Abstract
Free full text

Blood Coagulation, Inflammation and Malaria
I. ABSTRACT
Malaria remains a highly prevalent disease in more than 90 countries and accounts for at least 1 million deaths every year. Plasmodium falciparum infection is often associated with a procoagulant tonus characterized by thrombocytopenia and activation of the coagulation cascade and fibrinolytic system; however, bleeding and hemorrhage are uncommon events, suggesting that a compensated state of blood coagulation activation occurs in malaria. This article i) reviews the literature related to blood coagulation and malaria in a historic perspective, ii) describes basic mechanisms of coagulation, anticoagulation, and fibrinolysis, iii) explains the laboratory changes in acute and compensated disseminated intravascular coagulation (DIC), iv) discusses the implications of tissue factor (TF) expression in the endothelium of P. falciparum-infected patients, and v) emphasizes the pro-coagulant role of parasitized erythrocytes (pRBC) and activated platelets in the pathogenesis of malaria. This article also presents the ‘Tissue Factor Model’ (TFM) for malaria pathogenesis, which places TF as the interface between sequestration, endothelial cell activation, blood coagulation disorder and inflammation often associated with the disease. The relevance of the coagulation-inflammation cycle for the multiorgan dysfunction and coma is discussed in the context of malaria pathogenesis.
II. MALARIA: GENERAL ASPECTS
Malaria remains a major cause of morbidity and mortality in more than 90 countries and accounts for at least 1 million deaths every year and 450 million disease episodes annually [1–5]. Malaria is caused by Plasmodium sp. transmitted to the host by bite of the blood-feeding Anopheles sp. mosquitoes, which inject sporozoite-infected saliva [6–10]. There are four different types of Plasmodia (P. falciparum, P. vivax, P. ovale, P. malariae) that infect humans but P. falciparum causes almost all death [1–5]. Plasmodium parasite invades erythrocytes as part of its asexual life cycle within the human host [1–5, 8, 9]. As the P. falciparum parasites mature within the red blood cells (pRBC) to trophozoite and schizont stages [11, 12], they disappear from the peripheral circulation and localize specifically in the vascular beds of organs such as the brain, a process named sequestration [1–5]. Sequestration aids in parasite survival by avoiding clearance by the spleen and appears to be an important factor in malaria pathogenesis.
Clinically, malaria infection causes a range of symptoms from asymptomatic or mild flu-like illness (uncomplicated malaria)—particularly in immune individuals in endemic areas—to the uncommom complications of severe disease. This can present with a number of different syndromes, alone or in combination, including severe malarial anemia, respiratory complications and acidosis, renal failure, pulmonary edema, hepatic dysfunction, multiorgan failure, and cerebral malaria (CM) [1–5, 13–24]. Actually, CM is the most-studied complication, and it is usually described as a syndrome consisting of decreased consciousness and unrousable coma not attributable to other causes in a patient with P. falciparum parasitemia [1–5, 13–24]. When the patients survive, recovery is usually complete, but it can also leave some patients with long-term neurologic deficits [17]. The disease has a high mortality even when treated with appropriate antiparasite medication and intensive supportive care [24]. Because of the intensity of coma and the rapidity of its resolution, most authors agree that the underlying mechanisms are not compatible with primary vasocclusive pathology only, but resemble the picture of a toxic or metabolic encephalopathy [1–5].
While different theories have been proposed to explain the disease in humans [25, 26], including the sequestration and cytokine hypothesis, the mechanisms of malaria pathogenesis remain incompletely understood [1–5]. Nevertheless, a number of pathology studies in Southeast Asian adults and in African children have described an association between sequestration and coma in patients dying from malaria [27–32]. More recently, disease severity in P. falciparum malaria patients was demonstrated to be directly associated with the biomass of pRBC as estimated by plasma levels of a parasite-specific histidine-rich protein (PfHRP2). PfHRP2 is a marker of total population of pRBC undergoing schizogony [33] and accordingly has been used as an indirect marker for sequestration [34, 35]. In agreement with a prominent role of cytoadherence to EC in disease pathogenesis is the fact that P. vivax malaria is not consistently accompanied by sequestration and/or severe disease [30, 36, 37]. Moreover, in some hemoglobinopathies (e.g. hemoglobinopathy C), the host polymorphism that affects P. falciparum erythrocyte membrane protein-1 (PfEMP-1) may protect against malaria by impairing the parasite’s ability to cytoadhere to microvessels [38–40].
PfEMP-1 is the name for various antigenically diverse parasite-derived molecules located on the erythrocyte membrane mediating sequestration [41–43]. PfEMP-1 interacts with several endothelial cell (EC)-surface receptors such as CD36 [44–47], intercellular adhesion molecule 1 (ICAM-1) [48], thrombospondin [49], vascular cell adhesionmolecule 1 [50], E-selectin [50], and CD31 [51]. In addition, chondroitin sulfate A is bound by pRBC [52]and supports pRBC adhesion to both placental syncytiotrophoblast [53] and endothelium [54, 55]. Expression of some of these receptors appears tobe modulated, at least in part, by cytokines such as TNF-α, which is known to be increased in the plasma of malaria patients [56, 57]. Excess production of TNF-α is likely to be involved in the appearance of such symptoms as fever and headache associated with P. falciparum infection [58]. Actually, a systemic response characterized by increased levels of circulating cytokines such as IL-1β, IL-6, IL-8, IL-10, and HMGB-1 is observed in malaria in general and in cerebral malaria (CM) in particular [59–65]. Furthermore, postmortem studies of human brain tissue in patients who died from CM display expression of TNF-α and IL-1β [66, 67]. Of note, some reports have linked cytokine levels to disease severity and complications [56, 57, 64, 68–71]. It has been suggested that TNF-α and other cytokines up regulate adhesion molecules (e.g. ICAM-1) on vascular endothelium and thus modulate cytoadhrence [50, 72]. However, a relationship between in vitro cytoadhrence to CD36 and/or ICAM-1 and disease severity or cerebral symptoms has not been formally demonstrated [73–75].
Malaria pathogenesis has been associated with both localized and widespread activation of EC in uncomplicated and fatal cases (e.g. cerebral malaria). Accordingly, molecules such as ICAM-1, VCAM-1, E-selectin, MIF, iNOS, uPAR and TF have been identified in the endothelium of P. falciparum-infected patients, according to immunohistochemistry (IHC) studies [30, 76–82]. Increased plasma levels of soluble adhesion molecules sICAM-1, sVCAM-1, and sE-selectin [76, 83] and other markers of EC activation such as thrombomodulin [79, 84] and von Willebrand factor [85, 86] have also been reported in the same population. Further, both mild and severe cases of P. falciparum malaria have been found to be associated with thrombocytopenia [86–91], hemostatic alterations [86–106], and EC microparticle production [107], indicating a procoagulant state. It has thus been suggested that hemostatic alterations could be important in the disease progression and organ failure observed in malaria [92, 94, 98, 103, 104]. Of note, this concept has been applied in sepsis, a condition that shares with malaria a number of physiopathological mechanisms [108–113]. The potential role of the coagulation cascade in Malaria pathogenesis is discussed below. A revision of the literature related to blood coagulation in malaria, in a historic perspective, is found in the Supplementary Material (Section A, “Malaria and Blood Coagulation: Historic Perspective”).
III-THE ROLE OF THE COAGULATION CASCADE IN P FALCIPARUM MALARIA PATHOGENESIS: STILL A CONTROVERSIAL ISSUE IN 2007
This section attempts to explain why the coagulation cascade could be regarded as a critical component in falciparum infection [1–5]. A description of basic mechanisms of coagulation cascade, anticoagulation and fibrinolysis is discussed in the Supplementary Material (Section B, “Tissue Factor and the Blood Coagulation Cascade”) and presented diagrammatically in Figure 1 (see Figure Legends). Likewise, the role of protease-activated receptors (PAR), and the participation of blood-borne TF and microparticles in inflammation is described as a supplement to this article, and has been comprehensively reviewed elsewhere [108–113].
