Abstract
Free full text

Activation of Human Phospholipase C-η2 by Gβγ†
Abstract
Phospholipase C-η2 (PLC-η2) was recently identified as a novel broadly expressed phosphoinositide-hydrolyzing isozyme [Zhou, Y., et al. (2005) Biochem. J. 391, 667–676; Nakahara, M., et al. (2005) J. Biol. Chem. 280, 29128–29134]. In this study, we investigated the direct regulation of PLC-η2 by Gβγ subunits of heterotrimeric G proteins. Coexpression of PLC-η2 with Gβ1γ2, as well as with certain other Gβγ dimers, in COS-7 cells resulted in increases in inositol phosphate accumulation. Gβ1γ2-dependent increases in phosphoinositide hydrolysis also were observed with a truncation mutant of PLC-η2 that lacks the long alternatively spliced carboxy-terminal domain of the isozyme. To begin to define the enzymatic properties of PLC-η2 and its potential direct activation by Gβγ, a construct of PLC-η2 encompassing the canonical domains conserved in all PLCs (PH domain through C2 domain) was purified to homogeneity after expression from a baculovirus in insect cells. Enzyme activity of purified PLC-η2 was quantified after reconstitution with PtdIns(4,5)P2-containing phospholipid vesicles, and values for Km (14.4 µM) and Vmax [12.6 µmol min−1 (mg of protein)−1] were similar to activities previously observed with purified PLC-β or PLC-ε isozymes. Moreover, purified Gβ1γ2 stimulated the activity of purified PLC-η2 in a concentration-dependent manner similar to that observed with purified PLC-β2. Activation was dependent on the presence of free Gβ1γ2 since its sequestration in the presence of Gαi1 or GRK2-ct reversed Gβ1γ2-promoted activation. The PH domain of PLC-η2 is not required for Gβ1γ2-mediated regulation since a purified fragment encompassing the EF-hand through C2 domains but lacking the PH domain nonetheless was activated by Gβ1γ2. Taken together, these studies illustrate that PLC-η2 is a direct downstream effector of Gβγ and, therefore, of receptor-activated heterotrimeric G proteins.
Physiological responses to a variety of extracellular stimuli result from phospholipase C-catalyzed conversion of the minor membrane phospholipid PtdIns(4,5)P2 1 into the Ca2+-mobilizing second messenger, Ins(1,4,5)P3, and the protein kinase C-activating second messenger, diacylglycerol (1, 2). Membrane inositol lipids specifically bind to conserved PH, FYVE, PX (phox), and other domains found in key proteins in a broad range of signaling pathways (3, 4). Thus, by altering the ratio of membrane phosphoinositides, PLC isozymes also affect membrane association and activity of many signaling proteins.
A large family of PLC isozymes exists that includes six subfamilies of PLC (PLC-β, -γ, -δ, -ζ, -ε, and -η) and a total of 13 members (5). All PLC isozymes contain canonical PH, EF-hand, catalytic TIM barrel, and C2 domains, with the exception of sperm-specific PLC-ζ (6), which lacks a PH domain. PLC-δ isozymes are broadly distributed, but a description of their mechanism of regulation remains elusive (5). PLC-γ isozymes contain SH2 and SH3 (Src homology 2 and 3, respectively) domains and are regulated by receptor and nonreceptor tyrosine kinases (5, 7–10). PLC-γ2 also was shown recently to be regulated by Rac GTPases (11). PLC-β isozymes are elaborated with a helical C-terminus (12) and are activated directly by Gα subunits of the Gq family of heterotrimeric G proteins (13–15). Gβγ dimers released from heterotrimeric G proteins of the Gi family also activate PLC-β2 and PLC-β3 (16–18). Rac GTPases bind to the PH domain and activate PLC-β2 (19, 20), and a crystal structure of Rac in GTP-dependent complex with PLC-β2 has provided new insight into the molecular mechanism of G protein-mediated regulation of PLC-β isozymes (21). PLC-ε contains a CDC25 guanine nucleotide exchange factor domain at the amino terminus and two tandem RA domains at the carboxy terminus. Ras and Rho GTPases bind to the RA domains and the catalytic core, respectively, and directly regulate the activity of PLC-ε by distinct mechanisms (22–27).
Four research groups recently reported identification of a pair of novel PLC isozymes, PLC-η1 and PLC-η2 (28–31). PLC-η2 contains a long alternatively spliced variably expressed carboxy terminus (31), and the mouse ortholog was proposed to be involved in developmental aspects of neuronal function (30). We now illustrate that PLC-η2 is directly activated by Gβγ subunits of heterotrimeric G proteins by a mechanism that requires the canonical catalytic core of the isozyme.
