | PET |
| PTSD | Britton et al. (2005) (21) | 14 | 16 | Neutral and traumatic scripts | Trauma-neutral | Yes | PET |
| PTSD | Lanius et al. (2002) (27) | 10 | 7 | Traumatic script | Trauma-baseline | Yes | Functional magnetic resonance imaging (fMRI) |
| PTSD | Lanius et al. (2001) (28) | 9 | 9 | Traumatic script | Trauma-baseline | Yes | fMRI |
| PTSD | Lanius et al. (2003) (29) | 10 | 10 | Neutral and emotional scripts | Emotional-baseline for 1) traumatic, 2) sad, 3) anxious | Yes (only trauma) | fMRI |
| PTSD | Phan et al. (2006) (22) | 15 | 16 | Negative and neutral pictures | Negative-neutral (controls are combat) | No | PET |
| PTSD | Sakamoto et al. (2005) (30) | 16 | 16 | Masked traumatic and neutral images | Masked trauma-masked neutral | Yes | fMRI |
| PTSD | Shin et al. (1999) (31) | 8 | 8 | Neutral and traumatic scripts | Trauma-neutral | Yes | PET |
| PTSD | Shin et al. (2004) (6) | 19 | 17 | Neutral and traumatic scripts | Trauma-neutral for 1) male combat, 2) female nurse veterans | Yes | PET |
| PTSD | Shin et al. (2005) (7) | 13 | 13 | Fearful and happy faces | Fearful-happy | No | fMRI |
| PTSD | Williams et al. (2006) (8) | 13 | 13 | Fearful and neutral faces | Fearful-neutral | No | fMRI |
| PTSD | Yang et al. (2004) (32) | 6 | 5 | Neutral and traumatic images | Earthquake-neutral for 1) perception, 2) imagery | Yes | fMRI |
| Social anxiety disorder | Amir et al. (2005) (44) | 11 | 11 | Disgust and neutral faces | Disgust-neutral | Yes | fMRI |
| Social anxiety disorder | Kilts et al. (2006) (45) | 6 | 12 | Social anxiety and neutral imagery scripts | Social anxiety disorder-neutral script | Yes | PET |
| Social anxiety disorder | Lorberbaum et al. (2004) (9) | 6 | 8 | Speech anticipation | Anticipation-rest | Yes | fMRI |
| Social anxiety disorder | Phan et al. (2006) (10) | 10 | 10 | Harsh and happy faces | Harsh-happy | Yes | fMRI |
| Social anxiety disorder | Stein et al. (2002) (11) | 15 | 15 | Negative and happy faces | Negative-happy | Yes | fMRI |
| Social anxiety disorder | Straube et al. (2004) (12) | 10 | 10 | Angry and neutral faces | Angry-neutral for 1) implicit task, 2) explicit task | Yes | fMRI |
| Social anxiety disorder | Straube et al. (2005) (13) | 9 | 9 | Angry, happy, and neutral faces | Main effect for 1) angry, 2) happy, 3) neutral | Yes | fMRI |
| Social anxiety disorder | Tillfors et al. (2001) (14) | 6 | 18 | Public and private speaking | Public-private speaking | Yes | fMRI |
| Specific phobia | Dilger et al. (2003) (15) | 10 | 10 | Spider pictures | Spider pictures-baseline | Yes | fMRI |
| Specific phobia | Schienle et al. (2005) (16) | 13 | 10 | Phobia, fear, disgust, and neutral pictures | 1) Phobia-neutral, 2) fear-neutral, 3) disgust-neutral | Yes (only phobia) | fMRI |
| Specific phobia | Straube et al. (2006) (36) | 14 | 28 | Spider and control videos | Spider-control | Yes | fMRI |
| Specific phobia | Straube et al. (2004) (33) | 11 | 11 | Phobia and control words | Phobia-control | Yes | fMRI |
| Specific phobia | Straube et al. (2006) (17) | 12 | 11 | Spider and mushroom pictures | Spiders-mushrooms for 1) identification, 2) distraction tasks | Yes | fMRI |
| Specific phobia | Veltman et al. (2004) (18) | 6 | 12 | Spider and butterfly pictures | Spiders-butterflies | Yes | PET |
| Specific phobia | Wright et al. (2003) (34) | 10 | 10 | Fearful, neutral, and happy faces | Fearful-neutral | No | fMRI |
We constructed indicator maps (I, with values of 1 or 0) of whether each comparison resulted in activation coordinates within a 10-mm sphere surrounding each voxel in a 2×2×2 mm standard brain (Montreal, Que., Canada, Neurologic Institute avg152t1.img, SPM2 version; http://www.fil.ion.ucl.ac.uk/spm/spm2.html). Talairach coordinates were converted to Montreal Neurologic Institute coordinates by using Matthew Brett’s tal2mni.m script, implemented in Matlab (http://imaging.mrccbu.cam.ac.uk/imaging/MniTalairach) (62). The meta-analysis statistic at each voxel was the proportion of comparisons that activated within 10 mm of that voxel (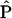 v), weighted by the square root of the sample size for each study. These weights allowed the larger, and thus more reliable, studies to carry more weight in the meta-analysis. Weights were normalized by the sum across comparisons so that for each voxel v in the brain,
v), weighted by the square root of the sample size for each study. These weights allowed the larger, and thus more reliable, studies to carry more weight in the meta-analysis. Weights were normalized by the sum across comparisons so that for each voxel v in the brain,
where wn is the weight for the nth of N comparison maps. To make statistical analysis tractable with the information available from published studies, we treated each comparison map as independent. However, we contacted investigators to determine the extent of nonindependence in subject cohorts (personal communications from Shin LM, Phan KL, Bremner JD, and Straube T). Of the studies for which this information was available, two studies of PTSD had complete overlap in subject cohorts (21, 22), and two pairs of studies had partial overlap (6, 7, 28, 29). In social anxiety disorder, two had partial overlap (12, 13), and in specific phobia, two had partial overlap (17, 36). To address the issue of partial nonindependence, we performed supplementary leave-one-study-out jackknife analyses, described below, that increased confidence that subject overlap did not qualitatively influence our results.
