Abstract
Free full text

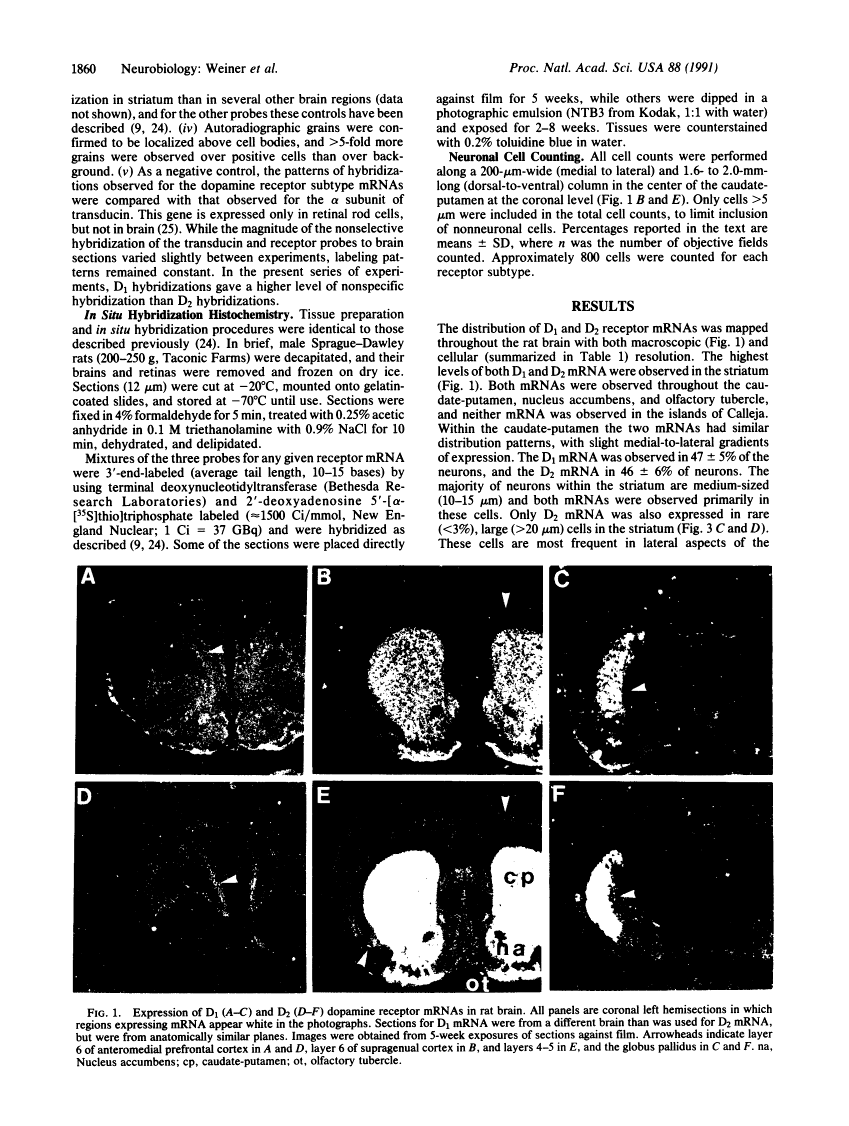
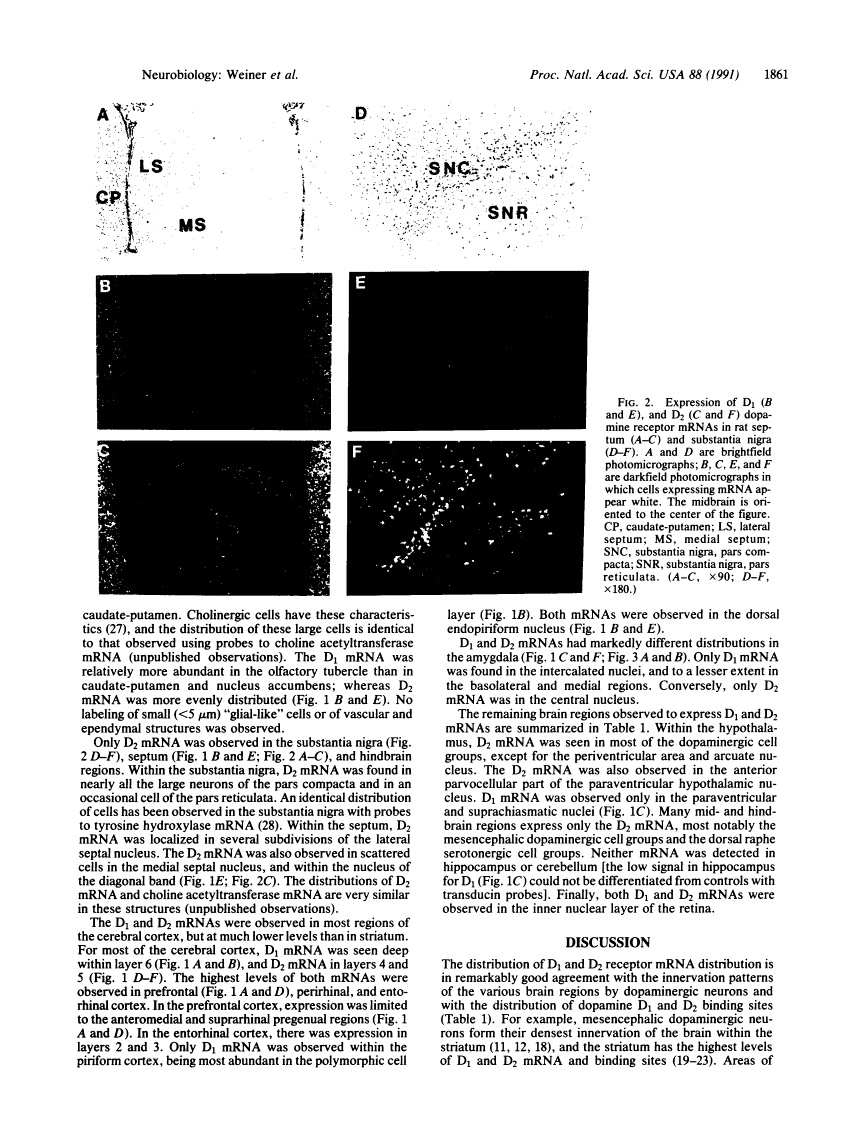
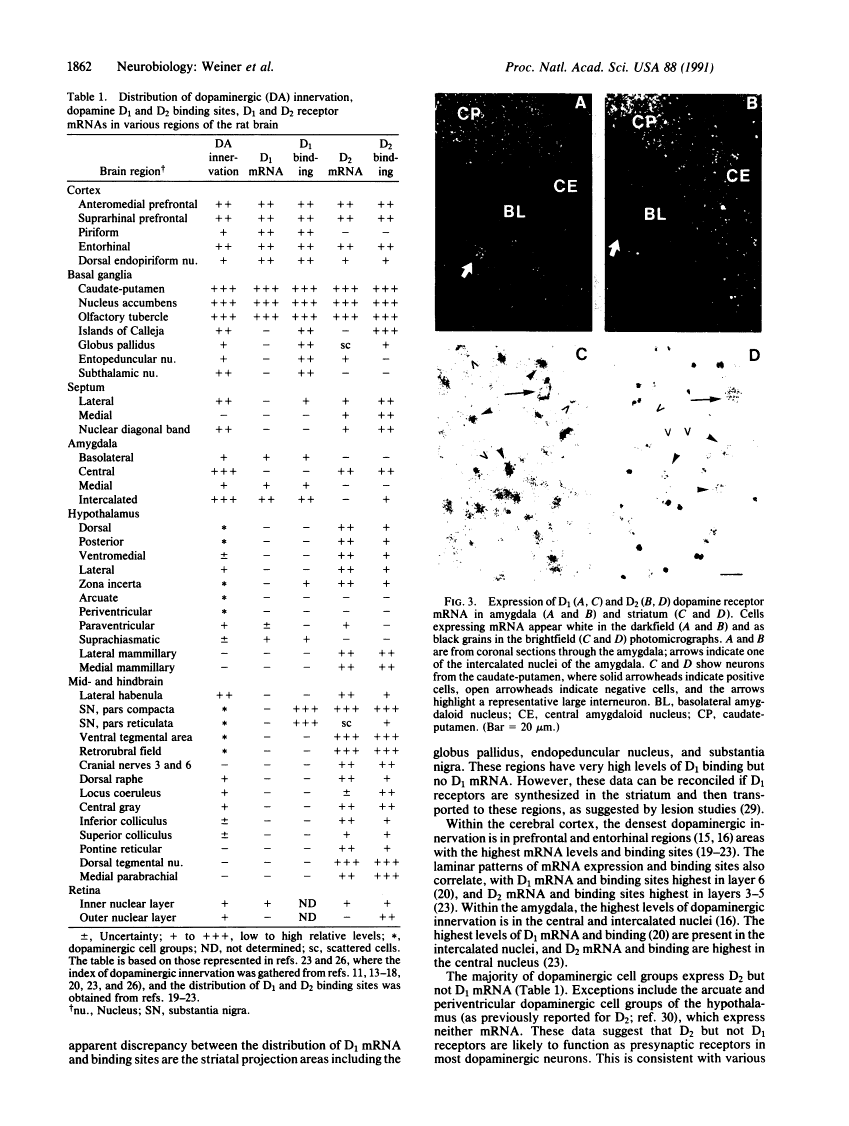
D1 and D2 dopamine receptor mRNA in rat brain.
Abstract
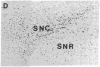
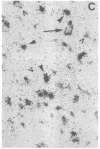
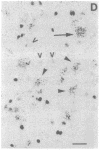
Physiological and pharmacological criteria have divided dopamine receptors into D1 and D2 subtypes, and genes encoding these subtypes have recently been cloned. Based on the sequences of the cloned receptors, we prepared oligodeoxynucleotide probes to map the cellular expression of the corresponding mRNAs in rat brain by in situ hybridization histochemistry. These mRNAs showed largely overlapping yet distinct patterns of expression. The highest levels of expression for both mRNAs were observed in the caudate-putamen, nucleus accumbens, and olfactory tubercle. Within the caudate-putamen, 47 +/- 6% and 46 +/- 5% of the medium-sized neurons (10-15 microns) expressed the D1 and D2 mRNAs, respectively, and only the D2 mRNA was observed in the larger neurons (greater than 20 microns). The D1 and D2 mRNAs were expressed in most cortical regions, with the highest levels in the prefrontal and entorhinal cortices. Within neocortex, D1 mRNA was observed primarily in layer 6 and D2 mRNA in layers 4-5. Within the amygdala, D1 mRNA was observed in the intercalated nuclei, and D2 mRNA in the central nucleus. Within the hypothalamus, D1 mRNA was observed in the suprachiasmatic nucleus and D2 mRNA in many of the dopaminergic cell groups. Within the septum, globus pallidus, superior and inferior colliculi, mammillary bodies, and substantia nigra only D2 mRNA was detected. These data provide insight into the neuroanatomical basis of the differential effects of drugs that act on D1 or D2 receptors.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.8M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchine JR. Drug therapy of parkinsonism. N Engl J Med. 1976 Oct 7;295(15):814–818. [Abstract] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. [Abstract] [Google Scholar]