MATERIALS AND METHODS
Materials
Inositol-free medium was purchased from ICN Biochemicals (Costa Mesa, CA). [inositol-2-3H]Phosphatidylinositol 4,5-bisphosphate substrate was purchased from Perkin-Elmer (Boston, MA), and brain l-α-phosphatidylinositol 4,5-bisphosphate and bovine liver l-α-phosphatidylethanolamine were purchased from Avanti Polar Lipids (Alabaster, AL). Protease inhibitor tablets were obtained from Roche Applied Science (Madison, WI). All other reagents were from sources previously reported (31).
We previously determined that 23 exons encode human PLC-η2 (31). DNA sequence of human PLC-η2 corresponding to exons 2–23 and amino acids 42–1416 [PLC-η2(2–23)] or corresponding to amino acids 42–872 [PLC-η2(PH-C2)] was cloned into the pcDNA3.1 vector at the 5′ EcoRI and 3′ XbaI sites. PLC-η2(2–23) was also cloned into the pcDNA4 vector with 5′ EcoRI and 3′ AgeI sites with a carboxy-terminal hexahistidine (6×His) tag (31). Mouse PLC-η1 (GenBank accession number BC055005) was obtained from ATCC (catalog number 9887980) and subcloned into the pcDNA4 vector at the 5′ EcoRI and 3′ AgeI sites with a carboxy-terminal 6×His tag. The DNA sequence of human PLC-β2 was cloned into the pcDNA3.1 vector at the 5′ EcoRI and 3′ XbaI sites (25). The sequence corresponding to human PLC-β2 lacking the Gαq-interacting carboxy terminus was cloned into the pFastbac HTc vector as previously described (21).
Purification of PLC-η2
The DNA sequence of human PLC-η2 corresponding to amino acids 42–872 [PLC-η2(PH-C2)] or corresponding to amino acids 168–872 [PLC-η2(EF-C2)] was subcloned into the pFastbac HTa vector at the 5′ EcoRI and 3′ XbaI sites with a 6×His tag and TEV (tobacco etch virus) cleavage site engineered at the amino terminus. Conditions for expression and subsequent purification of these two different forms of PLC-η2 were essentially identical. Thus, recombinant baculovirus for PLC-η2(PH-C2) or PLC-η2(EF-C2) was used to infect HighFive insect cells at a multiplicity of infection of 1. After 48 h of incubation at 27 °C, cells were harvested by low-speed centrifugation at 700g for 15 min at 4 °C and resuspended in buffer A [20 mM Hepes (pH 8), 400 mM NaCl, 1 mM CaCl2, 15 mM imidazole, 10 mM 2-mercaptoethanol, 5% glycerol, and protease inhibitor tablet (Roche)]. Cells were then lysed with an EmulsiFlex C5 cell homogenizer (Avestin), and the lysate was subjected to centrifugation at 100000g for 55 min. The clarified supernatant was filtered through a 0.2 µm bottle top filter (Fisher Scientific). Ni-NTA resin (1 mL of resin/L of culture) was added to the filtered supernatant, which was directly poured into a chromatography column. The flow-through was collected and passed over the column two more times to increase the level of protein binding with Ni resin. The column was then washed with 20 bed volumes of buffer A. The 6×His-tagged PLC-η2 was eluted with 2 bed volumes of buffer B (buffer A with 150 mM imidazole). The PLC-containing fractions of the eluant were combined, loaded onto a Superdex 200 gel filtration column, and subjected to resolution by fast protein liquid chromatography. Samples were eluted in buffer C [20 mM Hepes (pH 8), 150 mM NaCl, 1 mM CaCl2, 10 mM 2-mercaptoethanol, and 10% glycerol].