The approach described above has several advantages (63): 1) it treats comparison maps within studies as random effects, so that no single comparison map can contribute disproportionately, even if many peak coordinates are reported; 2) sample size weighting increases accuracy without the complexity of z-score-based weighting for studies with different types of analyses; and 3) the proportion of comparisons metric (P) is straightforward to interpret, unlike methods based on Gaussian or other kernels. Furthermore, unlike other methods that rely on z-score weighting, our meta-analysis approach is better suited to assess the replicability of activation across studies (63).
For each disorder, we created an activation mask consisting of voxels in which activation proportions 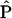 v exceeded the null-hypothesis density P0, established through Monte Carlo simulation. Probability values were corrected for multiple comparisons using false-discovery-rate control (64) at q<0.05, corrected. An additional threshold of at least two studies was imposed to ensure that one study could not create a significant meta-analytic result. The null hypothesis was a uniform random distribution of peaks within each comparison in a gray matter (plus 8-mm border) mask in the standard brain (SPM2 segmented avg152t1.img with 8-mm Gaussian smoothing). We then compared
v exceeded the null-hypothesis density P0, established through Monte Carlo simulation. Probability values were corrected for multiple comparisons using false-discovery-rate control (64) at q<0.05, corrected. An additional threshold of at least two studies was imposed to ensure that one study could not create a significant meta-analytic result. The null hypothesis was a uniform random distribution of peaks within each comparison in a gray matter (plus 8-mm border) mask in the standard brain (SPM2 segmented avg152t1.img with 8-mm Gaussian smoothing). We then compared 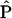 v for hyperactivations (patients > comparison subjects) versus hypoactivations (comparison subjects > patients) at each voxel within the activation mask using nonparametric chi-square tests (63). We used a statistical threshold of p<0.005 and a 10-voxel spatial extent.
v for hyperactivations (patients > comparison subjects) versus hypoactivations (comparison subjects > patients) at each voxel within the activation mask using nonparametric chi-square tests (63). We used a statistical threshold of p<0.005 and a 10-voxel spatial extent.
To directly compare the disorders, we constructed regions-of-interest using the WFU PickAtlas (65), including those in the amygdala, insula, and thalamus. A ventromedial prefrontal region of interest consisted of medial frontal voxels below z=0. A rostral anterior cingulate cortex region of interest was defined as areas 24 and 32 between z=−4 and z=+12, and a dorsal anterior cingulate cortex region of interest corresponded to the anterior cingulate cortex dorsal to z=+12 and anterior to y=+16. We computed 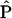 for hyper- and hypoactivation within each region of interest and compared them across disorders using nonparametric chi-square tests.
for hyper- and hypoactivation within each region of interest and compared them across disorders using nonparametric chi-square tests.
To assess whether any single study had a substantial impact on the meta-analytic results from the chi-square analyses, we calculated jackknife chi-square statistics and corresponding p values on each of the regions of interest (66). To implement the jackknife chi-square on the 40 comparisons within each region of interest, we recomputed the chi-square test 40 times, each time leaving out one of the 40 studies. We summarized the results in terms of the percentage of jackknife tests that were significant at a p<0.05 and the maximum p value with one study removed. A value of 100% at p<0.05 indicates that the p value was less than 0.05 for every test, and there was no single study whose omission changed the result at that alpha level.
