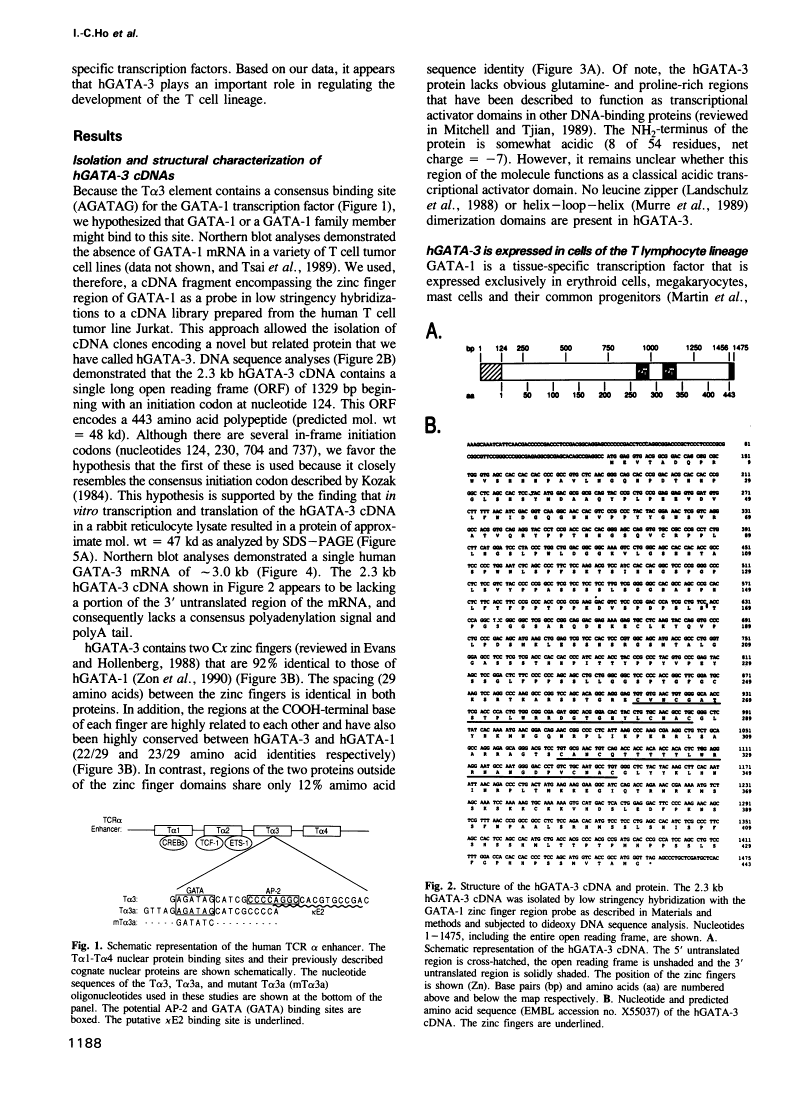
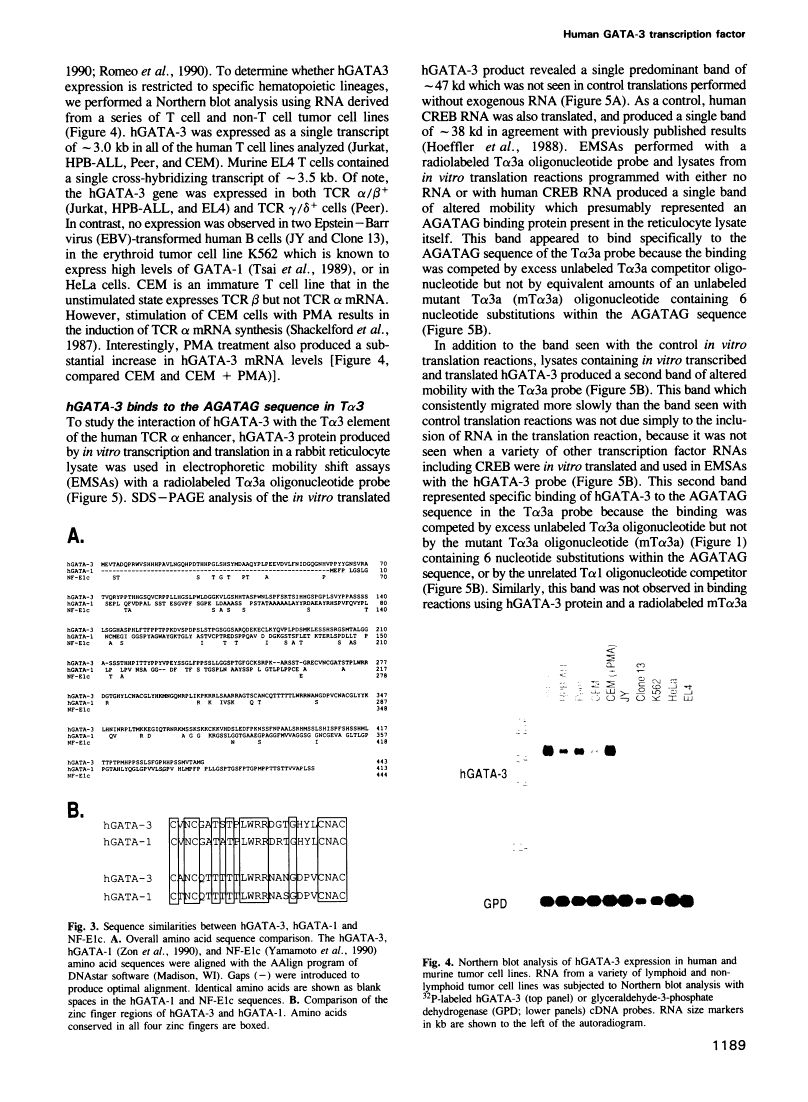
Abstract
Free full text

Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene.
Abstract
In addition to its role in the recognition of foreign antigens, the T cell receptor (TCR) alpha gene serves as a model system for studies of developmentally-regulated, lineage-specific gene expression in T cells. TCR alpha gene expression is restricted to cells of the TCR alpha/beta+ lineage, and is controlled by a T cell-specific transcriptional enhancer located 4.5 kb 3' to the C alpha gene segment. The TCR alpha enhancer contains four nuclear protein binding sites called T alpha 1-T alpha 4. In this report we describe the identification and characterization of a novel human cDNA, hGATA-3 that binds to the T alpha 3 element of the human TCR alpha enhancer. hGATA-3 contains a zinc finger domain that is highly related to the DNA-binding domain of the erythroid-specific transcription factor, GATA-1, and binds to a region of T alpha 3 that contains a consensus GATA binding site (AGATAG). Northern blot analyses of hematopoietic cell lines demonstrate that hGATA-3 is expressed exclusively in T cells. Overexpression of hGATA-3 in HeLa cells or human B cells specifically activated transcription from a co-transfected reporter plasmid containing two copies of the T alpha 3 binding site located upstream of the minimal SV40 promoter. Taken together these results demonstrate that hGATA-3 is a novel lineage-specific hematopoietic transcription factor that appears to play an important role in regulating the T cell-specific expression of the TCR alpha gene.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Leiden JM, Karpinski BA, Gottschalk L, Kornbluth J. Susceptibility to natural killer cell-mediated cytolysis is independent of the level of target cell class I HLA expression. J Immunol. 1989 Mar 15;142(6):2140–2147. [Abstract] [Google Scholar]
- Leung K, Nabel GJ. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. [Abstract] [Google Scholar]
- Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. [Abstract] [Google Scholar]
- Martin DI, Zon LI, Mutter G, Orkin SH. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. [Abstract] [Google Scholar]
- Mitchell PJ, Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. [Abstract] [Google Scholar]
- Müller MM, Ruppert S, Schaffner W, Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. [Abstract] [Google Scholar]
- Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. [Abstract] [Google Scholar]