Purification of Recombinant Gβ1γ2, Gαi1, and GRK2-ct
Recombinant baculoviruses encoding 6×His-Gαi1, Gβ1, and Gγ2 were used to co-infect HighFive cells at a multiplicity of infection ratio of 0.7 (6×His-Gαi1):0.35 (Gβ1):0.26 (Gγ2). After 48 h of incubation at 27 °C, cells were harvested by low-speed centrifugation and resuspended in buffer I [20 mM Hepes (pH 8), 100 mM NaCl, 10 mM EDTA, 2 mM MgCl2, 10 mM 2-mercaptoethanol, 10 µM GDP, and protease inhibitor tablet]. Cells were then lysed with an EmulsiFlex C5 cell homogenizer (Avestin), and the lysate was subjected to centrifugation at 100000g for 55 min. The particulate membrane fraction was resuspended in buffer II [20 mM Hepes (pH 8), 100 mM NaCl, 1 mM MgCl2, 10 mM 2-mercaptoethanol, 10 µM GDP, and protease inhibitor tablet], dounce-homogenized by 25 strokes, and solubilized with 1% sodium cholate at 4 °C for 1 h on a stir plate. The membrane extract was centrifuged at 100000g for 55 min. The clarified supernatant was batch-loaded with Ni-NTA resin at 4 °C for 3 h and poured over a column. The flow-through was collected, and the Ni resin was washed with 50 mL (25 bed volumes) of buffer III [20 mM Hepes (pH 8), 300 mM NaCl, 0.5% C12E10, 1 mM MgCl2, 10 mM 2-mercaptoethanol, 10 µM GDP, and 10 mM imidazole] followed by 10 mL (5 bed volumes) of buffer IV [20 mM Hepes (pH 8), 100 mM NaCl, 0.2% sodium cholate, 10 µM GDP, 10 mM 2-mercaptoethanol, 10 mM imidazole, and 1 mM MgCl2]. The column was brought to room temperature for 15 min and further washed three times with 2 mL (1 bed volume) of buffer IV (warmed to 30 °C). The Gβ1γ2 dimer was then eluted with six separate additions of 2 mL (1 bed volume) of buffer V [20 mM Hepes (pH 8), 50 mM NaCl, 1% sodium cholate, 10 µM GDP, 10 mM 2-mercaptoethanol, 10 mM imidazole, 50 mM MgCl2, 10 mM NaF, 30 µM AlCl3, and protease inhibitor tablet, warmed to 30 °C], and 6×His-GRi1 eluted with six separate additions of 2 mL (1 bed volume) of buffer VI [20 mM Hepes (pH 8), 100 mM NaCl, 1% sodium cholate, 10 µM GDP, 10 mM 2-mercaptoethanol, 150 mM imidazole, 1 mM MgCl2, and protease inhibitor tablet, warmed to 30 °C]. Two fractions of Gβ1γ2-containing eluant were combined and concentrated using a VIVASPIN6 10000 molecular weight cutoff column (Sigma-Aldrich) in a swing bucket rotor centrifuged at 2500 rpm according to the manufacturer’s protocol. The concentrated protein was passed over a G-25 gel filtration column to exchange the sample into buffer VII [20 mM Hepes (pH 8), 100 mM NaCl, 0.7% sodium cholate, and 10 mM 2-mercaptoethanol]. The desalted material was then concentrated as described above. Protein concentrations were determined by comparison to a BSA standard on a Coomassie-stained SDS-PAGE gel. Gαi1 was purified in the same fashion as described above except that infections were with baculoviruses for Gαi1 lacking a 6×His tag and with Gγ2 containing a 6×His tag. [35S]GTPγS binding assays were carried out to confirm that the purified Gαi1 stoichiometrically bound GTP and, therefore, was fully active.
A bacterial expression plasmid (pER28a) encoding the PH domain of G protein-coupled receptor kinase 2 (GRK2-ct; residues 548–641 with six C-terminal histidines) was a generous gift from A. Bohm (Tufts University, Medford, MA). GRK2-ct was purified as we previously described (20).
In Vitro PLC Assay
The enzyme activity of purified PLC-η2 and its potential regulation by Gβ1γ2 were quantified using phosphatidylethanolamine (PE)- and PtdIns(4,5)P2-containing vesicles as previously described in detail (32). Briefly, recombinant proteins were reconstituted with lipid vesicles containing 50 µM PtdIns(4,5)P2 (unless otherwise indicated; Avanti Polar Lipids), 500 µM PE (Avanti Polar Lipids), and ~8000 cpm of [3H]PtdIns(4,5)P2 (Perkin-Elmer) per assay. Final buffer conditions were as follows: 20 mM Hepes (pH 7.2), 70 mM KCl, 3 mM EGTA, 2 mM dithiothreitol, 0.16 mg/mL fatty acid-free bovine serum albumin, and 10 µM free Ca2+ in a final volume of 60 µL. All reactions were carried out at 32 °C for 10 min (unless otherwise indicated) and were terminated by the addition of 200 µL of 10% trichloroacetic acid and 100 µL of 10 mg/mL fatty acid-free bovine serum albumin. Centrifugation of the reaction mixture separated aqueous [3H]Ins(1,4,5)P3 from precipitated [3H]-PtdIns(4,5)P2, and soluble [3H]Ins(1,4,5)P3 was quantified by liquid scintillation counting.
For experiments determining the effects of Gβ1γ2 on phospholipase C activity, purified Gβ1γ2 dimer in cholate detergent was combined with phospholipid vesicles, therein diluting cholate to concentrations well below its critical micellar concentration. For experiments determining the effects of Gαi1 on the capacity of Gβ1γ2 to activate PLC, 10 µL of purified Gβ1γ2 and Gαi1 were combined in the presence of 10 µM GDP on ice for 20 min prior to lipid vesicle reconstitution. For experiments determining the effects of GRK2-ct on the Gβ1γ2-mediated activation of PLC, 10 µL of purified Gβ1γ2 and GRK2-ct were combined on ice for 20 min prior to lipid vesicle reconstitution.