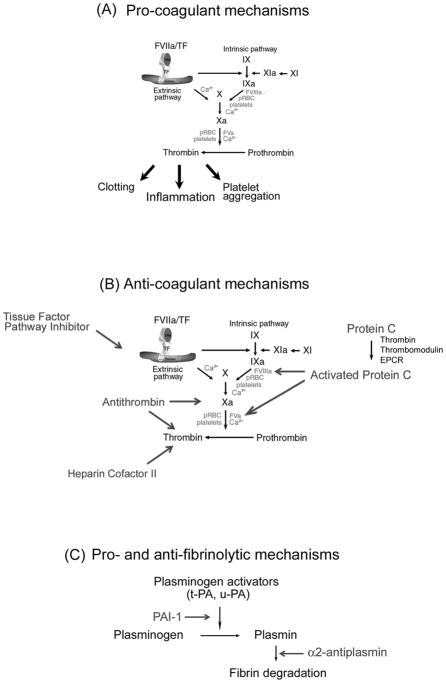
Coagulation cascade and its regulation. A) Pro-coagulant mechanisms. TF: a critical initiator of coagulation. Formation of a complex with Factor VIIa (FVIIa) leads to activation of FIX and FX. FXa in the presence of phosphatidyl serine and Ca2+ (prothrombinase complex) amplifies the coagulation cascade through conversion of prothrombin to thrombin, resulting in platelet aggregation, fibrin formation, and inflammation. Thrombin also activates FXI to XIa, which activates FIX to FIXa. FIXa in the presence of phosphatidyl serine and Ca2+ converts FX to FXa, consolidating the coagulation cascade. pRBC, parasitized red blood cells. B) Anticoagulant mechanism. TF pathway inhibitor (TFPI) binds to FXa and inhibits FVIIa/TF complex. Protein C is activated by thrombin (in the presence of thrombomodulin and EPCR), and APC inhibits the coagulation cascade through cleavage of cofactors FVa and FVIIIa. Antithrombin in the presence of heparin sulphate specifically interacts with and inhibits FXa and thrombin. Heparin cofactor II (in the presence of dermatan sulphate) inhibits thrombin. C). Pro- and antifibrinolytic mechanism. PAI-1, plasminogen activator inhibitor-1. The zymogen plasminogen is converted to the active serine protease, plasmin, through the action primarily of two-chain tissue plasminogen activator (tc-tPA) or two-chain urokinase (tc-uPA). Both tPA and uPA can be inhibited by plasminogen activator inhibitor-1 (PAI), while plasmin is inhibited by its major inhibitor, α2-antiplasmin, and to a lesser extent by α2-macroglobulin (not shown). For details, see Supplementary Material (Section B, “Tissue Factor and the Blood Coagulation Cascade”).
Controversy exists as to whether a coagulopathy is primarily or partially involved in the pathogenesis of malaria [94, 106]. In other words, a common question is whether coagulopathy has any “functional” importance in vivo in causing morbidity and/or fatal outcome. In actuality, controversy exists for a number of reasons: i) typical DIC (e.g., bleeding) is not present in most P. falciparum-infected patients [86, 92, 94, 96–99, 102–104] and occurs in 5% to 10% of severe malaria cases (1% of all cases) [1–5]; ii) thrombocytopenia in falciparum malaria [88–90] was indicative of prognosis in one study [88] but also occurs in vivax malaria [96, 101, 104, 114–116]; iii) fibrin and/or thrombus indicative of in vivo activation of the coagulation cascade have been clearly reported in some studies [99, 103, 117–122] but infrequently found [29, 80] or undetectable in others [123, 124]; iv) experimental malaria treated with anticoagulants such as heparin does not change the morbidity associated with Plasmodium infection in mice or rhesus monkeys [125, 126], with one exception [127]. In addition, case reports have shown that use of anticoagulants such as heparin to treat severe malaria in humans has not changed outcome for most patients [128–130]. Furthermore, a prospective and randomized study involving 97 humans infected with P. falciparum indicated that heparin did not affect the clinical course of the disease [131], and iv) laboratory changes reported in P. falciparum infection varies considerably from one study to another making it difficult for all authors to reach similar conclusions [86–106].
Despite the above, a careful study of the literature devoted to malaria in particular, and vascular biology in general, indicate otherwise, i.e., activation of the coagulation cascade could be involved in malaria pathogenesis.
Coagulation activity in malaria
Numerous studies with human and animals indicate that there is undoubtedly increased coagulation activity in malaria. It occurs in uncomplicated (mild) cases [14, 15] and, although clinically not significant, can be confirmed as a compensated state according to in vivo coagulation tests [86, 87, 92–101]. Most important, careful studies distinguishing mild, moderate, and severe P. falciparum infection as related to parasitemia have been reported in at least ten papers. These studies have demonstrated that the degree of coagulation derangement was often associated with disease severity [79, 86, 92, 94, 96–98, 101–104, 132]. Further, in some studies, it correlates with levels of parasitemia [86, 94, 97, 102, 104]. The fact that overt DIC (e.g., bleeding) is not present in severe malaria patients does not exclude an active role for the coagulation cascade in malaria pathogenesis. It is well known that severe organ dysfunction, including that seen in sepsis, is not necessarily accompanied by bleeding and/or hemorrhage [133–136] but associated with a certain degree of activation of coagulation, fibrinolytic systems, and inflammation [108–113].
Thrombocytopenia in malaria
The fact that platelet numbers are low in benign vivax malaria [96, 101, 104, 114, 115, 137], confirms rather than refutes, a role of the coagulation cascade in P. falciparum malaria pathogenesis because the mechanisms for thrombocytopenia in each infection differs. Thrombocytopenia in vivax malaria appears to be immune mediated [114] and occurs in the absence of blood coagulation activation [96, 101, 104]. In contrast, thrombocytopenia in falciparum malaria is most often accompanied by activation of the coagulation cascade [86, 87, 92–101], among other mechanisms (e.g. immune-mediated lyses, peripheral destruction) [91, 106, 138–141].
Fibrin and malaria
The fact that fibrin has been demonstrated in some cases [99, 103, 117–122] but infrequently found [29, 80] or undetectable [123, 124] in others could be explained by the fact that coagulation activation is accompanied by compensatory fibrinolysis [142, 143]. Other mechanisms may also be involved such as high levels of neutrophil elastase (which degrade fibrin-stabilization Factor XIII) found in the plasma of malaria patients [97] and/or high levels of (pro-fibrinolytic) urokinase-type plasminogen activator (uPAR) expression detected in the endothelium of the same population [81]. In fact, several reports indicate that D-dimer levels are increased in the circulation of many cases of P. falciparum-infected patients, in complicated or mild cases [86, 92, 96, 100–104, 144]. D-dimers are sensitive and specific in vivo markers of intravascular fibrin formation and fibrinolysis [142, 143]; this indicates that fibrin has been formed and digested intravascularly by plasmin in patients infected with P. falciparum. Of note, in 1972, O’Learly [93], studying DIC in monkeys, described: “Fibrin strands were sometimes seen in the engorged capillaries and veins, but organized thrombus were extremely rare. The most plausible explanation for this repeated observation is that the brisk fibrinolysis occurring secondary to DIC effectively digests the fibrin as it is elaborated and thus preventing the development of organized thrombus.” It is important to recognize that detection of fibrin in sepsis has also been reported in some studies [145–147], although undetectable in others [148]. Nevertheless, most authors will agree that the coagulation cascade contributes to sepsis pathogenesis, as discussed in the Supplementary Material (Part C) and comprehensively reviewed elsewhere [108–113].