- Redondo JM, Hata S, Brocklehurst C, Krangel MS. A T cell-specific transcriptional enhancer within the human T cell receptor delta locus. Science. 1990 Mar 9;247(4947):1225–1229. [Abstract] [Google Scholar]
- Romeo PH, Prandini MH, Joulin V, Mignotte V, Prenant M, Vainchenker W, Marguerie G, Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. [Abstract] [Google Scholar]
- Scheidereit C, Cromlish JA, Gerster T, Kawakami K, Balmaceda CG, Currie RA, Roeder RG. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988 Dec 8;336(6199):551–557. [Abstract] [Google Scholar]
- Shackelford DA, Smith AV, Trowbridge IS. Changes in gene expression induced by a phorbol diester: expression of IL 2 receptor, T3, and T cell antigen receptor. J Immunol. 1987 Jan 15;138(2):613–619. [Abstract] [Google Scholar]
- Singh H, Sen R, Baltimore D, Sharp PA. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. [Abstract] [Google Scholar]
- Sive HL, Roeder RG. Interaction of a common factor with conserved promoter and enhancer sequences in histone H2B, immunoglobulin, and U2 small nuclear RNA (snRNA) genes. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6382–6386. [Europe PMC free article] [Abstract] [Google Scholar]
- Staudt LM, Singh H, Sen R, Wirth T, Sharp PA, Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. [Abstract] [Google Scholar]
- Staudt LM, Clerc RG, Singh H, LeBowitz JH, Sharp PA, Baltimore D. Cloning of a lymphoid-specific cDNA encoding a protein binding the regulatory octamer DNA motif. Science. 1988 Jul 29;241(4865):577–580. [Abstract] [Google Scholar]
- Tsai SF, Martin DI, Zon LI, D'Andrea AD, Wong GG, Orkin SH. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. [Abstract] [Google Scholar]
- Waterman ML, Jones KA. Purification of TCF-1 alpha, a T-cell-specific transcription factor that activates the T-cell receptor C alpha gene enhancer in a context-dependent manner. New Biol. 1990 Jul;2(7):621–636. [Abstract] [Google Scholar]
- Winoto A, Baltimore D. A novel, inducible and T cell-specific enhancer located at the 3' end of the T cell receptor alpha locus. EMBO J. 1989 Mar;8(3):729–733. [Europe PMC free article] [Abstract] [Google Scholar]
- Winoto A, Baltimore D. Alpha beta lineage-specific expression of the alpha T cell receptor gene by nearby silencers. Cell. 1989 Nov 17;59(4):649–655. [Abstract] [Google Scholar]
- Yamamoto M, Ko LJ, Leonard MW, Beug H, Orkin SH, Engel JD. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. [Abstract] [Google Scholar]