Quantification of PLC Activity in COS-7 Cells
COS-7 cells were seeded in 12-well plates at a density of 65000 cells per well and maintained in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% (v/v) fetal bovine serum at 37 °C in an atmosphere of 90% air and 10% CO2. Three hundred nanograms of PLC-β2, 100 ng of PLC-η2(2–23), 100 ng of PLC-η2(PH-C2), or 300 ng of PLC-η1 plasmid DNA was transfected into COS-7 cells in the absence or presence of 200 ng each of expression vectors for Gβ and Gγ subunits using Fugene 6 (Roche) transfection reagent according to the manufacturer’s protocol. The medium was changed to inositol-free DMEM containing 1 µCi of [3H]inositol per well 24 h after transfection. After incubation for an additional 18 h, [3H]inositol phosphate accumulation was initiated by addition of LiCl to a final concentration of 50 mM. The reaction was stopped after 60 min by aspiration of the medium and the addition of 50 mM ice-cold formic acid. [3H]Inositol phosphates were quantified as described previously (33).
RESULTS
Introduction
With the goal of determining whether Gβγ subunits of heterotrimeric G proteins directly regulate PLC-η2, we initiated studies to purify the isozyme to homogeneity. However, multiple attempts to purify full-length PLC-η2 after expression from a baculovirus in insect cells yielded insuf- ficient amounts of purified protein, and two major degradation products of PLC-η2 were observed. Therefore, we considered whether Gβ1γ2-dependent regulation of a truncated form(s) of PLC-η2 occurred in intact cells, whether this truncated PLC-η2 protein(s) could be purified in adequate yield to be studied with phosphoinositide-containing vesicles, and whether direct Gβ1γ2-dependent activation could be observed with the purified isozyme.
Activation of PLC-η2 by Gβγ in COS-7 Cells
A mammalian expression vector was generated for a truncated version of PLC-η2 that encompassed the PH domain through C2 domain [amino acids 42–872 (Figure 1A)]. This truncated PLC-η2 was expressed in COS-7 cells, and its potential regulation by Gβ1γ2 was examined by cotransfection of cells with expression vectors for Gβ1 and Gγ2. As illustrated in Figure 1B, marked Gβγ-dependent increases in inositol phosphate accumulation occurred in cells also expressing the truncated form of the isozyme, and the extent of activation of PLC-η2(PH-C2) by Gηβ1γ2 was similar to that of PLC-η2(2–23), but lower than the activity observed when Gβ1γ2 was coexpressed with PLC-β2.
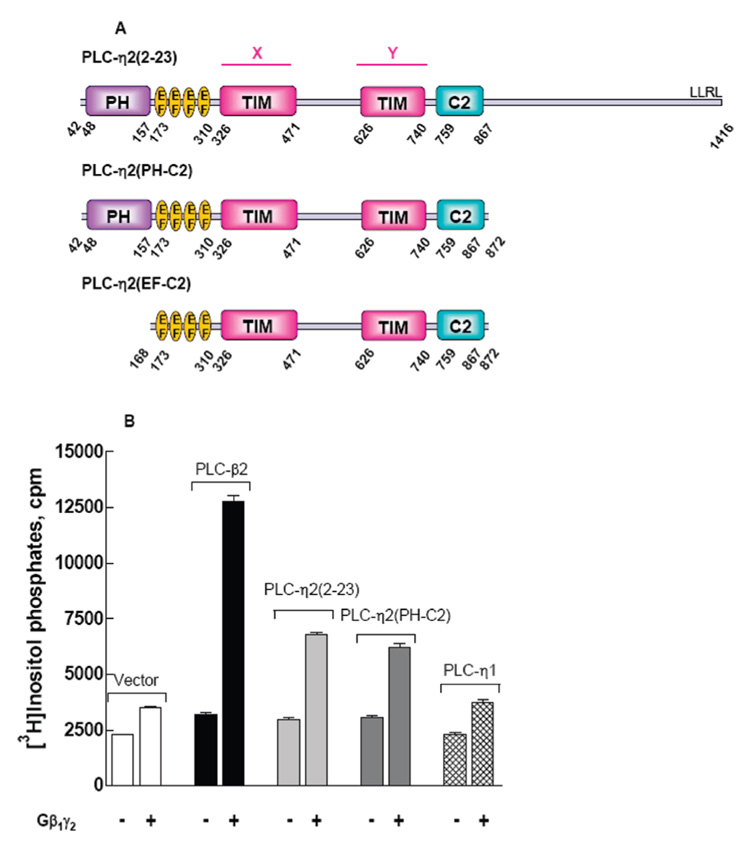
Activation of PLC-η2 by the G protein Gβ1γ2 dimer. (A) Schematic representation of PLC-η2(2–23) (residues 42–1416, top), PLC-η2(PH-C2) (residues 42–872, middle), and PLC-η2(EF-C2) (residues 168–872, bottom). PLC-η2(2–23) (exon 2–23) is an alternative splice variant that lacks the first exon (41 amino acids) of human PLC-η2 (GenBank accession number DQ176850). PLC-η2(PH-C2) lacks the C-terminal 545 amino acids of PLC-η2(2–23); PLC-η2(EF-C2) additionally lacks the N-terminal PH domain. (B) PLC-η2(2–23), PLC-η2(PH-C2), or mouse PLC-η1 was coexpressed as described in Materials and Methods with Gβ1γ2. [3H]Inositol phosphate accumulation was measured as described in Materials and Methods. PLC-β2 was coexpressed with Gβγ as a positive control. The results are presented as means ± SEM for triplicate determinations and are representative of results from three or more separate experiments.
