Abstract
Free full text

FUNCTIONAL VASODILATION IN THE RAT SPINOTRAPEZIUS MUSCLE: ROLE OF NITRIC OXIDE, PROSTANOIDS AND EPOXYEICOSATRIENOIC ACIDS
SUMMARY
The present study was designed to determine the mechanisms responsible for functional vasodilation of arterioles paired and unpaired with venules in the rat spinotrapezius muscle.
The spinotrapezius muscle (from Sprague-Dawley rats) was treated with combinations of the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 100 µmol/L), the cyclo-oxygenase inhibitor indomethacin (10 µmol/L) and the epoxygenase inhibitor 6-(2-propargyloxyphenyl) hexanoic acid (PPOH; 30 µmol/L) to determine vascular responses to muscle stimulation. Both paired and unpaired arcade arterioles were chosen for microcirculatory observation. Arteriolar diameter was measured following 2 min muscle stimulation before and 30 min after subsequent application of each inhibitor.
In all cases, l-NAME treatment resulted in decreased basal diameter that was restored to control levels by the addition of sodium nitroprusside (0.01–0.1 µmol/L) to the superfusion solution. NG-Nitro-l-arginine methyl ester significantly inhibited the functional dilation in both paired (−20 ± 3%) and unpaired (−29 ± 3%) arterioles, whereas these inhibitory effects of l-NAME were diminished after pretreatment with indomethacin and PPOH. Indomethacin treatment attenuated the dilation in paired (−33 ± 5%) but not unpaired (−6 ± 4%) arterioles. Treatment with PPOH had no effect on the functional dilation in either set of arterioles. Approximately 50% of the vasodilatory responses remained in the presence of l-NAME, indomethacin and PPOH.
These results suggest that both nitric oxide and vasodilator prostanoid(s) are involved in mediating functional vasodilation in the rat spinotrapezius. The vasodilator prostanoid(s) released from venules is responsible for a portion of the vasodilation of the paired arteriole. The results also suggest possible interactions between the synthesis of nitric oxide and prostaglandin or epoxyeicosatrienoic acids during muscle contraction.
INTRODUCTION
It is well established that during exercise, blood flow in skeletal muscle is controlled by regulation of local artery/arteriolar diameter (functional dilation) and, thus, metabolic demand and delivery are matched precisely. Several mechanisms have been proposed for the control of local blood flow in response to muscle contraction, such as metabolic,1 myogenic,2 conducted3 and flow-dependent4 mechanisms. Moreover, the explanations for hyperaemic responses have given rise to several metabolic vasodilators, such as nitric oxide (NO), prostacyclin (PGI2) and epoxyeicosatrienoic acids (EETs). Although several studies in humans and animals have been performed to test the contribution of these individual vasodilators to functional dilation and their interactions, their relative roles and interactions are unclear.
Previous studies from our laboratory have shown that inhibition of cyclo-oxygenase (COX) by indomethacin attenuates the functional dilation5–8 of paired arterioles in hamster cremaster muscle, but not the functional dilation of unpaired vessels, and that inhibition of NO by NG-nitro-l-arginine methyl ester (l-NAME) had no effect. These results suggested that, in hamster cremaster muscle, there are different mechanisms responsible for functional vasodilation of paired and unpaired arterioles and that vasodilator prostanoid(s) released from venules plays an obligatory role in mediating the hyperaemic vasodilation of paired arterioles.9 One limitation of these previous studies is that the cremaster muscle is a non-postural muscle and it is not clear whether the same mechanisms are important in postural muscle, which can increase metabolism through exercise. For example, an increase in the production of both NO and PGI2 in response to exercise has been demonstrated in animals10,11 and humans12,13 and there is evidence that different rat skeletal muscles exhibit different endothelium-dependent vasodilatory responses.14 The spinotrapezius muscle is considered a true postural skeletal muscle that can be contracted voluntarily, compared with the cremaster muscle. Therefore, the determination of the mechanisms responsible for the functional vasodilatory responses in the spinotrapezius muscle may be more representative of other postural skeletal muscles. In addition, the present study also examined the role of EETs on the functional dilation in paired and unpaired arterioles in spinotrapezius muscle and whether there are interactions between each possible mechanism. Indeed, no single factor has been identified to constitute the primary metabolic regulator of functional vasodilation in skeletal muscle. Published studies have tried to inhibit vasoactive pathways in animals running on a treadmill; however, changes in baseline blood flow and blood pressure (in response to both the inhibitors and exercise) make it difficult to determine the actual mechanism responsible for the functional vasodilation at the microcirculatory level. Taken together, the present study examines three potential pathways in a true skeletal muscle using a technique that allows direct observation of the microcirculation, avoiding differences in baseline flow or having to calculate conductance because of changes in blood pressure. The present study was designed to determine the factors and their interactions in mediating the functional vasodilation of paired and unpaired arterioles in the rat spinotrapezius muscle. We tested the hypothesis that NO, prostaglandins and EETs contribute to functional vasodilation in rat spinotrapezius muscle directly and indirectly via possible interactions.
METHODS
Animals
We obtained 12-week-old male Sprague-Dawley (SD) rats (n = 33) from Harlan Laboratories (Indianapolis, IN, USA). The experimental protocols for the study were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were performed according to both the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and the guidelines of the Animal Welfare Act (http://www.grants.nih.gov/grants/olaw/olaw.htm). All rats were housed two to three animals per cage at 22°C (12 h light–dark cycle), with free access to food and water.
Microcirculatory surgical preparation
The right spinotrapezius muscle was prepared for experimental observation as described previously.15 Briefly, rats were anaesthetized with sodium pentobarbital (65 mg/kg, i.p.) and the trachea was intubated. Animals spontaneously breathed a gas mixture containing 30% oxygen and 70% nitrogen. The left jugular vein was cannulated for supplemental addition of anaesthetic. At all times during the surgery and subsequent experiments, the spinotrapezius muscles were kept at in situ dimensions and superfused continuously with a physiological salt solution (of the following composition (in mmol/L): NaCl 118.07; KCl 6.17; CaCl2 2.55; NaHCO3 25) aerated with a gas mixture of 5% CO2, 95% N2 (pH = 7.4, 35°C). All chemicals were purchased from Sigma Chemicals (St Louis, MO, USA). At the completion of the study, animals were killed by cardiac injection of 10% potassium chloride. Death was confirmed by lack of a heart beat and spontaneous breathing.