- Andersen PH, Gingrich JA, Bates MD, Dearry A, Falardeau P, Senogles SE, Caron MG. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends Pharmacol Sci. 1990 Jun;11(6):231–236. [Abstract] [Google Scholar]
- Clark D, White FJ. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1(4):347–388. [Abstract] [Google Scholar]
- Sunahara RK, Niznik HB, Weiner DM, Stormann TM, Brann MR, Kennedy JL, Gelernter JE, Rozmahel R, Yang YL, Israel Y, et al. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990 Sep 6;347(6288):80–83. [Abstract] [Google Scholar]
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. [Abstract] [Google Scholar]
- Stormann TM, Gdula DC, Weiner DM, Brann MR. Molecular cloning and expression of a dopamine D2 receptor from human retina. Mol Pharmacol. 1990 Jan;37(1):1–6. [Abstract] [Google Scholar]
- O'Dowd BF, Lefkowitz RJ, Caron MG. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. [Abstract] [Google Scholar]
- Björklund A, Moore RY, Nobin A, Stenevi U. The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 1973 Mar 15;51:171–191. [Abstract] [Google Scholar]
- Björklund A, Lindvall O, Nobin A. Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Res. 1975 May 16;89(1):29–42. [Abstract] [Google Scholar]
- Lindvall O, Björklund A, Divac I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978 Feb 17;142(1):1–24. [Abstract] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978 Aug 1;180(3):509–532. [Abstract] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol. 1978 Aug 1;180(3):533–544. [Abstract] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978 Aug 1;180(3):545–580. [Abstract] [Google Scholar]
- Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986 Jun 11;375(2):291–301. [Abstract] [Google Scholar]
- Dawson TM, Gehlert DR, McCabe RT, Barnett A, Wamsley JK. D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci. 1986 Aug;6(8):2352–2365. [Abstract] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. [Abstract] [Google Scholar]
- Charuchinda C, Supavilai P, Karobath M, Palacios JM. Dopamine D2 receptors in the rat brain: autoradiographic visualization using a high-affinity selective agonist ligand. J Neurosci. 1987 May;7(5):1352–1360. [Abstract] [Google Scholar]
- Bouthenet ML, Martres MP, Sales N, Schwartz JC. A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience. 1987 Jan;20(1):117–155. [Abstract] [Google Scholar]
- Weiner DM, Brann MR. The distribution of a dopamine D2 receptor mRNA in rat brain. FEBS Lett. 1989 Aug 14;253(1-2):207–213. [Abstract] [Google Scholar]
- Raport CJ, Dere B, Hurley JB. Characterization of the mouse rod transducin alpha subunit gene. J Biol Chem. 1989 May 5;264(13):7122–7128. [Abstract] [Google Scholar]
- Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989 May-Jun;2(3):119–137. [Abstract] [Google Scholar]
- Levey AI, Wainer BH, Mufson EJ, Mesulam MM. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience. 1983 May;9(1):9–22. [Abstract] [Google Scholar]
- Young WS, 3rd, Bonner TI, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. [Europe PMC free article] [Abstract] [Google Scholar]
- Gale K, Guidotti A, Costa E. Dopamine-sensitive adenylate cyclase: location in substantia nigra. Science. 1977 Feb 4;195(4277):503–505. [Abstract] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Bunzow JR, Van Tol HH, Watson SJ, Jr, Civelli O. Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7625–7628. [Europe PMC free article] [Abstract] [Google Scholar]
- White FJ, Wang RY. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984 Nov;231(2):275–280. [Abstract] [Google Scholar]