Given the similarity in sequence between PLC-η2 and PLC-η1, we also tested whether Gβ1γ2 activates both members of this subfamily of PLC isozymes. As illustrated in Figure 1B, no Gβ1γ2-dependent increase in inositol phosphate accumulation occurred in cells coexpressing PLC-η1. Similar results were observed with transfection of a wide range of amounts of PLC-η1 DNA (data not shown). COS-7 cells overexpressing PLC-η1 or PLC-η2 were harvested 48 h after transfection. Immunoblots revealed that similar levels of expression of PLC-η1 and PLC-η2 occurred under the conditions of these experiments, and furthermore, coexpression of Gβ1γ2 did not alter the expression level of either PLC-η1 or PLC-η2 isozyme (data not shown). Therefore, we conclude within the limitations of transfection experiments that PLC-η2, but not PLC-η1, is subject to regulation by Gβ1γ2.
The possibility that Gβγ dimers in addition to Gβ1γ2 activate PLC-η2 also was examined in COS-7 cells transfected with equal amounts of DNA for expression of various Gβγ dimers (Gβ2γ2, Gβ3γ2, Gβ4γ2, and Gβ5γ2). Immunoblots illustrated similar levels of expression of all five different Gβxγ2-containing dimers tested, although the level of expression was reproducibly slightly higher with Gβ1γ2 (data not shown). The strongest activation of both PLC-η2 and PLC-β2 was observed with Gβ1γ2 (Figure 2), and the pattern of relative activities observed with the other Gβγ dimers was similar for PLC-η2 versus PLC-β2. Thus, Gβ2γ2 and Gβ4γ2 activated both PLC isozymes, whereas no reproducible activation was observed with Gβ3γ2. Gβ5γ2 slightly activated PLC-β2, but no reproducible activation of PLC-η2 was observed with this G protein dimer.
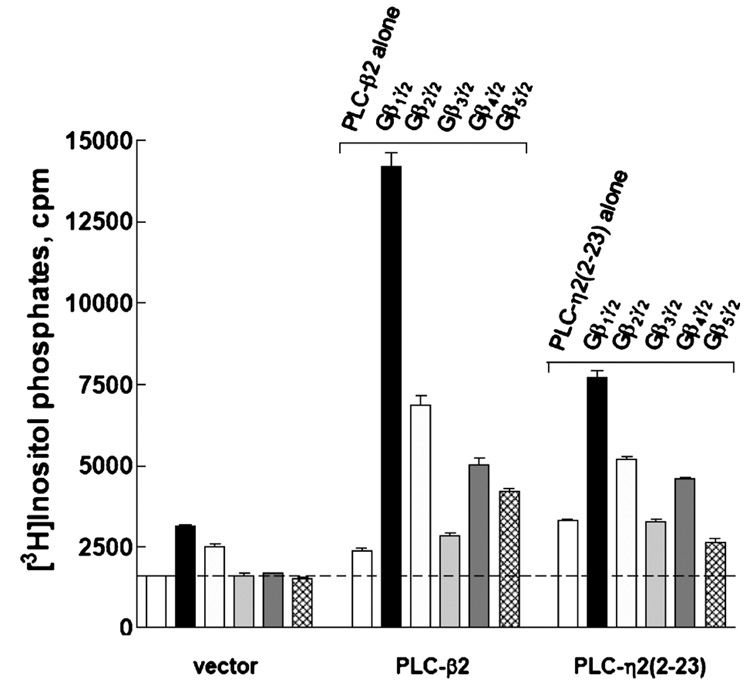
Activation of PLC-η2 by various G protein Gβγ dimers. PLC-η2(2–23) or PLC-β2 was coexpressed as described in Materials and Methods with Gβ1γ2, Gβ2γ2, Gβ3γ2, Gβ4γ2, or Gβ5γ2. [3H]Inositol phosphate accumulation was measured as described in Materials and Methods. The results are presented as means ± SEM for triplicate determinations and are representative of results from three or more separate experiments.