Anticoagulants
The fact that anticoagulants such as heparin dos not affect the outcome of malaria is not an entirely unexpected finding based on the increasingly recognized cross-talk between blood coagulation and inflammation [108–113]. In contrast to inhibition of thrombin by heparin [149, 150] or thrombin generation by active site-blocked Xa, which fails to reduce mortality [151], inhibitors of the TF initiation complex such as antibodies to TF [152], TFPI [153], or active site-blocked VIIa [154] reduce inflammatory responses in addition to their antithrombotic effects at least in some experimental models (e.g., sepsis). In other words, proximal inhibition of the coagulation cascade by TF blockade prevents coagulopathy, reduces inflammation, and attenuates lethality [152–156]. It has been suggested that signaling by the ternary FVIIa/TF/FXa initiation complex provides a mechanism to activate protease-activated receptors (PAR; see below) without requiring massive activation of fluid-phase proteases that drive DIC. Accordingly, blockade of ternary initiation complex by molecules such as TF inhibitors appears to be effective in the treatment of experimental sepsis [152–156].
Laboratory changes
The fact that laboratory changes are not the same in all studies dealing with the hemostatic disorder that follows P. falciparum infection [86–104, 132] could be the result of the selection of patient group and/or severity of the infection or because these hemostatic elevations are inherently fluctuant. Sensitivity of specific coagulation assays employed by each lab may have also introduced additional variability into the analysis.
IV. ACUTE VERSUS “COMPENSATED” DISSEMINATED INTRAVASCULAR COAGULATION (DIC): AN IMPORTANT DISTINCTION
Activation of the coagulation cascade as a consequence of inflammation is well known event and can be viewed as an essential part of the host defense of the body, for example, to infectious agents. While activation of the coagulation cascade is a physiologic response triggered in an effort to contain the invading entity and to keep the consequent inflammatory response to a limited area, an exaggerated or uncontrolled response may lead to a situation in which coagulation and microthrombosis contribute to disease. This is illustrated by the occurrence of systemic coagulation activation in combination with microvascular failure, which results from the systemic inflammatory response to severe infection or sepsis and contributes to multiple organ dysfunction [108–113]. Figure 2 summarizes this concept emphasizing the role of the coagulation cascade in regulating or driving inflammation [108]. It is remarkable that these two situations appear to occur in malaria.
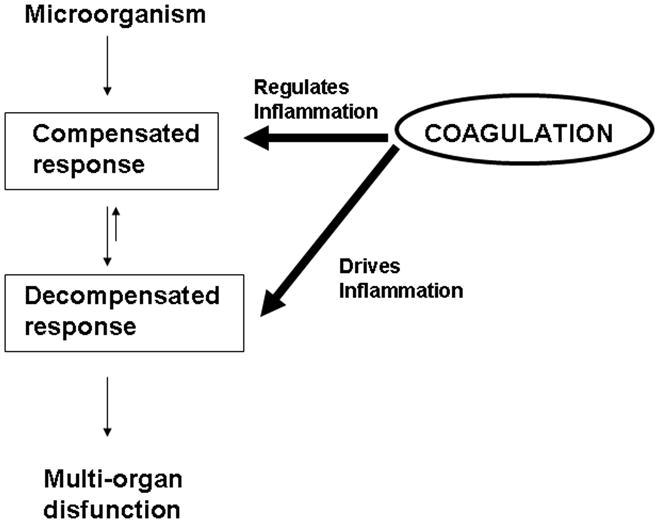
Compensated and decompensated responses and modulation by the coagulation cascade. Activation of coagulation cascade as a consequence of inflammation is an essential part of the host defense of the body. This physiologic response is triggered in an effort to contain the invading entity and to keep the consequent inflammatory response to a limited area. An exaggerated or uncontrolled response, however, may lead to a situation in which coagulation and microthrombosis contribute to disease. This is illustrated by the occurrence of systemic coagulation activation in combination with microvascular failure, which results from the systemic inflammatory response to severe infection or sepsis, and that contributes to multiple organ dysfunction. Modified from Ruf, 2004 [108].
Thus, in uncomplicated malaria, a coagulation disorder is a common laboratory finding, but bleeding and hemorrhage are not observed [14, 15, 19, 21, 22]. Coagulation disorder is also commonly observed in severe malaria (~ 1% of all cases), but only in 5% to 10% of these cases it is associated with bleeding [1–5, 86, 94, 96–98, 102, 104]. Therefore, an important distinction should be made between these two pathologic states, one where typical DIC is encountered (e.g., bleeding), and the other where a compensated state (e.g., laboratory changes only) is detectable.
DIC is a life-threatening acute, subacute, or chronic coagulation disorder occurring as a secondary complication in a variety of diseases. It is characterized by activation of the coagulation cascade, which leads to the formation of microthrombin through the microcirculation of the body. Sometimes the coagulopathy is localized to a specific organ, but it often presents an uneven distribution. Two major mechanism trigger DIC: release of TF into the circulation or endothelial injury [142, 143].
While acute DIC is the terminal phase of the coagulation disorder, it is often preceded by a period during which the coagulation cascade is already activated but the increased activation can be compensated for by the natural inhibitor systems, a state referred to as compensated DIC. In some cases, a continuous or intermittent slow rate of initiation of intravascular coagulation occurs and may or may never undergo decompensation to acute DIC. Under these conditions, the control mechanisms may effectively prevent severe clinical manifestations such as bleeding and hemorrhage by neutralizing active enzymes and/or by increasing the synthesis of the consumed hemostatic components. As the trigger for coagulation activation persists in DIC, inhibitors will be gradually exhausted, leading to more coagulation. In this process, many clotting factors—most notably, fibrinogen and platelets—are consumed, resulting eventually in complete impairment of the hemostasis system. This is why the term ‘consumptive coagulopathy’ is often used to denote this process. This results in bleeding tendency or decompensated DIC. In addition, activation of the coagulation cascade is accompanied by compensatory fibrinolysis where increase in plasminogen-dependent plasmin activity is detectable using markers such as D-dimers, as shown in Table 1. Table 1 also depicts the laboratory profile of acute and ‘compensated’ DIC, which has been comprehensively discussed in excellent reviews [142, 143].
TABLE 1
Profile of ‘compensated’ and acute DIC
| DIC | ||
|---|---|---|
| Characteristic | Acute | Compensated |
| Prothombin timea | ↑k | N or S |
| Partial thrombosplatin timeb | ↑ | N or S |
| Platelet countc | ↓ | ↓ or N |
| Fibrinogen leveld | ↓ | ↓ or N or ↑ |
| D-dimere | ↑ | ↑ or N |
| Fibrin(ogen) split productsf | ↑ | N or ↑ |
| Fibrin monomerg | ↑ | N or ↑ |
| F 1+2 fragmenth | ↑ | ↑ |
| Thrombin-antithrombin complexi | ↑ | ↑ |
| Plasmin-antiplasmin complexj | ↑ | ↑ |
| Bleeding and hemorrhage | + | − |
Clinically, the symptoms of acute DIC differ among individuals but fever, dyspnea, cyanosis, and extreme respiratory difficulty may predominate due to pulmonary dysfunction (acute respiratory distress syndrome). Neurologic signs and symptoms including convulsion and coma represent another pattern. Renal changes such as oliguria and acute renal failure may dominate, while circulatory failure and shock appear suddenly or develop progressively. Many of these symptoms are present in severe malaria [1–5]. In contrast, compensated DIC [142, 143] is not accompanied by bleeding or hemorrhage as is the case in uncomplicated malaria [14, 22].
A final important aspect related to the blood coagulation cascade in the context of malaria pathogenesis is physiologic changes in the plasma level of coagulation factors and inhibitors that occur in children [157] and in pregnancy [158]. In these populations, which are at high risk for developing severe malaria [23, 159], there is decreased fibrinolytic activity resulting in a hypercoagulable state. While these are considered to be physiologic changes [157, 158], they may nonetheless predispose children and women to a procoagulant tonus that may contribute to severe disease [24, 159].
V. TISSUE FACTOR AND THE COAGULATION-INFLAMMATION CYCLE
The TF pathway is the principal activator of clotting under physiologic conditions and in the presence of systemic inflammation and generalized infection [108–113]. In fact, accumulating evidence indicates that TF drives the coagulation-inflammation circuit through generation of coagulation factor, which induces cytokine production and expression of adhesion molecules. Conversely, and equally true, cytokine-driven inflammation leads to activation of coagulation. As an example, the best known contribution of inflammation to coagulation is probably induction of TF by TNF-α on the cell surface of monocytes and EC [160, 161]. Accordingly, evidence indicates that TF expression drives the vicious coagulation-inflammation cycle. The role of this cycle in multiorgan dysfunction is depicted in Figure 3, and has been comprehensive reviewed elsewhere [108–113].