Coactivation Analysis
We subjected data derived from a priori regions of interest to multivariate analysis to test whether coactivation patterns (the meta-analytic equivalent of functional connectivity) across comparison maps differed across disorders. An indicator matrix I (of size studies by regions) was constructed that encoded whether each comparison reported an activation coordinate within the region of interest. Patients > comparison subjects and comparison subjects > patients differences were integrated by coding them with values of 1 and −1. Associations between all pairs of regions were assessed using Kendall’s tau b (τ) (67, 68), a nonparametric measure of association. Positive τ values for a pair of regions indicate that across studies, observing activation in one region increases the likelihood of observing activation in the other (i.e., either hyper- or hypoactivation tends to co-occur). Negative τ values indicate that activation in one region predicts less frequent activation in another (i.e., hypoactivation in one region is associated with hyperactivation in another). These analyses, which exploit across-study variance in the directions of region-of-interest activation, can be distinguished from common forms of connectivity analyses in individual neuroimaging studies, which exploit within-study sources of variance (either across time or across subjects).
To visualize the relationships among all regions of interest, in Figure 1, we display regions and lines showing significant bivariate τ on a “flattened” map of the connectivity space determined by nonmetric multidimensional scaling (69-71). We first converted the interregion τ matrix into a dissimilarity matrix (D) using the formula Dij = (1−τij)/2. Nonmetric multidimensional scaling was used to find latent components without assuming that distances are euclidean with the s-stress criterion, as implemented in mdscale.m in Matlab 7.3 (Mathworks, Natick, Mass.). A three-dimensional space accurately reproduced the data (s-stress<0.05).
a (A) Three-dimensional rendering of the regions of interest and lines indicating significant coactivation correlations across the entire meta-analysis data set. (B) Patterns of coactivation correlations in either the positive (black lines) or inverse (blue lines) direction for the PTSD comparisons. (C) The same as for B, but for the combination of the social anxiety disorder and specific phobia data sets. For B and C, regions of interest are plotted along dimensions of the first two principal components on the x and y axes, respectively (axes not shown). Coactivation lines represent p<0.05, uncorrected.
Results
Common Mechanisms for Anxiety Disorders and Normal Fear
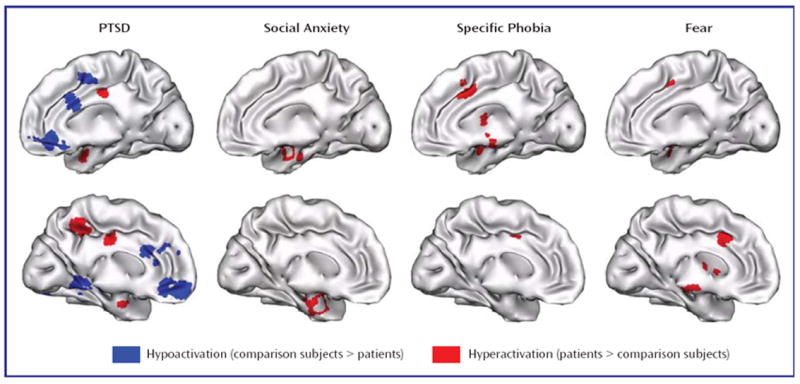
Studies and patient-comparison subject comparisons included in the meta-analysis, as well as overall differences in age and gender ratios, are summarized in Table 1. In patients with PTSD, we observed areas of both hyper- and hypoactivity. By contrast, in patients with social anxiety disorder and specific phobia, we only observed areas of hyperactivity. We first focused on hyperactivation clusters (patients > comparison subjects) to identify common mechanisms across anxiety disorders.
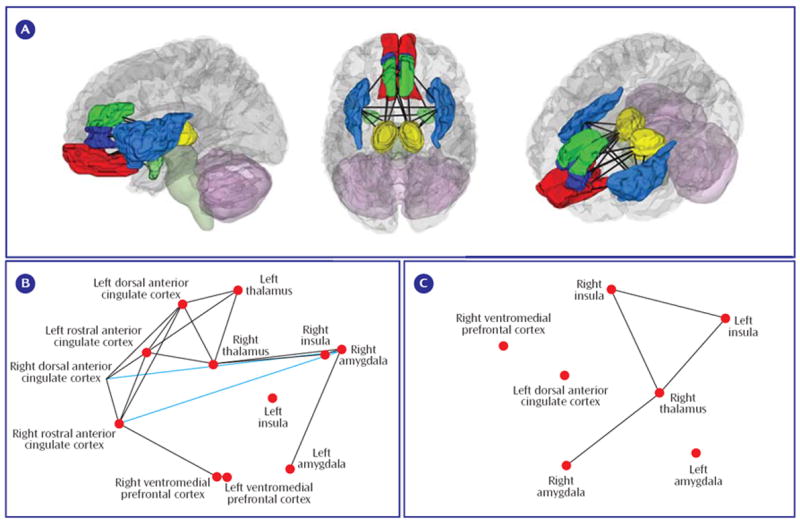
Patients with PTSD showed hyperactivity during emotional processing in the amygdalae, parahippocampal gyrus, insula, inferior parietal lobule, mid-cingulate, and precuneus (see data supplement Table 1 available at http://ajp.psychiatryonline.org). Patients with social anxiety disorder showed hyperactivity in the amygdalae, parahippocampal gyrus, fusiform gyrus, globus pallidus, insula, inferior frontal gyrus, and superior temporal gyrus (see data supplement Table 2). Finally, in patients with specific phobia, hyperactivity was seen in the amygdalae, fusiform gyrus, substantia nigra, insula, and mid-cingulate (see data supplement Table 3). Thus, hyperactivity was observed in all three disorders in only two structures—the amygdala (see Figure 2A) and the insula (see Figure 2B).