Purification of PLC-η2(PH-C2)
Given that Gβ1γ2-mediated regulation of PLC-η2 was retained in COS-7 cells in studies with a truncated version of this isozyme, we constructed a recombinant baculovirus for expression of a protein that encompasses the PH through the C2 domain [amino acids 42–872 (Figure 1A)] of PLC-η2. PLC-η2(PH-C2) was expressed at high levels without observable degradation products and was readily purified after overexpression in HighFive insect cells as described in Materials and Methods. Yields of purified protein averaged 5 mg/L of infected cells with approximately 90% purity obtained in a single Ni-NTA affinity purification step. Analyses of this purified fraction by size exclusion chromatography revealed that greater than 50% of PLC-η2 existed as soluble aggregates. Therefore, a Superdex 200 size-exclusion chromatography step was included in the standard protocol for purification of active PLC-η2. The final purified isozyme migrated as a monomer of the predicted size (approximately 100 kDa) and was essentially homogeneous as assessed by Coomassie staining of SDS-PAGE gels (Figure 3A inset). Assessment with a PLC-η2-specific antibody also indicated that little or no proteolysis of PLC-η2(PH-C2) occurred (data not shown).
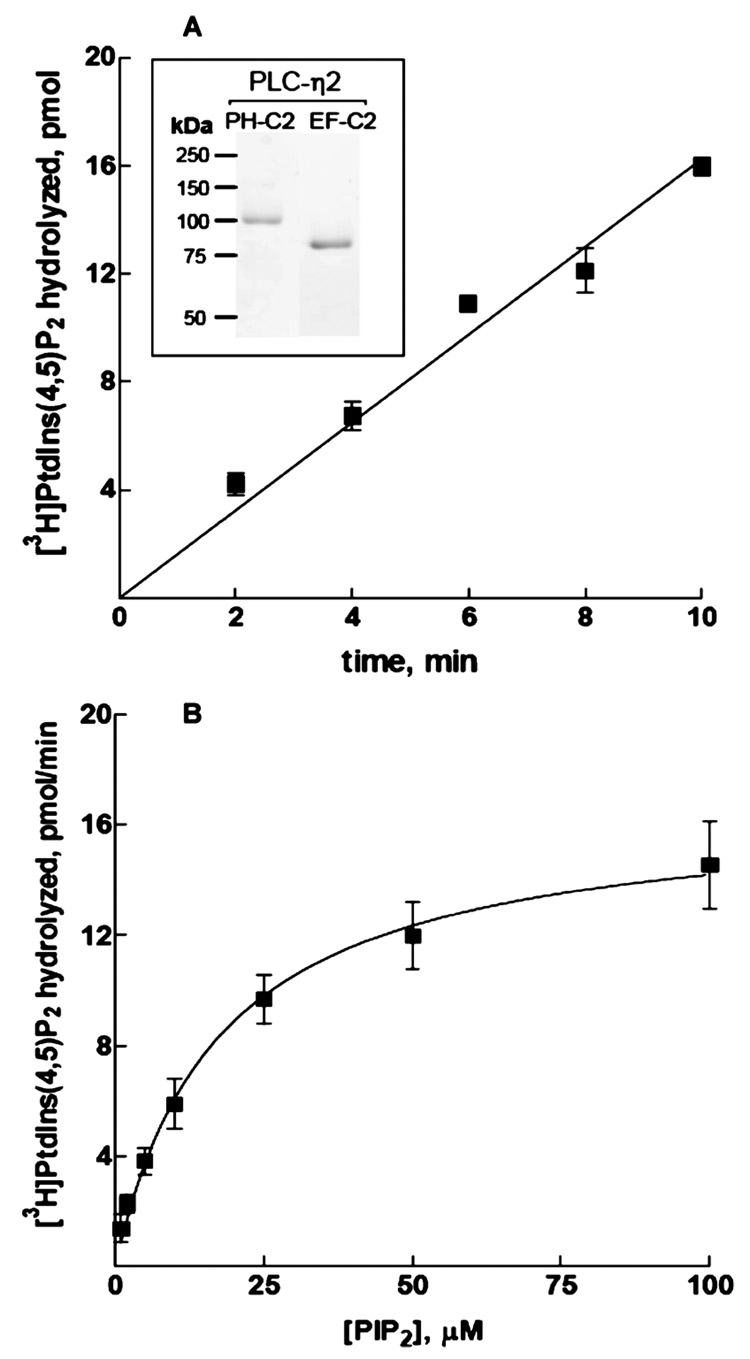
Characterization of the phospholipase activity of PLC-η2(PH-C2). (A) Time course of PtdIns(4,5)P2 hydrolysis in the presence of 1 ng of PLC-η2(PH-C2). (B) Lipase activity of 1 ng of PLC-η2(PH-C2) assayed with increasing concentrations of PtdIns(4,5)P2 substrate. The data are the means ± the standard error of three individual experiments. The inset shows a Coomassie-stained SDS–PAGE gel of purified PLC-η2(PH-C2) and PLC-η2(EF-C2).