Experimental measurements
The microcirculation of the spinotrapezius muscle was transilluminated and observed under a Nikon (Kanagawa, Japan) microscope fitted with a ×10 water immersion objective (numerical aperture = 0.30). The microscopic image was televised with a Dage (MIchigan, IN, USA) closed-circuit television camera and displayed on a Sony (Park Ridge, NJ, USA) monitor. The magnification of the image was ×660 from the tissue to the monitor screen. Vessel diameter was measured using a Texas A&M video analyser (Texas A&M University, College Station, TX, USA) modified to function as a video micrometer. The resolution of this system was ±1 µm.
Muscle stimulation
Two hooked silver–silver chloride electrodes (Grass Instruments, Quincy, MA, USA) were placed at each end of the spinotrapezius and connected to a Grass S44 stimulator. Diameters of vessels were obtained in the resting muscle and immediately following 2 min electrical stimulation (4–5 V, 1 Hz).
Pharmacological inhibitors
To assess the contribution of NO synthase (NOS), COX and cytochrome P-450 (CYP) epoxygenase enzymes to the functional dilation of arterioles, these enzymes were inhibited by l-NAME (100 µmol/L; Sigma Chemical), indomethacin (10 µmol/L; Sigma Chemical) and 6-(2-propargyloxyphenyl) hexanoic acid (PPOH; 30 µmol/L; Cayman Chemical), respectively.
Experimental protocol to test the inhibitory role of PPOH
In a separate set of SD rats (n = 6), we used the spinotrapezius preparation to test the inhibitory role of PPOH. After pretreatment of the spinotrapezius muscle with l-NAME (100 µmol/L) + indomethacin (10 µmol/L) for 30 min, arteriolar dilation was induced by topical application of 10 µmol/L AA before and 30 min after administration of 3 or 30 µmol/L PPOH. We hypothesize that the vasodilator response to AA following inhibition of NO with l-NAME and inhibition of PGI2 with indomethacin would be due to endothelium-derived hyperpolarizing factor (EDHF).
Experimental protocol to test functional vasodilation
Rats were divided into four groups based on the order of antagonist administration: (i) Group LIP (l-NAME, indomethacin and PPOH); (ii) Group ILP (indomethacin, l-NAME and PPOH); (iii) Group PLI (PPOH, l-NAME and indomethacin); and (iv) Group CTR (a time control group). Rats were allowed to stabilize for 15–30 min after completion of the surgical procedure. Segments of both paired and unpaired arcade arterioles (third order, approximately 9–15 µm) were selected for analysis. Diameters of the vessels were obtained in the resting muscle and immediately following the cessation of muscle stimulation. After the vessel had returned to its resting diameter, each inhibitor was added to the superfusion solution in the orders described above. Arterioles were incubated with the each inhibitor for 30 min prior to the muscle stimulation protocol. In the present study, l-NAME caused a decrease in basal diameter of approximately 20%. We have shown previously that the effects of l-NAME on basal diameter may lead to inappropriate conclusions.7,8 Therefore, to maintain the same control state, 0.01–0.1 µmol/L sodium nitroprusside (SNP) was added to the superfusion solution to restore basal diameters. Thus, this l-NAME treatment with subsequent SNP treatment would ‘fix’ the NO system, providing a background level required to maintain normal diameter but prevent any increase in NO release during the muscle stimulation. In the CTR group, three muscle contractions were performed every 30 min. At the end of the experiment, adenosine (10 µmol/L) was added to the superfusion solution to determine maximal diameter. Only preparations with significant basal tone, as determined by a minimal 40% increase in diameter in response to adenosine, were used for data analysis.
Analytical and statistical methods
Arteriolar diameter data was collected at 1 Hz using a computer equipped with a Computer Boards (Mansfield, MA, USA) analogue-to-digital converter and stored to disk for later analysis. The functional vasodilatory responses were normalized as percentage of maximal increase in diameter and presented as (functional vasodilation – basal diameter)/(maximal dilation – basal diameter). The total inhibition attributable to l-NAME, indomethacin and PPOH treatment was analysed using two-way anova. The decrease in basal diameter and the total inhibition of functional dilation in the presence of the three inhibitors between paired and unpaired arterioles were compared using t-test. All other data were analysed using one-way repeated-measures anova. Where significant main effects occurred, individual groups were compared using the Holm–Sidak method. All data are the mean±SEM. P < 0.05 was accepted as statistically significant for all comparisons.
RESULTS
Inhibitory role of PPOH
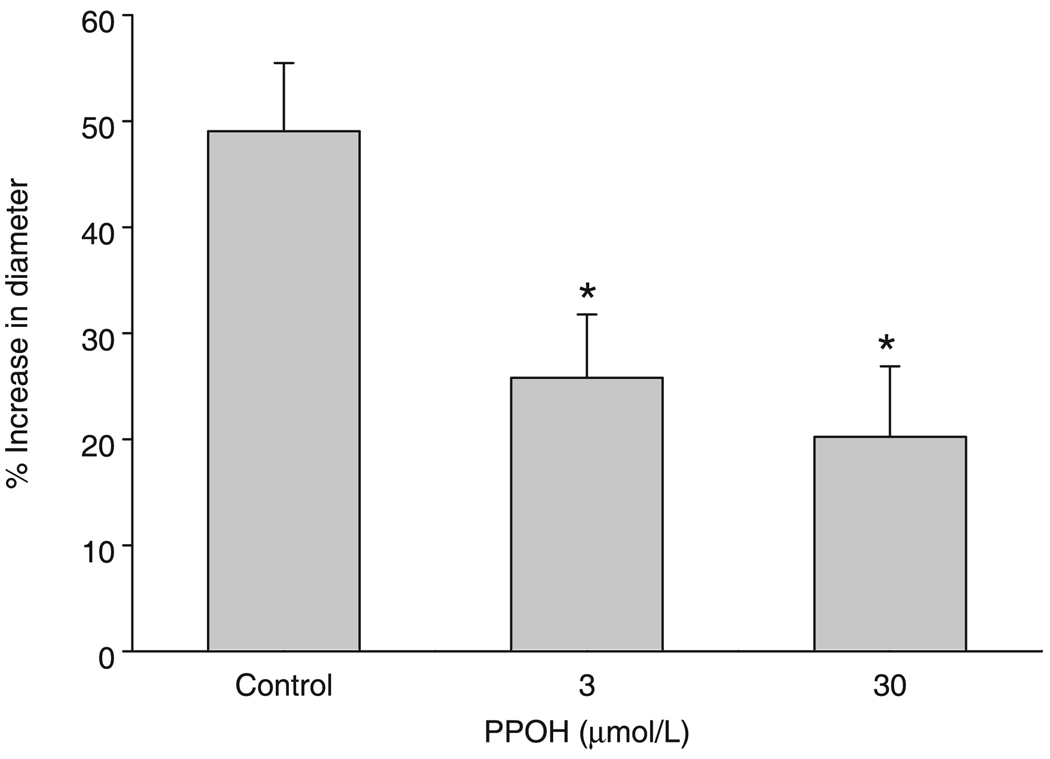
Figure 1 shows the effect of PPOH on AA-induced dilation after inhibition of NOS and COX. Administration of AA resulted in significant vasodilatory responses in the presence of l-NAME and indomethacin, which was inhibited by both 3 and 30 µmol/L PPOH.