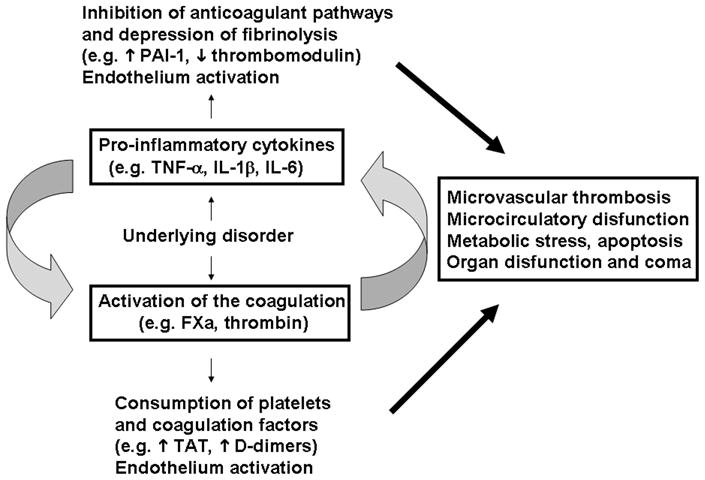
The coagulation-inflammation cycle. Diagrammatic representation of activation of coagulation and inflammation in response to an underlying disorder (e.g., infection). Infection is potentially associated with induction of pro-inflammatory cytokines and TF expression. Exposure of TF-bearing inflammatory cells (e.g., EC, monocytes) to blood results in thrombin generation, platelet aggregation, and conversion of fibrinogen to fibrin. Thrombin and other activated coagulation factors activate protease-activated receptors on inflammatory cells, inducing release of proinflammatory cytokines, which will further modulate coagulation through induction of TF, on one hand, and prevention of fibrinolysis through decrease in PAI-1 and thrombomodulin function, on the other. The vicious cycle between coagulation-inflammation leads to microvascular thrombosis, EC activation, increased vascular permeability, ROS generation, metabolic stress, apoptosis and ultimately to organ dysfunction. For details, see text and Supplementary Material (Section C, “Coagulation-inflammation cycle and the Relevance for Multiorgan Dysfunction and Coma in Malaria and Sepsis”).
Inflammatory cytokines induce Tissue Factor expression
Cytokines such as TNF-α, IL-1β, IL-2, IL-6, IFN-γ, VEGF, MCP-1, or PDGF are capable of inducing TF expression in vitro. P-selectin, E-selectin, and CD40 are other examples of positive modulators of TF synthesis in EC [108–113, 162, 163]. In vivo occurrence of coagulation/cytokine-dependent inflammation has also been demonstrated. Intramuscular injection of IL-6 results in thrombin generation in baboons, while infusion of TNF-α in humans produces a procoagulant state accompanied by inhibition of fibrinolysis [164, 165].
Blood coagulation factors induce cytokine expression
In vitro, FXa induces IL-6, IL-8, and MCP-1 production from EC and monocytes and promotes expression of E-selectin, ICAM-1, and VCAM-1, whereas thrombin induces IL-8 and IL-6 in mononuclear cells [108–113, 162, 163]. In vivo, it is well known that intravascular FXa and thrombin generation induces inflammation within the microcirculation via interaction with specific receptors on platelets, EC, and white blood cells. Accordingly, administration of FVIIa enhances IL-6 and IL-8 production in health volunteers [166]. In addition, thrombin, FXa, FVIIa/TF or peptides that activate PARs induce leukocyte rolling, adhesion, and extravasation in vitro or in vivo [166–176]. These effects are mediated by PAR, which induce gene expression, production of cytokines, and expression of adhesion molecules [167].
These adhesion molecules promote the initial rolling and tethering interactions between circulating granulocytes, monocytes, and lymphocytes to EC at sites of tissue injury. Interaction of monocytes with perturbed EC and the synergistic action [163, 177–180] of pro-inflammatory molecules potentially lead to exacerbated TF expression and further EC activation, which sustains the coagulation-inflammation cycle. Therefore, interplay between cytokines and coagulation factors promotes the “vicious” cycle of coagulation-inflammation of sepsis which appears to be critical in malaria pathogenesis, as well. Actually, severe malaria and sepsis share physiopathological mechanism and both cases are associated, to a certain degree, with i) uncontrolled infection, ii) systemic inflammation syndrome and cytokinemia, iii) increased capillary permeability, iv) hypoxia, v) acidosis, vi) EC activation, vii) microvascular coagulopathy, and viii) multiorgan dysfunction [1–5, 108–113]. The relevance of the coagulation-inflammation cycle for multiorgan dysfunction and coma in malaria, and sepsis is discussed in the Supplementary Material (Section C).
VI. TISSUE FACTOR: THE INTERFACE BETWEEN SEQUESTRATION, ENDOTHELIUM ACTIVATION, BLOOD COAGULATION DISORDER, AND INFLAMMATION IN MALARIA
Taking into consideration the concept that blood coagulation is initiated by TF expression [181–184], it was presumed that in vivo expression of TF was required to explain the coagulation disorder observed in malaria. TF expression by EC also appears to be important in the disease pathogenesis, because it is the only molecule that can be placed in the interface of all four pathologic features reportedly found in malaria, as shown in Figure 4.
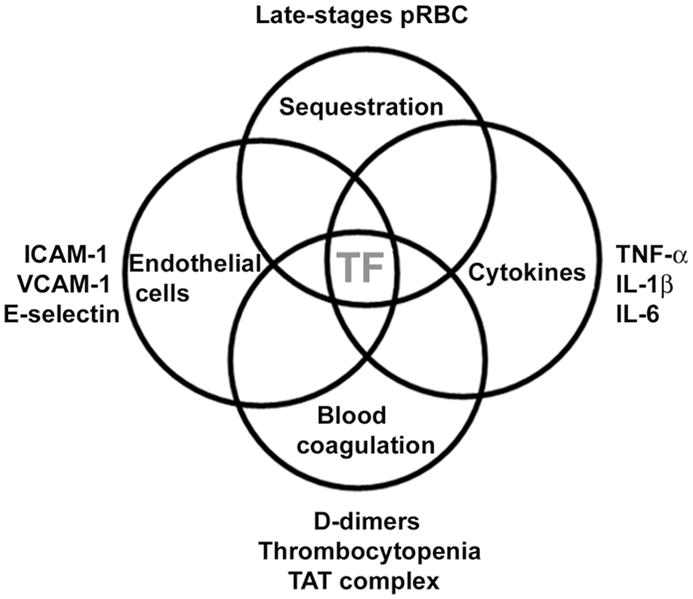
TF and the interface of pathologic features observed in malaria. Sequestration is associated with endothelial cell activation (estimated by an increase in ICAM-1, VCAM-1, E-selectin, and TF expression). TF expression is needed for activation of the coagulation cascade, which promotes thrombocytopenia, increase in TAT levels, and D-dimers. Coagulation factors also induce secretion of inflammatory cytokines (TNF-α, IL-1β, and IL-6), which in turn induce TF expression in different cell types promoting and sustaining a coagulation-inflammation cycle (for details, see text). TF, Tissue Factor.