Enzymatic Properties of Purified PLC-η2(PH-C2)
Purified PLC-η2(PH-C2) exhibited marked phospholipase C activity in assays using mixed detergent phospholipid micelles containing 50 µM [3H]PtdIns(4,5)P2 as a substrate. Phosphoinositide hydrolysis was linear for at least 10 min under these assay conditions (Figure 3A) and was proportional to the amount of purified enzyme used over a wide range of concentrations (data not shown). Kinetic analysis of PLC-η2(PH-C2)also was performed using PE-and[3H]PtdIns(4,5)P2-containing vesicles presented at various concentrations in the bulk medium in the absence of detergent. Assays carried out under these conditions with PLC-η2(PH-C2) revealed a Vmax of 12.6 µmol min−1 mg−1 and an apparent Km for PtdIns-(4,5)P2 of 14.4 µM (Figure 3B).
Activation of Recombinant PLC-η2 by Gβ1γ2
Gβ1γ2 directly activates PLC-β2 and PLC-β3, and although Gβ1γ2 also stimulates PLC-ε-dependent increases in inositol phosphate accumulation in intact cells (25), we have been unable to observe direct activation of several truncated constructs of PLC-ε in in vitro reconstitution assays. To determine whether Gβ1γ2 directly activates PLC-η2, Gβ1γ2 was expressed from recombinant baculoviruses in insect cells, purified to near homogeneity from detergent-extracted membranes (Figure 4A inset), and reconstituted in substrate-containing phospholipid vesicles. The capacity of 500 nM Gβ1γ2 to activate PLC-η2(PH-C2) was compared to its capacity to activate PLC-β2(PH-C2). The basal level of PtdIns(4,5)P2 hydrolysis was consistently higher in the presence of PLC-η2(PH-C2) than in the presence of PLC-β2(PH-C2), suggesting that PLC-η2 may exhibit less auto-inhibition (or basally interact with substrate-containing membranes more effectively) than PLC-β2. Coreconstitution with Gβ1γ2 resulted in increased activities of both isozymes, although the fold stimulation was greater for PLC-β2(PH-C2) than for PLC-η2(PH-C2) (Figure 4A).
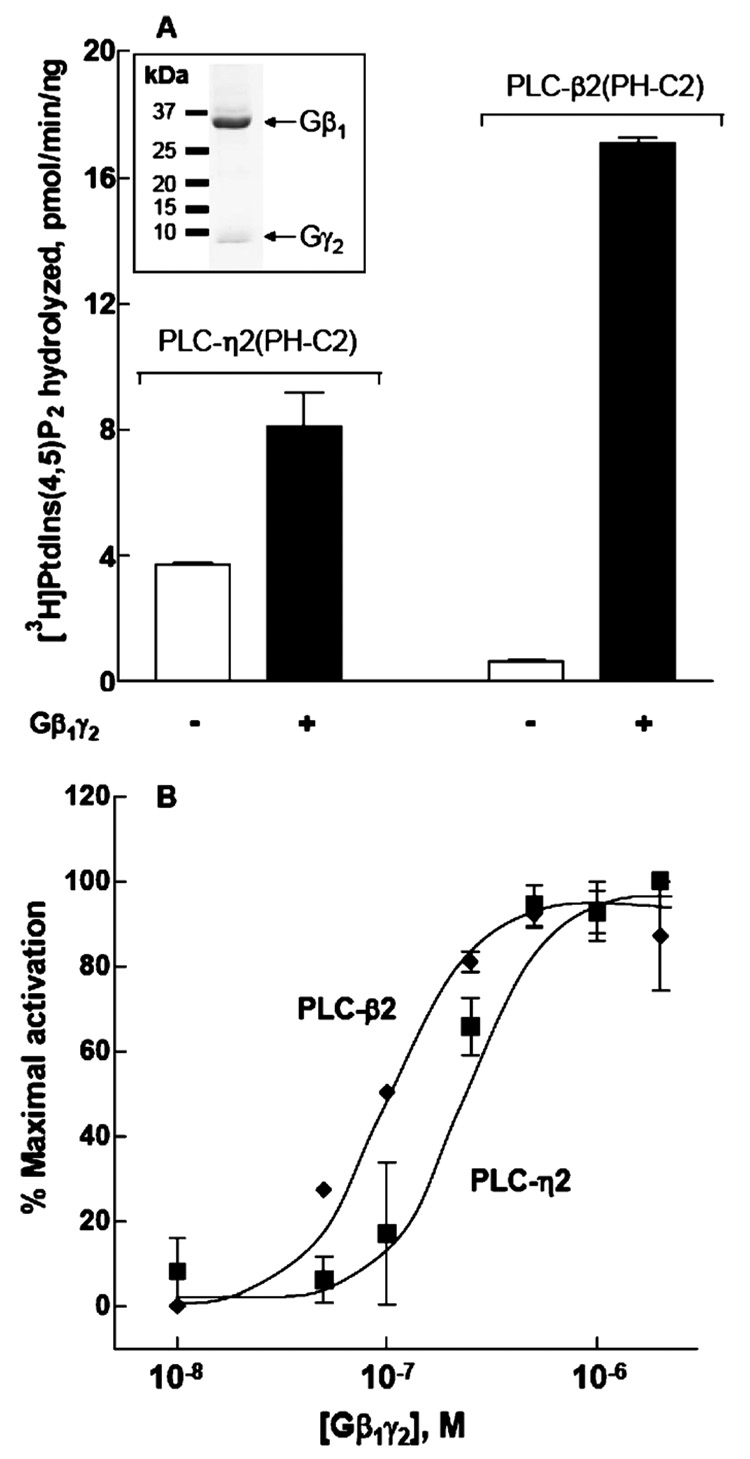