a Results are shown for the amygdalae (A) and insular cortices (B). Note that within the left amygdala there were two distinct clusters for PTSD, a ventral anterior hyperactivation cluster and a dorsal posterior hypoactivation cluster. The right side of the image corresponds to the right side of the brain.
We next examined activity during fear conditioning in healthy individuals, which involved 10 comparisons with 117 subjects (see data supplement Table 4). As shown in Figure 2 and Figure 3, fear conditioning also increased activity in the amygdala and bilateral insula (other regions are listed in data supplement Table 5).
Differences Between Anxiety Disorders
Hypoactivations (comparison subjects > patients) were seen only in PTSD, specifically in the inferior occipital gyrus, ventromedial prefrontal cortex, rostral anterior cingulate cortex, parahippocampal gyrus, lingual gyrus, dorsal amygdala and anterior hippocampus, orbitofrontal cortex, putamen, middle occipital gyrus, dorsomedial prefrontal cortex, dorsal anterior cingulate cortex, and mid-cingulate (see Figures 2A and and33 and data supplement Table 1). Of importance, five comparisons also reported correlations between PTSD symptom severity and brain activity (6-8, 21), and all five noted negative correlations in the medial prefrontal cortex, signifying that hypoactivity is associated with greater symptom severity.
We next directly compared regional frequencies of hyper- or hypoactivation between disorders within regions of interest for the ventromedial prefrontal cortex, rostral anterior cingulate cortex, dorsal anterior cingulate cortex, amygdala, and insula, as well as the thalamus because this region was highlighted in recent reviews of PTSD neuroimaging studies (40, 72).
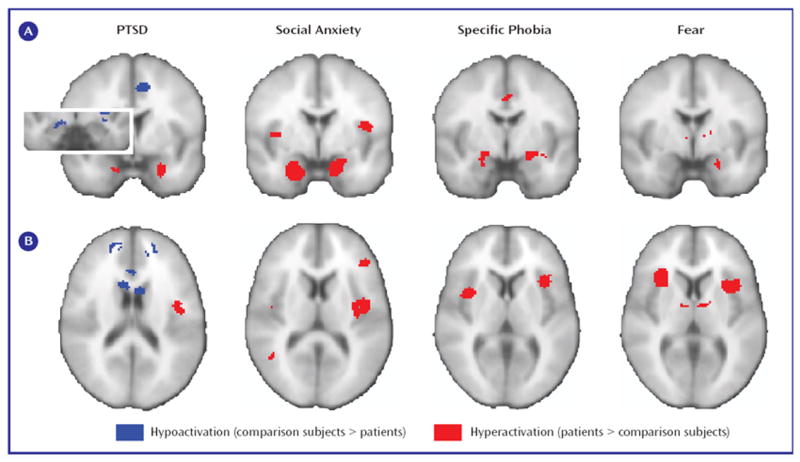
Hyperactivation in the amygdalae and insular cortices of patients was found more frequently in social anxiety disorder and specific phobia than in PTSD (see Figure 4, left). No difference in frequencies of hypoactivation were found in these regions (p>0.15, data not shown). By contrast, rostral anterior cingulate cortex, dorsal anterior cingulate cortex, and ventromedial prefrontal cortex hypoactivity was seen more frequently in PTSD than in either social anxiety disorder or specific phobia (see Figure 4, right). Finally, for the thalamus, hypoactivity was observed more frequently than hyperactivity in PTSD patients in relation to matched comparison subjects (left thalamus: p=0.004; right thalamus: p=0.06; data not shown). Thalamic hypoactivity was also more commonly seen in PTSD than either social anxiety disorder or specific phobia (see Figure 4, right).
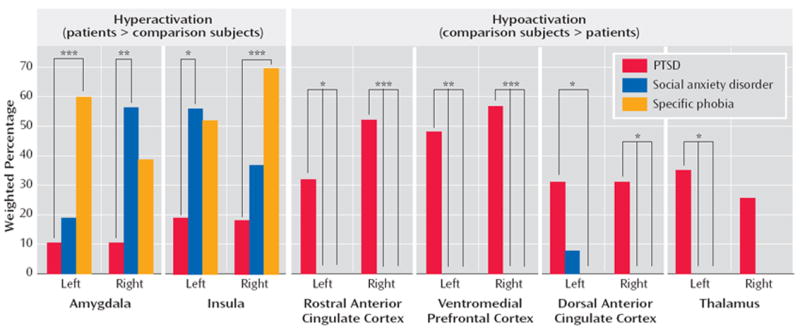
a (Left) Hyperactivation in patients, relative to comparison subjects, was observed more frequently in the amygdala and insula of patients with either social anxiety disorder or specific phobia than in patients with PTSD. (Right) Hypoactivation in the ventromedial prefrontal cortex, rostral and dorsal anterior cingulate cortices, and thalamus was specifically observed in patients with PTSD in relation to matched comparison subjects and not in patients with social anxiety disorder or specific phobia.