- Zon LI, Tsai SF, Burgess S, Matsudaira P, Bruns GA, Orkin SH. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. [Europe PMC free article] [Abstract] [Google Scholar]
- Auffray C, Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. [Abstract] [Google Scholar]
- Bhat NK, Fisher RJ, Fujiwara S, Ascione R, Papas TS. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. [Europe PMC free article] [Abstract] [Google Scholar]
- Dugaiczyk A, Haron JA, Stone EM, Dennison OE, Rothblum KN, Schwartz RJ. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. [Abstract] [Google Scholar]
- Evans T, Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. [Abstract] [Google Scholar]
- Evans RM, Hollenberg SM. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. [Abstract] [Google Scholar]
- Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. [Europe PMC free article] [Abstract] [Google Scholar]
- Gottschalk LR, Leiden JM. Identification and functional characterization of the human T-cell receptor beta gene transcriptional enhancer: common nuclear proteins interact with the transcriptional regulatory elements of the T-cell receptor alpha and beta genes. Mol Cell Biol. 1990 Oct;10(10):5486–5495. [Europe PMC free article] [Abstract] [Google Scholar]
- Ho IC, Leiden JM. Regulation of the human T-cell receptor alpha gene enhancer: multiple ubiquitous and T-cell-specific nuclear proteins interact with four hypomethylated enhancer elements. Mol Cell Biol. 1990 Sep;10(9):4720–4727. [Europe PMC free article] [Abstract] [Google Scholar]
- Ho IC, Yang LH, Morle G, Leiden JM. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. [Europe PMC free article] [Abstract] [Google Scholar]
- Ho IC, Bhat NK, Gottschalk LR, Lindsten T, Thompson CB, Papas TS, Leiden JM. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. [Abstract] [Google Scholar]
- Hoeffler JP, Meyer TE, Yun Y, Jameson JL, Habener JF. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. [Abstract] [Google Scholar]
- Ko HS, Fast P, McBride W, Staudt LM. A human protein specific for the immunoglobulin octamer DNA motif contains a functional homeobox domain. Cell. 1988 Oct 7;55(1):135–144. [Abstract] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. [Europe PMC free article] [Abstract] [Google Scholar]
- Kudla B, Caddick MX, Langdon T, Martinez-Rossi NM, Bennett CF, Sibley S, Davies RW, Arst HN., Jr The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. Mutations affecting specificity of gene activation alter a loop residue of a putative zinc finger. EMBO J. 1990 May;9(5):1355–1364. [Europe PMC free article] [Abstract] [Google Scholar]