Basal and adenosine-induced diameters
Changes in basal diameter after the administration of inhibitors are shown in Fig. 2. The four groups (LIP, ILP, PLI and CRT) were collapsed and the mean absolute values of all basal and maximal diameters were compared between paired and unpaired arterioles. The basal diameters of paired (12 ± 1 µm; n = 27) and unpaired (11 ± 1 µm; n = 21) arterioles were not different, whereas the adenosine-induced dilation was larger in paired arterioles compared with unpaired vessels (42 ± 3 vs 35 ± 2 µm, respectively; P = 0.03). Paired and unpaired arteriolar diameters were significantly reduced after treatment with 100 µmol/L l-NAME (24 ± 2 and 21 ± 2%, respectively). Neither 10 µmol/L indomethacin nor 30 µmol/L PPOH had any effect on basal diameter.
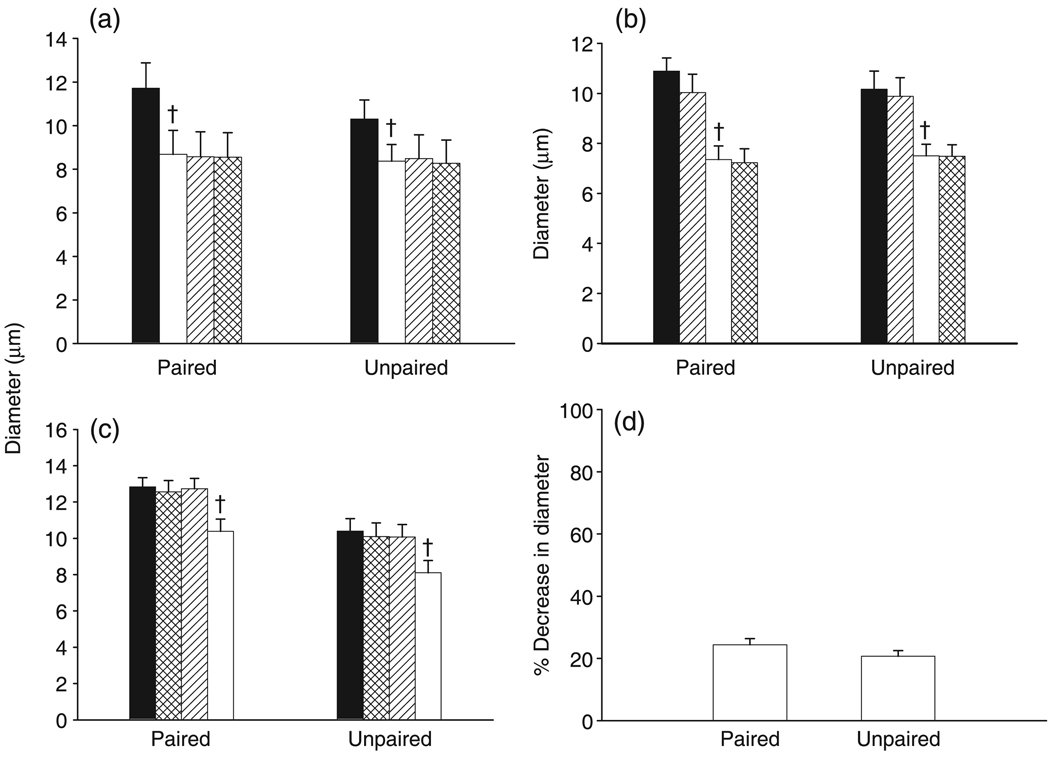
(a–c) Effect of NG-nitro-l-arginine methyl ester (l-NAME) on the basal diameters of paired and unpaired arterioles. For (a): (■), control; (□), l-NAME; (
), l-NAME + indomethacin; (

), l-NAME + indomethacin + 6-(2-propargyloxyphenyl) hexanoic acid (PPOH). For (b): (■), control; (
), indomethacin; (□), indomethacin + l-NAME; (

), indomethacin + l-NAME + PPOH. For (c): (■), control; (

), PPOH; (
), PPOH + indomethacin; (□), PPOH + indomethacin + l-NAME. (d) Relative contribution of nitric oxide in the maintenance of basal diameter (□, l-NAME). †P < 0.05 for l-NAME compared with indomethacin or PPOH. Data are the mean±SEM (a: n = 10 for paired and 6 for unpaired; b: n = 6 for paired and unpaired; c: n = for paired and 5 for unpaired; d: n = 22 for paired and 17 for unpaired).
Functional vasodilation in paired and unpaired arterioles
The stimulation parameters used to elicit muscle contraction resulted in vasodilatory responses that were significantly smaller than adenosine-induced vasodilation. The four groups (LIP, ILP, PLI and CRT) were collapsed and the functional dilatory responses before any administration of drugs were compared between paired and unpaired arterioles. There was no difference in the functional vasodilation between paired (72 ± 1%) and unpaired (72 ± 1%) vessels.
Functional vasodilation in CTR
Figure 3 shows the vasodilatory responses to muscle contraction preformed at 30 min intervals under control conditions. Functional vasodilation was no different between paired and unpaired arterioles and there were no time-dependent differences in functional vasodilation in either paired or unpaired arterioles.