The potential role of pRBC (sequestration) in TF expression by EC
We have recently demonstrated that expression of TF occurs in EC exposed to pRBC in vitro [82]. Expression of TF was blocked by TF inhibitor [185, 186] and was dependent on physical contact between pRBC and EC, as is apoptosis [187] and EC activation [188]. How pRBC induce TF expression remains to be understood at the molecular level, although it may involve receptor-mediated signaling [189], reactive oxygen species (ROS) [190], and NF-κB, which is nuclear translocated in brain EC incubated in vitro with pRBC [191]. Consistent with in vitro findings described above, ultrastructural studies using electron microscopy have shown that direct contact between pRBC and EC occurs in vivo by strictures called knobs [192]; presumably, this interaction is associated with EC activation in vivo [193, 194]. Moreover, sequestration of pRBC in the brain appears to be associated with coma according to studies on Southeast Asian adults and African children with fatal CM [27–31]. These studies imply that pRBC cytoadherence to cerebral EC may be an important and indispensable factor in P. falciparum malaria pathogenesis.
Strong support for the central role of parasite sequestration in disease severity has been recently published in a study involving 337 P. falciparum malaria patients. It was demonstrated that disease severity was associated with the number of sequestered pRBC as indirectly estimated by plasma levels of a parasite-specific, histidine-rich protein (PfHRP2) [30, 34, 35]. Further, a large trial on severe malaria showed that artesunate reduces mortality by 34% compared with quinine [35]. Both artesunate and quinine are active against sequestered parasites, preventing their development to schizonts, but artesunate is also active against the younger forms of the parasite, preventing their maturation and sequestration in the microcirculation of vital organs. Of note, the greatest mortality benefit in this trial compared with quinine was in patients with high parasitemias, indicating that prevention of sequestration, rather than prevention of schizont rupture, decreased mortality [35].
Also, host polymorphism that affects PfEMP-1 display (e.g. hemoglobinopathy C) may protect against malaria by impairing the parasite’s ability to adhere to microvessels and induce inflammation [38–40]. Likewise, CM is not observed in malaria caused by P. vivax, a condition where sequestration is not consistently observed [30, 36, 37]. Collectively, these in vitro and in vivo data support the view that sequestration is necessary for disease pathogenesis. It is clear, however, that cytoadherence per se cannot explain the coagulation disorder and neurologic syndrome observed during infection. Therefore, elements other than sequestration itself might account for malaria pathogenesis.
The role of pRBC in the amplification step of the coagulation cascade
We have recently demonstrated that late-stage pRBC inherently support the productive assembly of both intrinsic Xnase and prothrombinase complexes in vitro [82], amplifying by thousands of times the generation of FXa and thrombin, respectively [195, 196]. These findings are consistent with the fact that pRBC present changes in phospholipid composition as it matures and display PS, a negatively charged phospholipid that supports assembly of coagulation complexes [197]. Assemblies were attained at a remarkably low parasite concentration in vitro (0.025% hematocrit; 5% parasitemia); indicating that minute amounts of pRBC may successfully propagate the blood coagulation cascade in vivo when initiated by TF expression [82]. It should be emphasized, however, that platelets become pro-coagulant only when primed by low amounts of thrombin [195]. In contrast, pRBC are already ‘primed’ and as a consequence can immediately amplify blood coagulation independently of previous thrombin production and by a mechanism that is insensitive to platelet aggregation inhibitors such as aspirin. Of interest, only mature forms of pRBC are pro-hemostatic. These experimental data are important, as late- but not early-stage pRBC are found sequestered in the brain of most postmortem studies described so far [27–31].
It is noteworthy that while our coagulation studies used a laboratory-adapted parasite strain (3D7) [82], pRBC obtained directly from parasitized individuals had shortened recalcification time [198] and clotting time [199]. Both coagulation tests are sensitive to surface-mediated amplification of the coagulation cascade, indicating that pRBC are indeed procoagulant in vivo. Therefore, it is plausible to suggest that a procoagulant and proinflammatory microenvironment is encountered at sequestration sites and that this response may be overamplified by pRBC and activated platelets, leading to explosive thrombin generation if uncontrolled by coagulation inhibitors such TFPI [200], antithrombin [201], and the APC/thrombomodulin pathway [202]. It is likely that this reaction is particularly amplified in the vessels of the brain, a tissue where EC trombomodulin is not found [203, 204]. Thrombomodulin is a critical anticoagulant. Through interaction with thrombin, it changes the specificity of the enzyme that activates PC. APC has pleiotropic effects, among them inactivation of FVa and FVIIIa, and a direct antiinflammatory effect on EC [205]. Thus, lack of thrombomodulin in the brain could contribute to the enhanced blood coagulation and platelet aggregation observed in CM cases. More recenlty, it has been suggested that tissues which display low levels of thrombomodulin (e.g. brain and placenta) are prone to sequestration, as opposed to sites where higher levels of thrombomodulin/APC are found such as the heart and skeletal muscle [206].
The role of platelets in malaria pathogenesis
Platelets are considered crucial to malaria pathogenesis [1–5] and to other syndromes [207]. We suggest that amplification of the coagulation cascade through formation of coagulation complexes [195, 196] is the primary mechanism by which it contributes to disease pathogenesis. Amplification results in additional thrombin formation, which promotes recruitment of activated platelets to the site of injury [195, 196]. Activated platelets secrete a number of proinflammatory molecules through the so-called ‘release reaction’ and may contribute to local inflammatory processes by a number of mechanisms [208, 209]. Among these, platelets secrete such chemokines as TGF-β, which displays pro-apoptotic properties, and IL-1β, which activates white blood cells, induces TF expression, and promotes neutrophil and monocyte adherence [208, 209]. Platelets are a source of PAI-1, an important inhibitor of fibrinolysis [210]. Platelets also express P-selectin and promote neutrophil platelet EC interactions. Moreover, platelets are a major source of soluble CD40 ligand [211]. CD40 ligand belongs to the TNF superfamily of molecules and has multiple actions that may be of significance in microcirculation [211]. These actions include upregulation of cytokine expression on vascular smooth muscle cells and EC, increased expression of surface adhesion molecules on EC, and upregulation of TF synthesis on macrophages [108–113]. Finally, it has been recognized that thrombocytopenia in malaria is present in uncomplicated, mild, and severe cases [86–91] and is predictive of fatal outcome in one study [88].
TF Expression in vivo in Cerebral Malaria Cases
We have recently demonstrated that sequestration is associated with TF expression by brain EC of P. falciparum-infected children [82]. A typical staining for TF in EC from a pediatric CM case is shown in Figure 5. This finding strongly suggests that activation of the coagulation cascade took place in vivo, a contention corroborated by histopathologic findings of platelet and monocyte accumulation and fibrin deposition in the brain of some CM patients [29, 212, 213]. Notably, in our series of IHC studies TF expression in EC was associated with sequestration of mature forms in some cases, while in others, the presence of hemozoin (malaria pigments) indicative of schizont rupture (previous sequestration) was observed [82]. In still others, no pRBC or hemozoin were found. The events associated with malaria in general and CM in particular thus appear to be a highly dynamic process where sequestration occurs and is followed by schizont rupture and then clearance. Although the pathologic studies [27–32, 76–78] are only a snapshot of this dynamic and complex process, they clearly indicate that TF was present in EC at the time of death of all CM patients [82]. Finally, while it is not known whether TF-bearing microparticles contribute to TF staining in EC [82], it is known that uncomplicated cases of malaria—in contrast to CM cases—do not display circulating EC microparticles [107]. Because 4 of 13 P. falciparum patients we studied died from other causes (nonmalarial coma) but were positive for TF staining [82], it is concluded that, at least in these cases, TF was indeed expressed by EC.
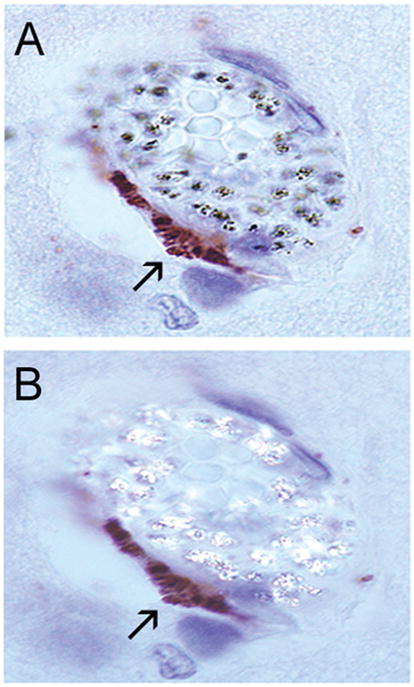
TF staining of EC associated with sequestration in a pediatric CM case. IHC was performed with anti-TF monoclonal antibodies. A) Regular light showing positive staining in the EC. B) Polarized light that detects hemozoin, indicative of sequestration. Staining was negative in the absence of primary antibody. For details, see text and [82].