- Landolfi NF, Capra JD, Tucker PW. Interaction of cell-type-specific nuclear proteins with immunoglobulin VH promoter region sequences. Nature. 1986 Oct 9;323(6088):548–551. [Abstract] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1991.tb08059.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc452772?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1002/j.1460-2075.1991.tb08059.x
Article citations
The role of transcription factors in shaping regulatory T cell identity.
Nat Rev Immunol, 23(12):842-856, 19 Jun 2023
Cited by: 26 articles | PMID: 37336954
Review
METTL3 promotes SMSCs chondrogenic differentiation by targeting the MMP3, MMP13, and GATA3.
Regen Ther, 22:148-159, 29 Jan 2023
Cited by: 5 articles | PMID: 36793308 | PMCID: PMC9923043
Endothelial-to-osteoblast transition in normal mouse bone development.
iScience, 26(2):105994, 16 Jan 2023
Cited by: 2 articles | PMID: 36798441 | PMCID: PMC9926118
DOCK8-expressing T follicular helper cells newly generated beyond self-organized criticality cause systemic lupus erythematosus.
iScience, 25(1):103537, 02 Dec 2021
Cited by: 3 articles | PMID: 34977502 | PMCID: PMC8689056
Comparative Transcriptomics of IBD Patients Indicates Induction of Type 2 Immunity Irrespective of the Disease Ideotype.
Front Med (Lausanne), 8:664045, 31 May 2021
Cited by: 3 articles | PMID: 34136502 | PMCID: PMC8200538
Go to all (196) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A T-cell specific TCR delta DNA binding protein is a member of the human GATA family.
EMBO J, 10(7):1809-1816, 01 Jul 1991
Cited by: 104 articles | PMID: 2050118 | PMCID: PMC452855
Erythroid-specific activity of the glycophorin B promoter requires GATA-1 mediated displacement of a repressor.
EMBO J, 11(11):4095-4102, 01 Nov 1992
Cited by: 40 articles | PMID: 1396593 | PMCID: PMC556919
Identification and functional characterization of the human T-cell receptor beta gene transcriptional enhancer: common nuclear proteins interact with the transcriptional regulatory elements of the T-cell receptor alpha and beta genes.
Mol Cell Biol, 10(10):5486-5495, 01 Oct 1990
Cited by: 82 articles | PMID: 2144610 | PMCID: PMC361259
Transcriptional regulation of T cell receptor genes.
Annu Rev Immunol, 11:539-570, 01 Jan 1993
Cited by: 83 articles | PMID: 8476572
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: HL32259
NIAID NIH HHS (1)
Grant ID: AI-29673