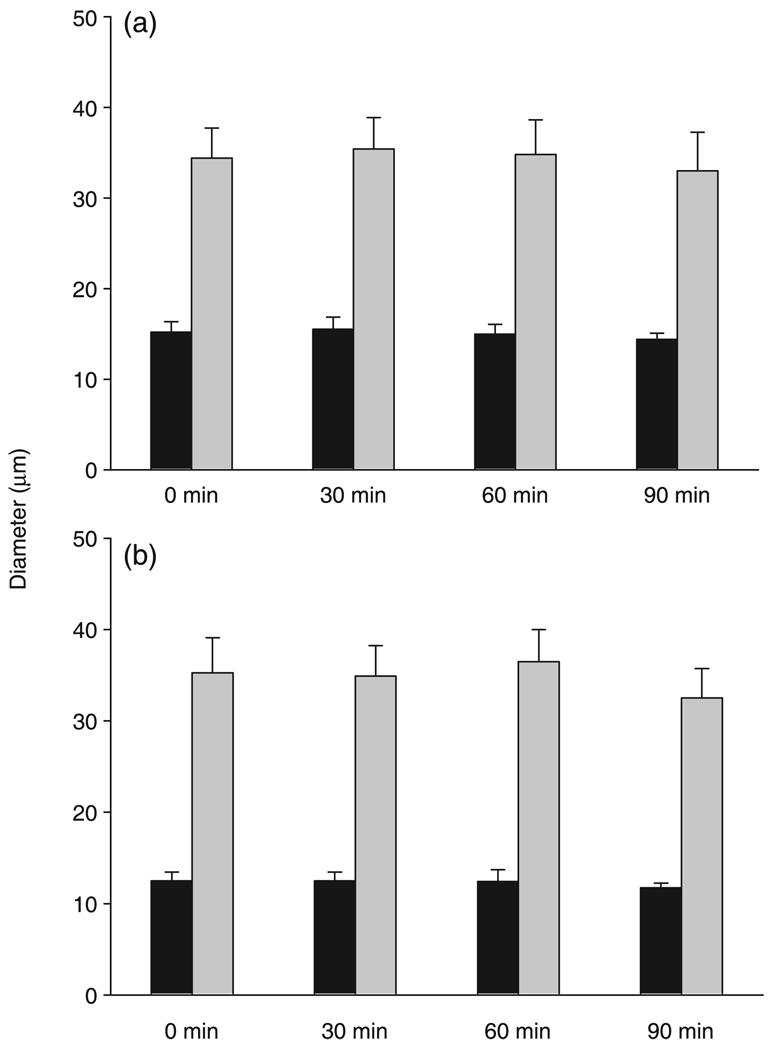
Functional dilation in response to muscle contraction in both (a) paired (n = 5) and (b) unpaired (n = 4) arterioles. Functional vasodilation did not differ between paired and unpaired arterioles and there were no time-dependent differences in functional vasodilation in either paired or unpaired arterioles. Data are the mean±SEM. (■), basal; (

), stimulation.
Functional vasodilation in Group LIP
Figure 4 shows the vasodilatory responses to muscle contraction in response to the sequential administration of l-NAME, indomethacin and PPOH. Basal diameters were normalized using SNP in the superfusion solution for both paired and unpaired arterioles (12 ± 1 and 10 ± 1 µm, respectively, before l-NAME; 12 ± 1 and 10 ± 1 µm, respectively, after l-NAME ± SNP). Nitric oxide synthase inhibition resulted in a significantly decreased functional dilation in both paired and unpaired arterioles. Cyclo-oxygenase inhibition in the presence of NOS inhibition resulted in a significant reduction in functional dilation in the paired but not unpaired arterioles. Inhibition of CYP epoxygenase had no effect on the vasodilatory response in either paired or unpaired vessels.
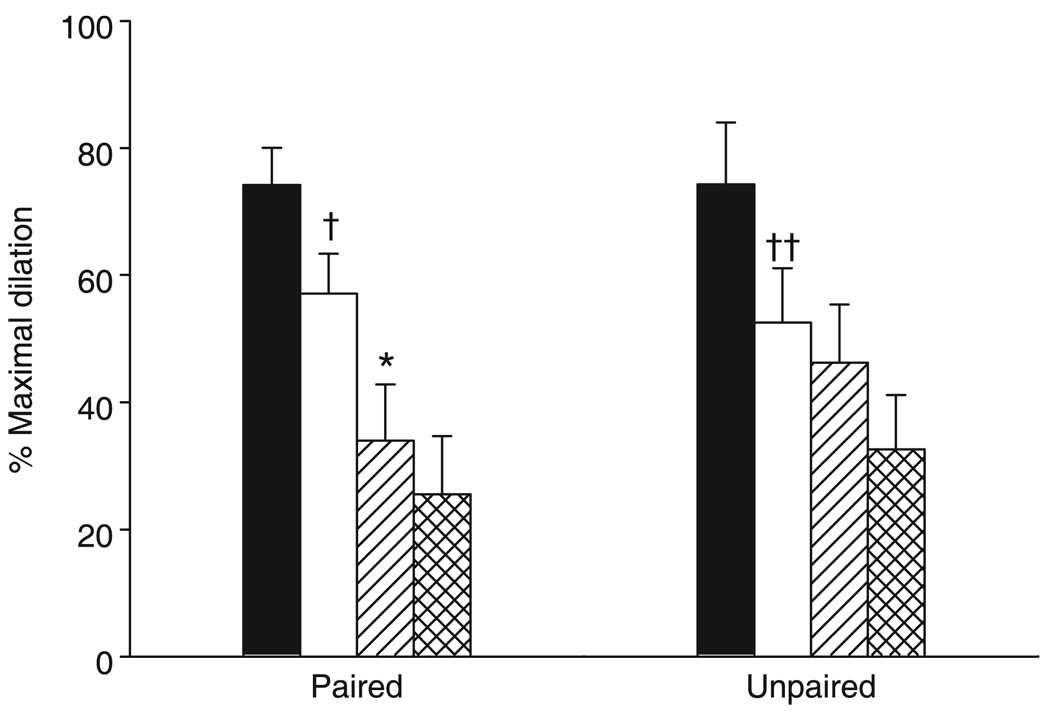
Functional vasodilation in response to the sequential administration of NG-nitro-l-arginine methyl ester (l-NAME), indomethacin and 6-(2-propargyloxyphenyl) hexanoic acid (PPOH) in Group LIP. †P = 0.018, ††P = 0.007 compared with control; *P = 0.002 compared with l-NAME. Data are the mean±SEM (n = 10 for paired and 6 for unpaired). (■), control; (□), l-NAME; (
), l-NAME + indomethacin; (

), l-NAME + indomethacin + PPOH.