Our IHC studies show that some vessels contain sequestration and no TF staining [82], suggesting that the tightly regulated mechanism of TF expression [182], among other mechanisms [214], may play a role in preventing uncontrolled EC activation with widespread TF expression. Of note, in vivo TF staining in human EC has been reported in only a few studies [203, 215–219], and it is remarkable that our studies consistently show positive staining and even, in some cases, at sites of sequestration [82]. Thus, a picture is emerging where relevant processes described in malaria presumably trigger TF expression in EC (e.g., sequestration and/or sequestration-associated events) while others may converge to express TF (e.g., cytokines) and vice-versa.
TF Expression in vivo in Parasitemic Patients Who Died from Other Causes
Our IHC studies also show variable degrees of TF staining in the five of six parasitemic children who died from nonmalarial causes [82]. This suggests that uncomplicated cases display TF expression in EC but formal evidence is still needed using appropriate samples. The one asymptomatic ‘parasitemic control’ [220] with no evidence of TF expression died from direct head trauma [82]. Therefore, TF expression in EC appears to be a general feature of malaria, and it presumably occurs in uncomplicated cases and it is not specific for CM. Consistent with this notion, widespread EC activation has been reported in uncomplicated malaria [76, 78], a condition where sequestration [76, 78], thrombocytopenia [86–91], and activation of blood coagulation are generally observed [86, 94, 96–98, 104]. These noncomatose patients, however, present no or few clinical manifestations compatible with a coagulation disorder (e.g., bleeding and hemorrhage) and their laboratory profiles [142, 143] resembles compensated DIC (Table I). Accordingly, most of these patients display normal or close to normal PT and PTT, while PC and PC-1 levels are low and D-dimer and TAT levels high in the absence of bleeding [86, 94, 96–98, 104].
Compensated versus Non-compensated States
It remains to be revealed why some patients develop CM while others present ‘uncomplicated’ malaria, a condition where sequestration and activation of blood coagulation also occurs. It may be that activation of blood coagulation, while normally homeostatic, may contribute to disease when uncontrolled or undergoing decompensation [108, 112]. Consistent with this view, platelet accumulation in the vessels of the brain [212], apoptosis [221], and formation of EC microparticles [107] have been specifically reported in CM (or complicated malaria) but not in uncomplicated cases. The finding that microparticles known to express TF [222] and PS and to participate in inflammation [223, 224]—are present in severe cases suggests that it may contribute to the inflammation-coagulation cycle at late stages of the disease. Microparticle formation could be also regarded as detectable markers of decompensation in P. falciparum-infected patients. Other reports have demonstrated that high levels of soluble uPAR [225], thrombomodulin [79], HMGB1 [226] and overall activation of the coagulation cascade [98] occurs in severe (sometimes lethal) malaria as opposed to healthy or less severe cases.
Considering that compensated states occur in malaria, specific risk factors for decompensation could be identified, in theory, in parasitemic patients. Numerous possibilities can be listed including patient immune status, polymorphism of pro- and/or anticoagulant inflammatory molecules [227, 228], PfEMP-1 and/or other parasite molecules [39, 229]. Heterogeneity of platelets [230], EC [231], and parasite population [232], associated diseases and/or a combination of factors may play a role in decompensation. Consistent with the notion that compensated states do occur in malaria is the fact that it shares physiopathologic mechanisms with sepsis [5], a condition in which compensatory mechanisms of the coagulation cascade have been studied in in vivo escalation models after E. coli challenge in baboons [112, 233]. These models have allowed investigators to conclude that the TF pathway of coagulation drives inflammation and that blockade of TF reduces lethality [152–156]. It is thus possible to envision TF inhibitors as prototypes to prevent development of CM or perhaps as drugs to be used in treatment of established CM.
Sequestration in Other Vascular Beds
Sequestration in malaria occurs (although not equally distributed) in sites other than the brain [27–32] including the eye, heart, liver, kidney, intestine and lung [27, 32], placenta [159], adipose tissue [27], dermis [76], and skeletal muscle [78] with widespread EC activation [76, 77]. Of note, pathology studies have demonstrated signs of inflammation in some of these tissues. For example, inflammatory infiltrates and fibrin have been detected in the lung of malaria patients who develop acute respiratory distress syndrome [15, 234, 235], a pathology where the role for TF has been described [236]. Also, “placental malaria”, a disease often associated with poor birth outcomes [237], is accompanied by TF expression by macrophages [238] with fibrin deposition and monocyte accumulation in the villae [239]. Placenta is a tissue particularly rich in TF [240] and presents a hemostatic system that appears to play a critical role in maternal-fetal homeostasis [241, 242]. At last, it remains to be investigated whether other cerebral and non-cerebral tissues display TF expression in the endothelium, as reported for the frontal cortex of patients who died from malaria [82].
VII. THE “TISSUE FACTOR MODEL” OF HUMAN MALARIA PATHOGENESIS
Previous models, namely the ‘sequestration’ and ‘cytokine’ hypotheses, have tried to explain malaria pathogenesis [25, 26]; immunopathologic mechanisms have also been described [243]. The ‘sequestration’ hypothesis suggests that “the presence of infected erythrocytes is an essential event” [26]. The ‘cytokine’ hypothesis proposes that, “while significant cerebral sequestration is often present and sometimes block vessels (with predictable sequelae), it is not essential for the onset of the cerebral manifestation of malaria” [25]. We propose an alternative model, here named the “Tissue Factor Model” (TFM) for human malaria pathogenesis. This model, summarized in Figure 6 (see Figure Legends), attempts to explain the disease by combining concepts evolved from both ‘sequestration’ and ‘cytokine’ hypotheses. In contrast to previous models, however, it classifies TF expression in the endothelium, and the amplification of the coagulation cascade by pRBC and platelets, as critical steps in disease pathogenesis. The TFM is based on experimental and clinical findings and on concepts from vascular biology.