*p<0.05. **p<0.01. ***p<0.005.
Potential Confounds for Between-Disorder Differences
We explored several potential confounds for between-disorder regional differences, including the use of symptom-provocation designs, medication status, PET versus fMRI imaging, and whether effects were driven by single outlying studies. First, we tested whether the lower proportion of PTSD studies with symptom-provocation designs affected the results (Table 1). Restricting our meta-analysis to symptom-provocation studies, however, largely confirmed our previous findings: hypoactivation was more frequent in PTSD than in social anxiety disorder or specific phobia in the ventromedial prefrontal cortex, rostral anterior cingulate cortex, and thalamus (data not shown). Similarly, hyperactivation was more frequently found in specific phobia than PTSD in the amygdala (the PTSD versus social anxiety disorder comparison was nearly significant) and in the insular cortices for both social anxiety disorder and specific phobia versus PTSD. Second, we examined whether medication status was a confounder. Most studies reported this information, and in only one (44) were some subjects taking medication at the time of the study. Thus, current medication status did not confound our findings.
Third, we assessed whether the greater proportion of PET studies in the PTSD data set may have affected our results in the amygdala region-of-interest analyses because each method offers different advantages and disadvantages for detecting amygdalar activity (59). Therefore, we restricted our amygdala region-of-interest analyses to fMRI studies. These results confirmed our original findings, with amygdala hyperactivation observed more frequently in social anxiety disorder and specific phobia than in PTSD (data not shown).
Finally, we assessed whether any individual studies included in the meta-analysis had a disproportionate effect on the results. This was done through a jackknife analysis, in which we iteratively left each study out of the chi-square test for each region of interest with significant effects. Doing so provided strong evidence for the robustness of our findings because the reported between-disorder differences in the frequencies of hyperactivation or hypoactivation remained significant at p<0.05 for 100% of the leave-one-out analyses for all regions of interest except for the left dorsal anterior cingulate cortex (35% of leave-one-out tests significant at p<0.05, maximum leave-one-out p=0.12), left insula (95% significant at p<0.05, maximum p<0.07), and right amygdala (92% significant at p<0.05, maximum p<0.08).
Consistency of Relationships Between Regions of Interest
Individual studies in our analysis frequently reported sets of activated regions as “networks,” even in the absence of evidence that the regions are intercorrelated. We quantified interregional relationships using a novel coactivation analysis on the region-of-interest data (see Methods and Figure 4A). Because our results suggest a similarity between social anxiety disorder and specific phobia, we pooled the data for these disorders, thus preserving statistical power. One study in the social anxiety disorder/specific phobia data set (44), however, was a clear outlier because it was the only such study to activate a large number of frontal regions. Therefore, we report results excluding this study and for completeness publish results including this study in supplemental data, although doing so did not alter our overall findings (see data supplement Figure 1).
Notable in the PTSD coactivation map is the consistency of coactivation—in this case, cohypoactivation—among diverse medial frontal regions of interest (see Figure 1B). Also, we observed significant negative coactivation between the dorsal or rostral anterior cingulate cortex and the amygdala or insula, indicating that hypoactivation of frontal regions was associated with hyperactivation in limbic and perilimbic regions (see Figure 1B). Direct comparison of frontal-limbic coactivation in PTSD with that in social anxiety disorder/specific phobia was not possible because frontal hyper- or hypoactivity was not observed in social anxiety disorder or specific phobia, and there was thus no variance in frontal regions to correlate with limbic activation. Thus, limbic hyperactivity associated with decreased frontal inhibition appears to be a distinguishing feature of PTSD.
Discussion
The Amygdala, the Insula, and Fear Responses in Anxiety Disorders
It has been repeatedly suggested that there is exaggerated amygdala activity in clinical anxiety (4, 37, 39, 40). Empirical support for this hypothesis, however, has been inconsistent across neuroimaging studies and between anxiety disorders. Our quantitative meta-analysis revealed consistent amygdalar hyperactivity in all three disorders. Because we also observed consistent amygdala activation during fear conditioning in healthy subjects, we conclude that amygdalar hyperactivation in PTSD, social anxiety disorder, and specific phobia reflects a common exaggerated engagement of fear circuitry, which results in shared symptoms among the disorders.