Functional vasodilation in Group ILP
Figure 5 shows the vasodilatory responses to muscle contraction in response to the sequential administration of indomethacin, l-NAME and PPOH. Treatment with l-NAME resulted in a vasoconstriction of arteriolar diameters. Arteriolar diameters were normalized using SNP in the superfusion solution. The diameters of paired and unpaired arterioles were 11 ± 1 and 10 ± 1 µm, respectively, before l-NAME and 11 ± 1 and 10 ± 1 µm, respectively, after l-NAME + SNP. Similar to the results shown in Fig. 4, COX inhibition resulted in an attenuated functional vasodilation in paired arterioles with no effect in unpaired vessels. However, the addition of NOS inhibition in the presence of COX inhibition attenuated the vasodilatory response to muscle contraction in unpaired arterioles, with no effects in paired vessels. No significant differences were observed in the vasodilatory response during inhibition of CYP epoxygenase.
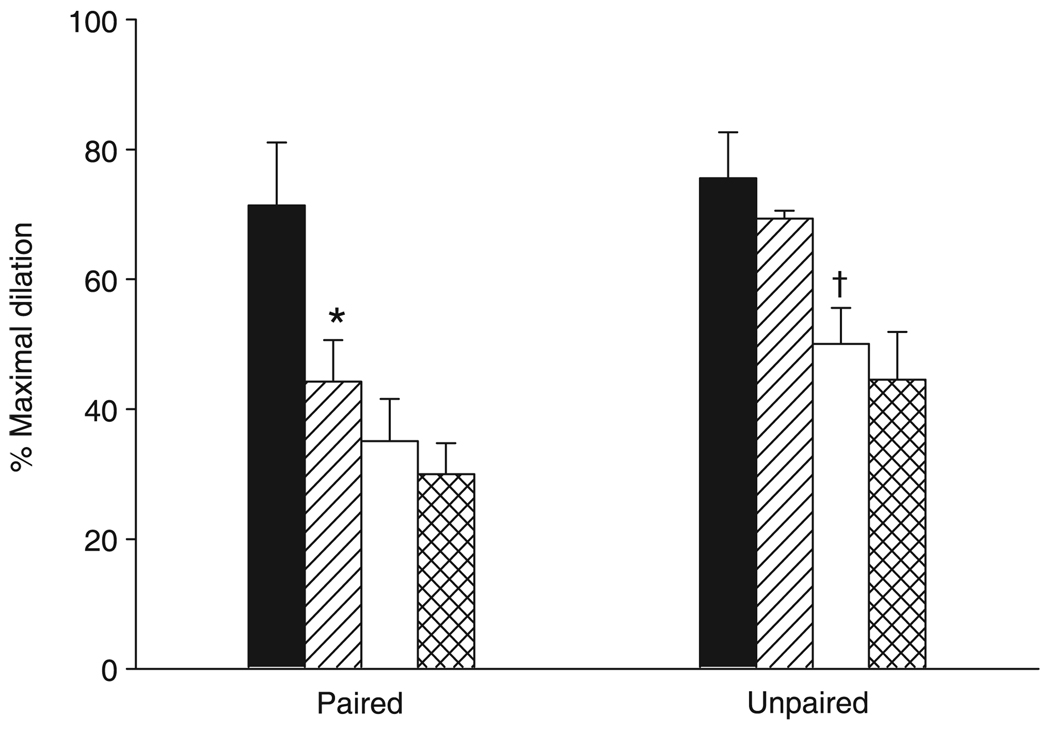
Functional vasodilation in response to the sequential administration indomethacin, NG-nitro-l-arginine methyl ester (l-NAME) and 6-(2-propargyloxyphenyl) hexanoic acid (PPOH) in Group ILP. *P = 0.001 compared with control; †P = 0.011 compared with indomethacin. Data are the mean±SEM (n = 6 for paired and unpaired). (■), control; (
), indomethacin; (□), indomethacin + l-NAME; (

), indomethacin + l-NAME + PPOH.
Functional vasodilation in Group PIL
Figure 6 shows the vasodilatory responses to muscle contraction in response to the sequential administration of PPOH, indomethacin and l-NAME. Arteriolar diameters were normalized using SNP in the superfusion solution following l-NAME treatment for both paired and unpaired arterioles (13 ± 1 and 10 ± 1 µm, respectively, before l-NAME; 13 ± 1 and 11 ± 1 µm, respectively, after l-NAME + SNP). Neither paired nor unpaired arterioles exhibited an altered vasodilatory response following inhibition of CYP epoxygenase. Inhibition of COX significantly attenuated the functional vasodilation in paired but not unpaired arterioles. Treatment with l-NAME failed to cause a further inhibition of the vasodilatory responses in either paired or unpaired arterioles.
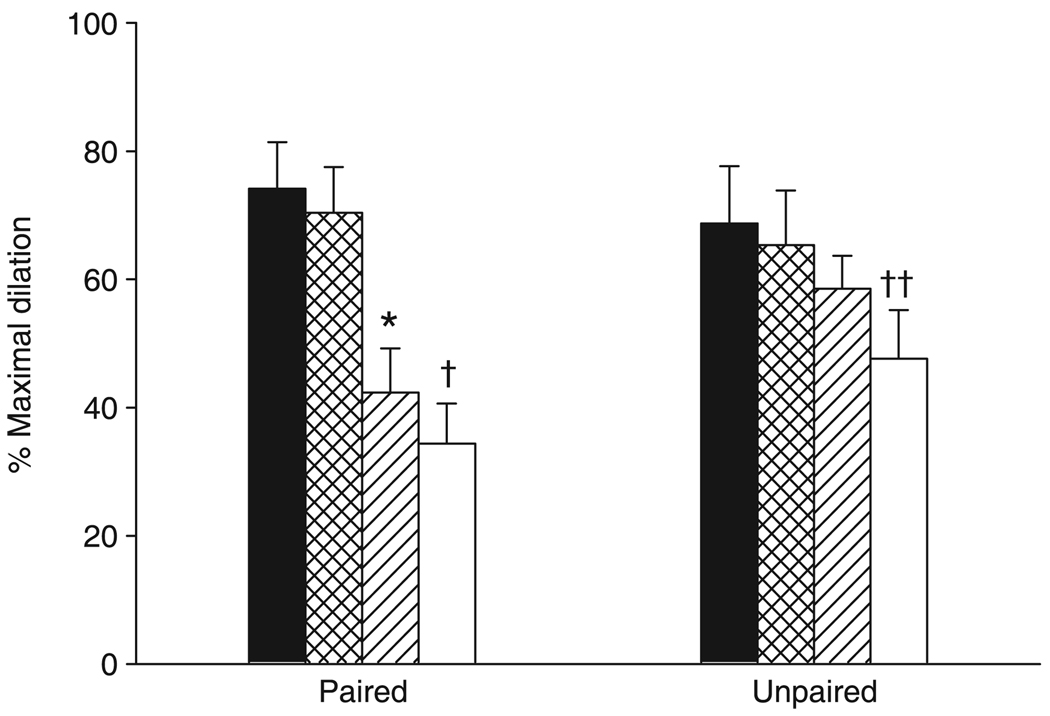
Functional vasodilation in response to the sequential administration of 6-(2-propargyloxyphenyl) hexanoic acid (PPOH), indomethacin and NGnitro-l-arginine methyl ester (l-NAME) in Group PIL. *P = 0.001 compared with PPOH; †P = 0.004, ††P = 0.013 compared with control. Data are the mean±SEM (n = 6 for paired and 5 for unpaired). (■), control; (

), PPOH; (
), PPOH + indomethacin; (□), PPOH + indomethacin + l-NAME.