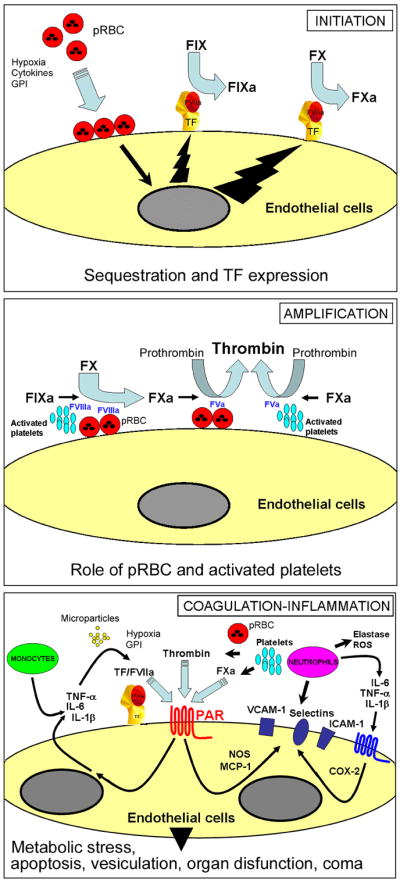
The TF model of human CM: Initiation: Normal, quiescent endothelium does not express TF in the absence of biologic stimulation. According to this model, sequestration and/or sequestration-associated events (e.g. cytokines, fibrin, hypoxia, apoptosis, proinflammatory molecules released by pRBC which activate TLRs such as GPI and plasmodium DNA-containing hemozoin) primarily induces EC activation in the microvessels of the brain and in other vascular beds. This contributes to TF expression, at sequestration sites, and/or possibly paracrinally. Monocytes may also be a source of TF in malaria. Mechanistically, TF initiates the coagulation cascade through binding to coagulation FVIIa and the substrate FIX and FX (extrinsic Xase). In this ternary initiation complex, FIXa and FXa are generated. Amplification: FXa, FVa, and prothrombin assemble in the pRBC surface and/or activated platelets, with formation of the prothrombinase complex leading to explosive thrombin formation and amplification of the coagulation cascade. Thrombin thus formed promotes fibrin deposition and induces platelet aggregation. Thrombin also activates FXI to FXIa (not shown), which activates FIX to FIXa. FIXa, FVIIIa, and FX assemble in the membrane of activated platelets or pRBC with formation of the intrinsic Xnase complex required for production of additional FXa owing to feedback inhibition of the FVIIa/TF complex by TFPI(not shown). Therefore, the TF model of human CM proposes that initiation of blood coagulation by TF expression and the amplification phase supported by pRBC (and/or activated platelets) - particularly at sequestration sites where the concentration of pRBC-derived phosphatydilserine is presumably very high - are critical for disease pathogenesis. Coagulation-inflammation: TF/VIIa, TF/FVIIa/FXa, and thrombin activate protease-activated receptors (PARs) in different cell types including mononuclear cells and EC at sequestration sites and/or paracrinally. PAR activation in EC is accompanied by upregulation of molecules (e.g., ICAM-1, VCAM-1, E-selectin, COX-2, NO synthase) and production of proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) reportedly found in CM. Because cytokines act synergistically with coagulation factors and induce upregulation of TF and adhesion molecules, they both are critical to perpetuate the inflammatory response that promotes increased interaction of monocytes, platelets, and/or pRBC with activated EC. Hypoxia, GPI, DNA-containing hemozoin and other events may also contribute to the coagulation-inflammation cycle. The result is a convergence of signals leading to exacerbated TF expression that sustains the coagulation-inflammatory cycle. This cycle may leads to microvascular thrombosis, additional EC activation, increased vascular permeability, ROS generation, metabolic stress, apoptosis and ultimately to organ dysfunction (e.g. SARS) that is often associated with poor prognosis in malaria. In extreme cases, death occurs. For details, see text.
Specifically, the TFM takes into account the following assumptions: i) sequestration is associated with disease pathogenesis [31, 32, 34, 35]; ii) P. falciparum infection is accompanied by EC activation [30, 76–82] and correlates with disease severity according to some studies [76, 78]; iii) malaria patients develop a coagulation disorder [86–104, 132]; iv) the degree of hemostatic alterations is often associated with the severity of malaria [79, 86, 92, 94, 96–98, 101–104, 132] and correlate with parasitemia according to some studies [86, 94, 97, 102, 104]; v) antiparasitic treatment halts the coagulation disorder and improve clinical outcome [79, 92, 94, 96–98, 132, 244]; vi) P. falciparum infection is accompanied by increased plasma concentration of various cytokines [5, 59–64]; vii) TF is expressed in the endothelium of parasitemic patients [82], viii) pRBC, in addition to activated platelets, support the assembly of prothrombinase and intrinsic Xnase complexes in vitro [82] and are procoagulant in vivo [198, 199]; ix) TF is reportedly in the interface of blood coagulation and inflammation [108–113]; x) similarities exist between malaria and other clinical conditions (e.g., sepsis) with abnormal expression of TF expression and sustained coagulation-inflammation cycle [111]; and finally, xi) TF can be placed in the interface of all four pathologic features reported in malaria (this article). These findings indicate that TF expression by endothelium is not an epiphenomenon but a major and differential component of the disease.
Favoring a prominent role for sequestration-associated TF expression by the endothelium in falciparum malaria pathogenesis is the fact that high levels of circulating cytokines (e.g. TNF-α) [245] and thrombocytopenia, apparently immune-mediated [114], are present in vivax malaria [101, 102, 115, 116]. However, vivax malaria is a benign condition where sequestration of pRBC is not a consistent finding and coagulation cascade activation or DIC does not take place [30, 36, 37, 96, 101]. Therefore, TNF-α and other cytokines do not account for TF expression in the endothelium of falciparum malaria patients, despite its pro-coagulant effect in vitro [160, 161]. In other words, it is not only the absence of consistent sequestration but the lack of blood coagulation activation as well two important factors that distinguishe vivax from falciparum malaria, pathobiologically. Finally, procoagulant activity of monocytes occurs in both P. falciparum and P. vivax malaria, indicating that events other than or besides monocyte procoagulant activity are critical for the genesis of severe malaria [96].
The TFM also differs from the previous hypothesis in five major respects: i) sequestration and/or sequestration-associated events is (are) the critical and perhaps the most important steps leading to disease pathogenesis primarily because it is accompanied by EC activation, TF expression, and initiation of the coagulation cascade [82]; ii) sequestered pRBC and activated platelets play an active role in amplifying, propagating, and consolidating the coagulation cascade initiated by TF expression, particularly at sequestration sites [82, 198, 199] where the kinetics of the coagulation reactions occur favorably because the concentration of phosphatydilserine is presumably very high; iii) the two previous steps are critical in mounting and sustaining a coagulation-inflammatory cycle that presumably leads to organ dysfunction by a combination of various molecular mechanisms [108–113, 246]; iv) there are compensated and decompensated states in malaria. This supposition is based on numerous studies comparing the clinical profile and activation status of the coagulation cascade in uncomplicated, moderate, and severe cases of malaria [86, 94, 96–98, 104] and experimental models of decompensation in sepsis [112, 233]; v) the TFM also introduces orderly steps to didactically explain the disease, namely initiation, amplification, and coagulation-inflammation steps. Figure 6 depicts these events diagrammatically.
The TFM model thus highlights the fundamental importance of sequestration-associated activation of EC to trigger the blood coagulation cascade, on one hand, and the role of pRBC and activated platelets to inherently amplify this response through formation of multimolecular blood coagulation complexes, on the other [82]. In other words, TF is needed but is not sufficient alone (as is the case for sequestration) to produce disease without an amplification step. It should be emphasized that the revised theory of the blood coagulation proposes that TF is critical but not enough to sustain normal hemostasis [182]. Under normal conditions, the level of FXa initially produced by the extrinsic pathway (FVIIa/TF) is feedback inhibited by TFPI [182]. Thus, the intrinsic Xnase complex (FXIa and FVIIIa, Ca2+ and PS) is required for production of additional FXa that amplifies and consolidate the coagulation cascade. In this context, lack of amplification by the intrinsic Xnase explains why hemophiliacs bleed despite normal levels of TF [247]. Likewise, TF expression by the endothelium and the amplification of the coagulation cascade by pRBC and/or activated platelets (particularly at sequestration sites) appears to be critical events in mounting and sustaining a coagulation-inflammation cycle which contributes to organ dysfunction and coma, in P. falciparum malaria.
The TFM also considers that events potentially associated with sequestration such as hypoxia [1–5], fibrin formation [99, 103, 117–122], and apoptosis [221, 248–250] may contribute to a procoagulant/-inflammatory cycle in malaria. Further, cytokine production [5] may play a critical role in the disease pathogenesis. TNF-α levels, for example, are increased in P. falciparum-infected patient plasma [56, 57], which has been reported to induce TF expression and NF-κB activation in EC in vitro [251]. Moreover, TNF-α upregulates expression of adhesion molecules on EC and suppresses thrombomodulin expression [252]. Thus, it favors adhesion of leukocytes which in turn damage EC by releasing radical oxygen species (ROS) and preformed proteases [111, 253]. It is also remarkable that TNF-α [254], and IL-1β [255] induce increased vascular permeability of EC through a FVIIa- and TF-dependent manner. It may be that the coagulation-inflammation cycle [108–113, 256] contributes to the brain-barrier dysfunction observed in malaria [257]. One may also speculate that this cycle creates an appropriate environment that favors sequestration in vivo.