- Morelli M, Mennini T, Di Chiara G. Nigral dopamine autoreceptors are exclusively of the D2 type: quantitative autoradiography of [125I]iodosulpride and [125I]SCH 23982 in adjacent brain sections. Neuroscience. 1988 Dec;27(3):865–870. [Abstract] [Google Scholar]
- Brann MR, Young WS., 3rd Dopamine receptors are located on rods in bovine retina. Neurosci Lett. 1986 Sep 12;69(3):221–226. [Abstract] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7050–7054. [Europe PMC free article] [Abstract] [Google Scholar]
- Battaglia G, Norman AB, Hess EJ, Creese I. Forskolin potentiates the stimulation of rat striatal adenylate cyclase mediated by D-1 dopamine receptors, guanine nucleotides, and sodium fluoride. J Neurochem. 1986 Apr;46(4):1180–1185. [Abstract] [Google Scholar]
- Plaznik A, Stefanski R, Kostowski W. Interaction between accumbens D1 and D2 receptors regulating rat locomotor activity. Psychopharmacology (Berl) 1989;99(4):558–562. [Abstract] [Google Scholar]
- Scatton B. Effect of dopamine agonists and neuroleptic agents on striatal acetylcholine transmission in the rat: evidence against dopamine receptor multiplicity. J Pharmacol Exp Ther. 1982 Jan;220(1):197–202. [Abstract] [Google Scholar]
- Robinson SE, Malthe-Sørenssen D, Wood PL, Commissiong J. Dopaminergic control of the septal-hippocampal cholinergic pathway. J Pharmacol Exp Ther. 1979 Mar;208(3):476–479. [Abstract] [Google Scholar]
- Miller R, Wickens JR, Beninger RJ. Dopamine D-1 and D-2 receptors in relation to reward and performance: a case for the D-1 receptor as a primary site of therapeutic action of neuroleptic drugs. Prog Neurobiol. 1990;34(2):143–183. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.88.5.1859
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc51125?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/120693810
Article citations