Total inhibition on functional vasodilation
The LIP, ILP and PLI groups were collapsed to compare the contribution of NOS, COX and CYP epoxygenase to functional vasodilation. The degree of inhibition attributable to l-NAME, indomethacin and PPOH treatments is shown in Fig. 7. The amount of inhibition due to l-NAME did not differ between paired and unpaired arterioles. Indomethacin significantly attenuated the vasodilatory response in paired compared with unpaired arterioles. Moreover, the amount of inhibition in paired vessels attributable to indomethacin was significantly greater than that inhibited by l-NAME. The total inhibition of functional dilation in the presence of the three inhibitors was not significantly different between paired and unpaired vessels.
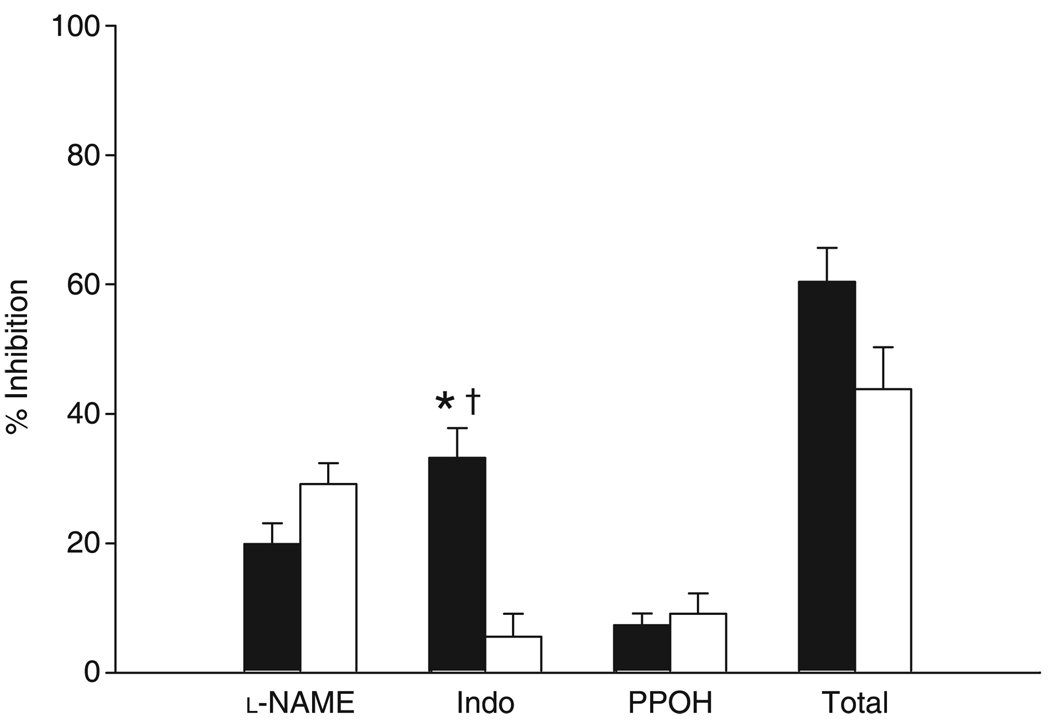
Relative contribution of nitric oxide synthase, cyclo-oxygenase and epoxygenases to functional vasodilation in paired (■) and unpaired (□) arterioles. *P = 0.001 compared with unpaired within the indomethacin (Indo) group; †P = 0.004 compared with NG-nitro-l-arginine methyl ester (l-NAME) within paired arterioles. Data are the mean±SEM (n = 22 for paired and 17 for unpaired). PPOH, 6-(2-propargyloxyphenyl) hexanoic acid.
DISCUSSION
The major findings of the present study are that: (i) arcade arterioles in the rat spinotrapezius muscle exhibited a marked increase in diameter in response to muscle contraction; (ii) inhibition of NOS, but not COX or epoxygenases, resulted in a significantly decreased basal diameter; (iii) inhibition of NOS significantly attenuated functional dilation in both paired and unpaired arterioles, whereas these inhibitory effects of l-NAME were diminished after pretreatment with indomethacin and PPOH; (iv) COX inhibition attenuated the vasodilatory responses in paired vessels only; and (v) inhibition of CYP epoxygenase had no effect on functional dilation. These results suggest that NO and prostanoid(s) are important in mediating functional vasodilation. However, for paired and unpaired arterioles, there are differences in the mediators responsible for the functional vasodilation in that prostanoid(s) are important in the regulation of diameter in paired but not unpaired arterioles. These results also suggest the possible interactions between NO and prostaglandin or EETs during muscle contraction and the contribution of other unidentified mediator(s) responsible for approximately 50% of the vasodilatory response in this preparation.