Because sequestration is followed by schizogony, it is plausible that molecules released by or leaked from pRBC [258] positively modulate inflammation in vivo. For example, the malaria toxin - glycosylphosphatidylinositol anchor (GPI) - is a potent pro-inflammatory stimulus known to induce adhesion molecule expression in ECs, and to activate macrophages through TLR-2/-4 [259–262]. Of note, injection of GPI mimics several aspects of the pathology observed after infection of mice with P. berghei ANKA [263]. At last, plasmodium DNA-containing hemozoin activates TLR-9 and promotes IL-12p40 production by dendritic cells in vitro [264] and display pro-inflammatory effects [260, 265, 266]. The role of TLR, monocytes and the immune system in inflammation/coagulation is concisely discussed in the Supplementary Material (Section D, “Monocytes, Toll-like Receptors, Tissue Factor, and the Immune System”) and reviewed elsewhere [111].
It is important to recognize that host response such as activation of the “contact” pathway [95] and complement system reported in malaria patients [267, 268] and its crosstalk with the coagulation cascade [111, 269–272] may contribute to the inflammatory tonus observed in the disease. Further, neutrophils should be regarded as effectors in malaria pathogenesis [273]. Neutrophils interact with coagulation proteins and plasma neutrophil elastase is increased in patients with severe falciparum infection [95, 97, 132] and correlates inversely with platelet counts, antithrombin [97] and FXIII levels [132]. Of note, elastase appears to account, at least in part, for the pivotal role of neutrophils in sepsis [109, 274, 275]. Elastase proteolytically inhibits thrombomodulin function and may therefore interfere with activation of PC [276]. Additionally, elastase damages EC by promoting EC detachment, and degrades fibrinolytic enzymes or anticoagulants such as TFPI [273, 274, 277], thus favoring a procoagulant tonus. Finally, ROS are involved in oxidative stress and apoptosis in malaria [278, 279] and in other conditions where the coagulation-inflammation cycle contributes to disease pathogenesis (e.g. SARS)[1–5, 111, 274, 280]. The molecular mechanisms by which activation of the coagulation cascade (and coagulation-inflammation cycle) is associated with metabolic stress and organ dysfunction is highly complex and discussed in the Supplementary Material (Part C, “Coagulation-inflammation Cycle and the Relevance for Multiorgan Dysfunction and Coma in Malaria and Sepsis”) [108–113].
While the TFM for malaria pathogenesis should be regarded as an oversimplification of the pathologic processes involved in malaria, it should also be regarded as a flexible platform for future studies, and not a static model. Hopefully it will be modified, revised, and challenged as our understanding of malaria pathogenesis progresses. This model, however, identifies malaria as a disease with a strong vascular component where EC are the primary substrate of pathology. This is consistent with the fact that the endothelial surface area is vast and estimated to comprise 1012 EC in the adult human (there are ~1010 blood monocytes) [281]. It seems likely, therefore, that EC expression plays a prominent role in malaria and that one can consider malaria as a disease of the EC and/or microcirculation. Of interest, sickle-cell anemia, which shares many pathologic aspects with malaria such as activation of the endothelium (and coagulation cascade), is also considered an EC disease [282]. Whether the TF-bearing EC are apoptotic and vice versa is not yet known, but it is certainly an important issue that deserves further investigation [283]. Apoptosis occurs in experimental or falciparum malaria in vitro and in vivo [187, 221, 248–250, 278]. It also occurs in sepsis and has been implicated as a critical mechanism associated with organ dysfunction [246]. Of note, apoptotic cells become pro-coagulany and display PS, which assembles the coagulation complexes [284–287]. In other words, apoptotic cells potentially support the coagulation cascade and appear to participate in inflammation in vivo [284–286]. Therefore, one may speculate that in malaria there is an apoptotic-coagulation-inflammation imbalance. Finally, monocytes may play an important role in the disease pathogenesis and/or contribute to the procoagulant/inflammatory tonus observed in falciparum malaria [96], as described for sepsis [5]. Not surprisingly, malaria has been regarded as an inflammatory sepsis-like syndrome [5], a condition where cytokines, TF expression by EC and/or monocytes reportedly plays a pivotal role [108–113, 216].
VIII. CONCLUSIONS
The concept that TF is a potentially critical mediator of CM suggests that therapeutics targeting TF, and/or the endothelial cells may be successful in the treatment of malaria [154, 288, 289]. While anticoagulation in general might attenuate the local inflammatory escalation by reducing thrombin levels the antiinflammatory benefits of TF inhibition in particular are unique, as it blocks a proximal step of the coagulation cascade while levels of TNF-α—an important pathway for fighting infection—remains intact [154, 288]. Finally, identification of affordable therapeutics targeting EC and hemostatic components are particularly important vis-à-vis the increasingly reported resistance of Plasmodium sp. to antimalarial drugs [290].
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, USA. Because IMBF, is government employees and this is a government work, the work is in the public domain in the United States. Notwithstanding any other agreements, the NIH reserves the right to provide the work to PubMedCentral for display and use by the public, and PubMedCentral may tag or modify the work consistent with its customary practices. You can establish rights outside of the U.S. subject to a government use license.
We are grateful to Drs. Jose Marcos C. Ribeiro, Thomas E. Wellems, Robert W. Gwadz, and Kathryn C. Zoon (NIAID) for continuous encouragement and support. We express our thanks to Drs Richard O. Whitten, Jerrold M. Ward and Terrie E. Taylor for immunohistochemistry studies. We thank the Reviewers for their time, comments and suggestions. We acknowledge NIAID intramural editor Brenda Rae Marshall for assistance.
References
Full text links
Read article at publisher's site: https://doi.org/10.1080/10739680701451516
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1080/10739680701451516
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1080/10739680701451516
Article citations
Acquired thrombotic thrombocytopenic purpura in a patient with plasmodium vivax malaria: A case report.
Rev Soc Bras Med Trop, 57:e008072024, 02 Sep 2024
Cited by: 0 articles | PMID: 39230164 | PMCID: PMC11374122
Revolution in malaria detection: unveiling current breakthroughs and tomorrow's possibilities in biomarker innovation.
Ann Med Surg (Lond), 86(10):5859-5876, 17 Jul 2024
Cited by: 0 articles | PMID: 39359838 | PMCID: PMC11444567
Review Free full text in Europe PMC
Exploring coagulation parameters as predictive biomarkers of Plasmodium infection: A comprehensive analysis of coagulation parameters.
PLoS One, 19(4):e0301963, 16 Apr 2024
Cited by: 2 articles | PMID: 38626035 | PMCID: PMC11020526
Alteration of prothrombin time in Plasmodium falciparum and Plasmodium vivax infections with different levels of severity: a systematic review and meta-analysis.
Sci Rep, 14(1):9816, 02 May 2024
Cited by: 1 article | PMID: 38698102 | PMCID: PMC11066112
Review Free full text in Europe PMC
Dynamic intravital imaging reveals reactive vessel-associated microglia play a protective role in cerebral malaria coagulopathy.
Sci Rep, 13(1):19526, 09 Nov 2023
Cited by: 0 articles | PMID: 37945689 | PMCID: PMC10636186
Go to all (106) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Does activation of the blood coagulation cascade have a role in malaria pathogenesis?
Trends Parasitol, 24(6):258-263, 06 May 2008
Cited by: 48 articles | PMID: 18467176 | PMCID: PMC2882796
Plasmodium falciparum-infected erythrocytes induce tissue factor expression in endothelial cells and support the assembly of multimolecular coagulation complexes.
J Thromb Haemost, 5(1):155-165, 26 Sep 2006
Cited by: 63 articles | PMID: 17002660 | PMCID: PMC2892312
A simple protocol for platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes in a resource poor setting.
J Vis Exp, (75):e4316, 16 May 2013
Cited by: 0 articles | PMID: 23711755 | PMCID: PMC3684037
Plasmodium falciparum picks (on) EPCR.
Blood, 123(2):163-167, 18 Nov 2013
Cited by: 29 articles | PMID: 24246501 | PMCID: PMC3888284
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Intramural NIH HHS (2)
Grant ID: Z99 AI999999
Grant ID: Z01 AI000810-11