The role of the amygdala in PTSD, however, appears to be more complicated than previously thought. We found a ventral anterior hyperactive cluster and a dorsal posterior hypoactive cluster. Although the exact relevance of these two clusters is uncertain, we note that the amygdala is composed of multiple subregions. The basal and lateral nuclei (together the basolateral complex) lie ventral to the central nucleus and extended amygdala. The basolateral complex is the primary site of sensory input into the amygdala, whereas the central nucleus contains efferent subnuclei mediating autonomic, endocrine, and behavioral responses to threat (4, 73). We speculate that the ventral hyperactive cluster relates to the basolateral amygdala and may be relevant to acquired fear responses in PTSD, given the role of this region in forming emotional memories (3). The dorsal hypoactive cluster lies at the border between the dorsal amygdala and anterior hippocampus. Hypoactivation of the dorsal amygdala, containing the central nucleus, may be relevant for the autonomic blunting associated with emotional numbing or dissociation in PTSD (74). Hypoactivation of the anterior hippocampus may be important for both declarative memory (75) and regulation of the hypothalamic-pituitary-adrenal axis (76, 77), both of which are perturbed in PTSD (78, 79). The rodent homologue of the anterior hippocampus in humans has also been shown to play a role in endogenous anxiety (80, 81). These interesting subregional distinctions might serve as a guide for more directed investigations of PTSD with high-resolution fMRI methods.
Methodological factors may also contribute to amygdalar hyperactivity versus hypoactivity. Specifically, amygdala activity strongly habituates to repeated presentation of emotional stimuli (48, 82, 83), which may result in below-baseline levels of activity (48, 82). Although such habituation favors elimination of patient-comparison subject differences rather than hypoactivity (84), it suggests that greater attention should be paid to the time course of amygdala effects.
Unlike for the amygdala, a role for the insular cortex in anxiety disorders has not been frequently highlighted. The insula is heavily interconnected with the amygdala, hypothalamus, and periaqueductal gray matter (73), regulates the autonomic nervous system (85), and is activated during the processing of a variety of negative emotions (86). Thus, it is notable that insular hyperactivity was consistently observed in PTSD, social anxiety disorder, and specific phobia, as well as during normal fear conditioning. Insular hyperactivity therefore likely also reflects increased activation of a network responsible for generating fear responses to symptom-provoking stimuli.
Of interest, amygdalar and insular hyperactivity was more common in social anxiety disorder and specific phobia than in PTSD, a finding not previously suggested in the literature. An intriguing explanation, however, may be that while each of the three disorders involves an excessive fear component, PTSD is a more complex disorder in which the fear part per se is only one element. Thus, PTSD symptoms may be more attributable to dysfunctional emotion regulation systems, whereas the symptoms of social anxiety disorder and specific phobia may be more readily described as intense states of fear.
A Final Common Pathway for Anxiety?
Biological commonalities may be sought among different psychiatric disorders at many levels, ranging from genetic vulnerabilities to alterations in widespread neuronal networks (87). Our data argue that amygdala and insula hyperactivation may be key components of a common neurobiological pathway for at least the three anxiety disorders studied, which may reflect overactivation of a core fear system (88). Whether “fear” or other descriptors best explain the common hyperactivations, the activation of similar areas in neuroimaging studies is one kind of evidence for shared mechanisms (89-91), and these results may provide an anatomical basis in which to explore other molecular, cellular, and circuit-level commonalities and differences among anxiety disorders.
Additional evidence for our argument comes from a recent study of “anxiety-prone” individuals, which noted both amygdalar and insular hyperactivity (92). Furthermore, administration of the anxiolytic drug lorazepam decreases activity in these regions in a dose-dependent fashion during an emotional processing task (93). Thus, identification of a neural signature common to anxiety disorders may be useful in terms of both diagnosis and nosology (43, 87), as well as for the development of novel therapeutics (94). The presence of amygdalar and insular hyperactivity may eventually become a useful aspect of disorder categorization in DSM-V. A focus on the shared role of both of these regions in fear may also yield new molecular targets for novel therapeutics.
Emotion Regulation, the Medial Prefrontal Cortex, and Anxiety Generalization
Conceptual models of PTSD have not distinguished between the functional relevance of abnormalities in the dorsomedial prefrontal cortex/dorsal anterior cingulate cortex and those in the rostral anterior cingulate cortex/ventromedial prefrontal cortex, which represent anatomically dissociable subregions of the medial prefrontal cortex. Therefore, we propose a distinction between these regions based on their roles in emotion generation and regulation, respectively. Moreover, we distinguish between the type of emotion regulation subserved by the medial prefrontal cortex and that subserved by the lateral prefrontal cortex, a region not implicated in previous models of PTSD.