Maintenance of basal vascular tone
The reduction in resting diameter by l-NAME is consistent with previous findings in animals and humans16–18 suggesting that, under resting conditions, in vivo vascular tone is due, in part, to a continuous generation of endogenous NO. A possible mechanism may be flow-induced NO release from the endothelium, which stimulates cGMP in smooth muscle cells and causes vasodilation. Vequaud and Freslon showed that, in rat isolated coronary artery, either l-NAME or destruction of the endothelium significantly reduced both flow-induced dilation and shear stress values, whereas indomethacin had no effect.19 Although prostacyclin (PGI2) and EETs have been demonstrated to be important vasodilators, the present study suggests that their contribution to the regulation of vascular tone under resting conditions is minor, consistent with previous findings.20,21
Role of NO in functional vasodilation
In the present study, inhibition of NOS significantly attenuated functional arteriolar dilation in rat spinotrapezius muscle, suggesting that an increased NO production in response to the muscle contraction results in an enhanced arteriolar diameter and blood flow. However, although increased NO production in response to exercise has been demonstrated in animals10 and humans,12,13 studies examining blood flow during exercise under NOS blockade have yielded conflicting results. For example, skeletal muscle blood flow was shown to be reduced both at rest and during continuous, dynamic exercise in response to NG-monomethyl-l-arginine,16,22 whereas other studies have shown that NOS blockade only significantly decreased muscle blood flow at rest but had no effect during muscle contraction.23,24 This controversy may be explained by differences in the vascular bed, muscle type and techniques used. In addition, NOS inhibition may reduce basal vascular diameter. This change in basal diameter may result in differential responses to vasoactive metabolites such that the overall vasodilatory responses are different. In addition, the percentage change in diameter may appear to be the same, although the absolute change may be less than normal.
As mentioned above, the increase in shear stress may account, in part, for the NO production in response to muscle contraction. However, there is evidence that flow-mediated vasodilation is already maximally activated at rest,25 implying that there may be an alternative mechanism responsible for the increase in NO production during exercise. Berg et al. showed that, in hamster cremaster muscle, local coupling between capillary flow and muscle contraction includes a conducted vasodilation,26 which depends on NO production. 3,27 However, the signalling mechanism between the local and remote dilatation is unclear. Boegehold et al. showed that a shear-dependent increase in venular NO production dilates nearby arterioles in rat spinotrapezius muscle.28 In the present study, l-NAME attenuated basal arteriolar diameter and functional dilation to the same degree in both paired and unpaired arterioles, suggesting that venular endothelium-derived NO is not important in mediating arteriolar diameter at rest or during exercise.
NG-Nitro-l-arginine methyl ester is a non-selective NOS inhibitor, so it is unclear whether the blunted functional dilation in the present study was due to decreased NO production from other tissues, such as skeletal muscle, instead of endothelium. For example, NO produced by neuronal NOS in skeletal muscle is also proposed to regulate blood flow in exercising muscle by diffusing from the skeletal muscle fibres to the nearby microvessels.10,29,30 These published studies present the possibility that muscle-derived NO may play an important vasodilatory role. The cellular source(s) of NO and how it plays a role in mediating the functional vasodilatory response to muscle contraction needs to be determined.
Role of vasodilator prostanoids in functional vasodilation
Although prostanoids (such as prostacyclin) have been accepted as important vasodilators, their contribution to the functional vasodilation and hyperaemia is unclear. For example, the concentration of prostacyclin in the venous effluent has been shown to be elevated during exercise.11,13 Inhibition of prostacyclin production by indomethacin has either significantly reduced exercise-induced vasodilation31 or had no effect.32 These inconsistencies may be due, in part, to the different anatomical arrangements of the arteries/arterioles (i.e. paired with veins/venules). Consistent with our previous findings in the hamster cremaster muscle,5 the present study shows that indomethacin only inhibits the functional dilation of paired and not unpaired arterioles in rat spinotrapezius muscle. Taken together, these results suggest that, in response to muscle contraction, vasodilator prostanoids are released from the venules, resulting in a vasodilatory response in the adjacent arteriole. Moreover, Fig. 6 shows that, in paired vessels, COX inhibition with indomethacin results in a greater inhibition than NOS inhibition, suggesting a larger role of prostanoids in mediating functional dilation in paired arterioles. Further studies are needed to determine precisely the cellular source of prostaglandin production in response to muscle contraction in the spinotrapezius muscle.
In addition to the proposed mechanisms, such as blood flow-induced and conducted vasodilation, venular–arteriolar communication may be an additional mechanism that contributes to functional vasodilation. This theory is based on the findings that vasoactive metabolites can diffuse from venules to arterioles and influence arteriolar tone.33–36 Moreover, there is evidence to suggest that, in rat spinotrapezius muscle, the functional dilation and hyperaemia depends more on venular Po2 levels than on arteriolar and capillary Po2 levels.2 In addition to a role for oxygen, Koller et al. demonstrated that, in rat skeletal muscle venules, an increase in shear stress stimulated the release of endothelium-derived prostaglandins.37 Future studies will be needed to determine whether and how venules release prostag-landins and regulate blood flow.
Role of EETs and other factors in functional vasodilation
Epoxyeicosatrienoic acids are formed by specific CYP expoxygenase pathways and can cause vasodilation through calcium-activated potassium (KCa) channels. A previous study has shown that PPOH (1–50 µmol/L) decreases microsomal arachidonate epoxygenase activity in a concentration-dependent manner, but has almost no effect on ω-hydroxylation (20-HETE formation).38 In the present study, both 3 and 30 µmol/L PPOH inhibited AA-induced dilation in the presence of l-NAME and indomethacin, suggesting that if EETs were involved in mediating functional dilation, the 30 µmol/L PPOH used in the present study should be sufficient to attenuate their effect. However, 30 µmol/L PPOH did not affect the functional dilation in paired or unpaired arterioles, suggesting that epoxides are not important in functional vasodilation in rat spinotrapezius muscle. Unfortunately, there is a lack of evidence regarding the involvement of EETs in exercise hyperaemia. Although a recent study found that in hamster cremaster arterioles flow-dependent dilation was reduced by approximately 50% following treatment with 17-octadecynoic acid (a non-selective CYP450 inhibitor), it is unknown whether the increase in metabolic rate in response to muscle contraction can stimulate vasodilator EETs release.39
Future studies need to test possible interactions of enzymes and other mechanisms involved
We noticed that the effectiveness of l-NAME pretreatment was dependent on the order of inhibitor application. We speculate that an increased production of prostagladins or EETs from the venules in response to muscle contraction may contribute to the synthesis of NO in paired arterioles. For example, l-NAME inhibited the functional dilation in the LIP group, whereas it failed to further reduce the vasodilatory responses in the ILP or PIL groups after pretreatment with indomethacin or PPOH. These results suggest that inhibition of COX or epoxygenase may also attenuate NO production via cross-talk between enzymes during muscle contraction, resulting in the diminished effect of l-NAME. Indeed, l-NAME caused a significant decrease in the functional vasodilation compared with control in group PIL, suggesting accumulated inhibition of NO. In addition, basal production of NO was not affected after pretreatment with indomethacin or PPOH, supporting our former conclusion that the basal production of PGI2 or EETs is not Blackwell Publishing Asia Pty Ltd important in mediating the resting vascular tone. However, although many studies have demonstrated the possible interactions between NO, PGI2 and EETs biosynthesis in vivo,40,41 one clinical study has suggested that NO and prostaglandin signals appear to contribute independently to forearm exercise hyperaemia in humans.42 Moreover, combined blockade of prostaglandin and NO only attenuated blood flow during steady state exercise and failed to delay the onset of the hyperaemic response, suggesting that a separate mechanism(s) may contribute to the immediate and early increase in blood flow in response to exercise.43 Therefore, further studies are needed to determine the possible ‘interactions’ during the functional hyperaemic response in the rat spinotrapezius muscle.