Acute Ongoing Nociception Delays Recovery of Consciousness from Sevoflurane Anesthesia via a Midbrain Circuit.
J Neurosci, 44(34):e0740242024, 21 Aug 2024
Cited by: 0 articles | PMID: 39019613
Rationale and Development of Tavapadon, a D1/D5-Selective Partial Dopamine Agonist for the Treatment of Parkinson's Disease.
CNS Neurol Disord Drug Targets, 23(4):476-487, 01 Jan 2024
Cited by: 3 articles | PMID: 36999711 | PMCID: PMC10909821
Review Free full text in Europe PMC
Amphetamine-induced dopamine release in rat: Whole-brain spatiotemporal analysis with [11C]raclopride and positron emission tomography.
J Cereb Blood Flow Metab, 44(3):434-445, 26 Oct 2023
Cited by: 0 articles | PMID: 37882727 | PMCID: PMC10870964
Role of dopamine D1 receptor in the modulation of memory consolidation by passive and self-administered heroin and associated conditioned stimuli.
Sci Rep, 13(1):12614, 03 Aug 2023
Cited by: 0 articles | PMID: 37537211 | PMCID: PMC10400648
Dopamine agonists in Parkinson's disease: Impact of D1-like or D2-like dopamine receptor subtype selectivity and avenues for future treatment.
Clin Park Relat Disord, 9:100212, 07 Jul 2023
Cited by: 4 articles | PMID: 37497384 | PMCID: PMC10366643
Review Free full text in Europe PMC
Go to all (275) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Visualization of dopamine D1, D2 and D3 receptor mRNAs in human and rat brain.
Neurochem Int, 20 Suppl:33S-43S, 01 Mar 1992
Cited by: 63 articles | PMID: 1365451
Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene.
Proc Natl Acad Sci U S A, 88(10):4205-4209, 01 May 1991
Cited by: 182 articles | PMID: 1827915 | PMCID: PMC51627
Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain.
Neuropsychopharmacology, 5(4):231-242, 01 Dec 1991
Cited by: 160 articles | PMID: 1839499
Molecular characterization of dopamine receptors.
Am J Hypertens, 3(6 pt 2):29S-33S, 01 Jun 1990
Cited by: 3 articles | PMID: 2143386
Review