Emotion generation versus regulation
Emotion regulation has been investigated using tasks that instruct subjects to deliberately decrease their emotional responses using distraction, reappraisal, suppression, or detachment strategies (95-100). These studies consistently point to involvement of the lateral prefrontal cortex, a locus of executive control for nonemotional stimuli (99, 101). A different picture emerges when one considers “reflexive” forms of emotion regulation, in which the generation and/or modulation of emotion is based on an individual’s expectations about stimuli but without any self-reflective focus on the emotions themselves or the explicit goal of regulating them.
Etkin et al. (102) recently found that monitoring of emotional conflict was associated with dorsomedial prefrontal activation, whereas resolution of emotional conflict was associated with rostral anterior cingulate cortex increases and amygdala decreases, consistent with its top-down inhibition. Extinction of conditioned fear also involves increased activity in the rostral anterior cingulate cortex and ventromedial prefrontal cortex and decreased activity in the amygdala (54). Likewise, rostral anterior cingulate cortex activation and amygdala decreases have been observed during placebo anxiolysis (103), and placebo-induced increases in mu-opioid activity have been found in the rostral anterior cingulate cortex, the ventromedial prefrontal cortex, and the amygdala while the subject was experiencing pain (104). Based on these data, we have previously proposed that emotional control processes mediated by the rostral anterior cingulate cortex/ventromedial prefrontal cortex may reflect an individual’s emotional coping or resilience mechanisms (102), which normally function in absence of explicit task instructions to regulate emotion.
Our meta-analysis demonstrated robust hypoactivations in PTSD in the rostral anterior cingulate cortex and ventromedial prefrontal cortex but failed to show alterations in the lateral prefrontal cortex. Thus, we propose that hypoactivation of the rostral anterior cingulate cortex and ventromedial prefrontal cortex in patients with PTSD reflects a deficit in reflexive emotion regulation processes occurring in the absence of self-reflection about emotion or deliberate attempts at emotional control and is reflected clinically in emotional dysregulation symptoms and anxiety generalization. A reflexive emotion regulation deficit may thus encompass and extend beyond a fear extinction deficit in PTSD, as has been proposed previously (42, 105). The work on the rostral anterior cingulate cortex and the ventromedial prefrontal cortex in humans parallels animal data showing that ventromedial prefrontal cortex lesions in rats impair the ability of these animals to extinguish learned fear (106). Electrical stimulation of this region, which has direct inhibitory projections to the amygdala (107, 108), facilitates fear extinction (109). Although the ventromedial prefrontal cortex in rodents is not an exact homologue of the human rostral anterior cingulate cortex and the ventromedial prefrontal cortex, the shared roles of these areas in fear extinction suggest some degree of analogous function (105) and support a translational approach to anxiety.
Unlike the regulatory roles proposed for the rostral anterior cingulate cortex and ventromedial prefrontal cortex, activity in the dorsomedial prefrontal cortex and adjacent dorsal anterior cingulate cortex seems to relate to emotional experience (59) or awareness (110). These regions are activated by emotional conflict (102), track levels of emotional arousal (111), correlate with autonomic activity (112, 113), and respond in anticipation of an aversive event (96), among related functions. Thus, inasmuch as the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex function in the experience of negative emotion, hypoactivation of these regions in PTSD may be related to a decrease in the experience or impact of negative emotion. Because these regions may help recruit rostral anterior cingulate cortex emotion regulation mechanisms (102), dysfunction of the dorsomedial prefrontal cortex and dorsal anterior cingulate cortex may indirectly further contribute to emotional dysregulation in PTSD. Likewise, hypoactivation of the thalamus may relate to decreased processing of sensory information and thereby decreased experience of negative emotion, as suggested previously (40, 72), although the thalamus also plays diverse roles in cortical-cortical interactions.
Therapeutic implications
Studies of the rodent ventromedial prefrontal cortex not only shed light on the consequence of rostral anterior cingulate cortex/ventromedial prefrontal cortex dysfunction in PTSD but also suggest avenues for novel therapeutics. Animals who have been exposed to inescapable stress display subsequent potentiation of fear and anxiety in unrelated tasks (114-116), whereas control over the stressor promotes resilience (114-116), an effect that depends on the ventromedial prefrontal cortex (114). In humans, perceived controllability during a trauma is related to the severity of subsequent PTSD symptoms (117), an idea that is incorporated into the diagnostic criteria in DSM-IV (1). New treatments, both psychotherapeutic and psychopharmacologic, aimed at bolstering medial prefrontal emotion regulation systems may therefore allow PTSD patients to exert greater control over subsequently encountered fear- and anxiety-producing stimuli or thoughts. Work in monkeys suggests that early life exposure to mild stress can have an inoculating effect against fear and anxiety in unrelated situations later in the animal’s life (118). Although the neural substrates of stress inoculation have not yet been described, the rodent data mentioned above on control over stress suggest that the medial prefrontal cortex may play an important role. As such, stress inoculation may inform approaches at enhancing medial prefrontal emotion regulation systems.