In the present study, the remaining vasodilatory response after the administration of the three inhibitors may be due to other vasoactive factors, an incomplete inhibition of the enzymes or both. Although the protocols and concentrations of l-NAME and indomethacin applied in the present studies are based on our and other published work, we notice that it is hard to achieve 100% inhibition. This limitation complicates the explanation of the remaining vasodilatory response. Even if incomplete inhibition exists, it is very possible that other mechanisms and factors may contribute to the functional hyperaemic response in addition to NO and PGI2. For example, blockade of adenosine receptors was reported to decrease blood flow in exercising humans4 and the circulating levels of ATP have been shown to increase in an exercise intensity dependent manner.44 Indeed, our previous study demonstrated that blockade of ATP-sensitive potassium (KATP) channels can attenuate functional vasodilation in hamster cremaster muscles.45 Although both NO- and PGI2-mediated vasodilation has been shown to involve KATP channels,46 it is not clear whether other endogenous vasodilators, such as adenosine, also activate KATP during muscle contraction and the role of KATP channel activation in functional vasodilation in rat spinotrapezius muscle is not clear. In addition, the present study cannot exclude the possibility that other EDHF are involved in the functional vasodilatory response. For example, there is evidence that the increase in interstitial potassium concentration surrounding the vasculature in response to muscle contraction results in vasodilation through activation of smooth muscle Kir channels.47,48 In addition, endothelial cell calcium has been shown to increase in response to muscle contraction,49 implying hyperpolarization of the endothelial cell membrane potential. This suggests a role for the conduction of a hyperpolarization through gap junctions to the underlying smooth muscle.50 Alternatively, the remaining dilation may be a compensatory effect in response to NOS and COX inhibition by a yet to be determined factor. Further studies are needed to determine the mechanisms responsible for the additional 50% of the remaining functional vasodilation.
Conclusions
The present study was designed to determine the vasoactive factors involved in the functional vasodilation in the rat spinotrapezius muscle. The results indicate that NO is important in maintaining basal arteriolar diameter. Vasodilator prostanoids and NO, but not epoxygenases, are involved in functional vasodilation. However, unlike NO, prostanoids appear to play a major role in paired but not unpaired arterioles. Further studies are needed to determine the interactions between NO and prostaglandin or EETs synthesis and the factors responsible for the remaining vasodilatory response.
ACKNOWLEDGEMENTS
The authors thank Jennifer Dearman for her technical help with these experiments. These studies were supported by National Institutes of Health grants HL-51971 and HL-63958 and an American Heart Association–Southeast Affiliate Postdoctoral Award.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1440-1681.2007.04864.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2788941?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1440-1681.2007.04864.x
Article citations
Endothelium-dependent responses in the microcirculation observed in vivo.
Acta Physiol (Oxf), 224(2):e13111, 10 Jul 2018
Cited by: 16 articles | PMID: 29873936
Review
Angiotensin II Receptor Blockers Inhibit the Generation of Epoxyeicosatrienoic Acid from Arachidonic Acid in Recombinant CYP2C9, CYP2J2 and Human Liver Microsomes.
Basic Clin Pharmacol Toxicol, 121(4):239-245, 10 May 2017
Cited by: 8 articles | PMID: 28374982
Non-steroidal anti-inflammatory drugs attenuate the vascular responses in aging metabolic syndrome rats.
Acta Pharmacol Sin, 35(11):1364-1374, 29 Sep 2014
Cited by: 7 articles | PMID: 25263337 | PMCID: PMC4220071
Oxygen transport in the microcirculation and its regulation.
Microcirculation, 20(2):117-137, 01 Feb 2013
Cited by: 45 articles | PMID: 23025284 | PMCID: PMC3574207
Review Free full text in Europe PMC
Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers.
J Lipid Res, 53(8):1662-1669, 24 May 2012
Cited by: 69 articles | PMID: 22628615 | PMCID: PMC3540841
Go to all (10) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Exercise training enhances flow-induced vasodilation in skeletal muscle resistance arteries of aged rats: role of PGI2 and nitric oxide.
Am J Physiol Heart Circ Physiol, 292(6):H3119-27, 02 Mar 2007
Cited by: 50 articles | PMID: 17337602
Skeletal muscle arteriolar function following myocardial infarction: Analysis of branch-order effects.
Microvasc Res, 81(3):337-343, 27 Jan 2011
Cited by: 2 articles | PMID: 21276804 | PMCID: PMC3078981
Gender-specific compensation for the lack of NO in the mediation of flow-induced arteriolar dilation.
Am J Physiol Heart Circ Physiol, 280(6):H2456-61, 01 Jun 2001
Cited by: 55 articles | PMID: 11356598
Role of endothelium-derived relaxing factors in arteriolar dilation during muscle contraction elicited by electrical field stimulation.
Microcirculation, 1(3):195-201, 01 Oct 1994
Cited by: 16 articles | PMID: 8790590
Funding
Funders who supported this work.
NHLBI NIH HHS (6)
Grant ID: R01 HL063958-03
Grant ID: HL-63958
Grant ID: R01 HL063958
Grant ID: HL-51971
Grant ID: P01 HL051971-159001
Grant ID: P01 HL051971