Limitations and Future Directions
Our study has several limitations. First, despite the large number of subjects studied, these numbers still represent a relatively limited population size given significant across-study variation in subject characteristics and study methodologies. This may have compromised our ability to detect more subtle, but highly informative, changes in neural activity. Second, there is some overlap in the published subject cohorts across studies, producing a partial nonindependence between studies. This partial nonindependence is an aspect of neuroimaging data that may be addressed by further refinements of the statistical techniques. The jackknife analyses, however, mitigate in part against results driven by pairs of studies with overlapping cohorts by eliminating one member of any pair. To our knowledge, subjects were not shared across more than two studies.
Finally, we noted age and gender ratio differences between subjects in studies of the three anxiety disorders. These differences did not affect within-study results because patient and control groups were well matched. It is possible, however, that age or gender differences between disorders may affect the sensitivity for detecting differences between patients and comparison subjects—that is, age-by-disorder or gender ratio-by-disorder interactions—a possibility not generally addressed in the psychiatric neuroimaging literature. Although possible, this is unlikely, given that the limbic structures in question are among those particularly spared during aging (119) and that none of our cohorts were elderly. Moreover, in our neuroimaging data, the social anxiety disorder and specific phobia data sets showed similar effects (both of which differed from the PTSD results), despite being different in both age and gender. Thus, neither of these potential confounds produced clear brain differences between social anxiety disorder and specific phobia.
Conclusion
This meta-analysis of functional neuroimaging studies compared the neural correlates of emotional processing in PTSD, social anxiety disorder, and specific phobia. Meta-analyses provided a unique opportunity to assess replicability of regional activations across individual studies and the overlap in affected brain systems across disorders. This approach yielded several key findings. First, patients with all three disorders demonstrated hyperactivity (patients > comparison subjects) in the amygdala and insula. Second, this pattern of activation was also noted for healthy subjects experiencing anticipatory anxiety during fear conditioning. In combination, these data support the hypothesis that shared symptoms—an exaggerated fear response—might be reflected in shared neurobiology. Of interest, amygdala and insula hyperactivity was more commonly observed in social anxiety disorder and specific phobia than in PTSD. Third, we also proposed that PTSD-specific alterations would be related to the emotional dysregulation symptoms characteristic of this disorder. Only PTSD featured prominent hypoactivations (comparison subjects > patients), which were seen in the ventromedial prefrontal cortex, rostral and dorsal anterior cingulate cortex, and thalamus, regions associated with the experience or regulation of emotion. Extension of these findings to other anxiety disorders on which there is currently less information, as well as affective disorders, may lead to advances in the understanding of their psychopathological mechanisms and provide further insight into the relationships between these disorders.
Acknowledgments
The authors thank Eric Kandel, Alan Schatzberg, Thomas Neylan, and Lorrin Koran for their comments on this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1176/appi.ajp.2007.07030504
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3318959?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1176/appi.ajp.2007.07030504
Article citations
The altered resting-state functional connectivity of thalamic subregions in patients with globus pharyngeus.
Brain Imaging Behav, 17 Oct 2024
Cited by: 0 articles | PMID: 39417942
Resting-state functional connectivity in anxiety disorders: a multicenter fMRI study.
Mol Psychiatry, 04 Oct 2024
Cited by: 0 articles | PMID: 39367057
A meta-analysis of cognitive flexibility in aging: Perspective from functional network and lateralization.
Hum Brain Mapp, 45(14):e70031, 01 Oct 2024
Cited by: 1 article | PMID: 39360550 | PMCID: PMC11447525
Neural processing of audiovisual and painful analogue trauma and its relationship with subsequent audiovisual and pain intrusions.
Eur J Psychotraumatol, 15(1):2388429, 16 Sep 2024
Cited by: 0 articles | PMID: 39282770 | PMCID: PMC11407396
Neural activation changes following attention bias modification treatment or a selective serotonin reuptake inhibitor for social anxiety disorder.
Psychol Med, 1-13, 10 Sep 2024
Cited by: 0 articles | PMID: 39252484 | PMCID: PMC11496228
Go to all (1,736) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder.
Arch Gen Psychiatry, 63(2):184-192, 01 Feb 2006
Cited by: 91 articles | PMID: 16461862
Increased amygdala and insula activation during emotion processing in anxiety-prone subjects.
Am J Psychiatry, 164(2):318-327, 01 Feb 2007
Cited by: 493 articles | PMID: 17267796
Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli.
Am J Psychiatry, 164(8):1250-1258, 01 Aug 2007
Cited by: 63 articles | PMID: 17671289
Is posttraumatic stress disorder a stress-induced fear circuitry disorder?
J Trauma Stress, 22(5):409-415, 09 Sep 2009
Cited by: 43 articles | PMID: 19743481
Review
Funding
Funders who supported this work.
NIMH NIH HHS (2)
Grant ID: R01 MH076136
Grant ID: R01 MH076136-01A1