Activation of PLC-η2(PH-C2) by purified Gβ1γ2. Recombinant Gβ1γ2 at 500 nM (A) or at various concentrations (B) was reconstituted in phospholipid vesicles as described in Materials and Methods. PLC-η2(PH-C2) or PLC-β2(PH-C2) was added, and the assay was performed at 32 °C for 10 min. The data from multiple experiments were transformed and pooled by subtracting basal activities (2–4 pmol min−1 ng−1 for PLC-η2 and 0.5–1 pmol min−1 ng−1 for PLC-η2) from maximal Gβ1γ2-stimulated activities (6–9 pmol min−1 ng−1 for PLC-η2 and 12–15 pmol min−1 ng−1 for PLC-β2) to provide values for “maximal activation”. Identically calculated values for each concentration of Gβ1γ2 were then divided by the values for maximal activation to provide values for “percent maximal activation”. The results presented are the means ± the standard error of three separate experiments. The inset shows recombinant Gβ1γ2 purified from detergent-extracted HighFive insect cell membranes. Proteins were resolved by SDS–PAGE and stained with Coomassie blue.
Gβ1γ2 was reconstituted in phospholipid vesicles over a wide range of concentrations (Figure 4B), and Gβ1γ2-dependent increases in PtdIns(4,5)P2 hydrolysis were observed with both purified PLC-η2(PH-C2) and purified PLC-β2(PH-C2). Half-maximal activation occurred at a concentration of Gβ1γ2 of 284 ± 94 nM (n = 3 experiments) for PLC-η2(PH-C2) and at 98 ± 23 nM (n = 3 experiments) for PLC-β2(PH-C2).
To establish that the effects observed with Gβ1γ2 represented effector activation that depended on the presence of free Gβγ dimer, the natural binding partner of Gβγ, Gαi1, was purified after expression in HighFive cells (Figure 5A inset) and reconstituted in substrate-containing phospholipid vesicles with purified Gβ1γ2 in the presence of GDP. As illustrated in Figure 5A, Gβ1γ2-dependent stimulation of phospholipase activity of PLC-η2(PH-C2) was inhibited to basal levels in the presence of GDP-bound Gαi1, suggesting that the activation of PLC-η2(PH-C2) was indeed Gβγ-specific. Similar effects of Gαi1 on Gβ1γ2-stimulated PLC-β2 activity were observed. Gαi1 was further reconstituted over a wide range of concentrations in phospholipid vesicles with a fixed amount of Gβ1γ2. The addition of Gαi1 inhibited Gβ1γ2 activation of both PLC-η2(PH-C2) and PLC-β2(PH-C2) in a concentration-dependent manner (Figure 5B). The curve for Gαi1-mediated reversal of activation of PLC-η2(PH-C2) was shifted to the left compared to that for inhibition of PLC-β2(PH-C2) activation, which was consistent with the fact that higher concentrations of Gβ1γ2 were necessary for activation of PLC-η2(PH-C2) compared to PLC-β2(PH-C2).
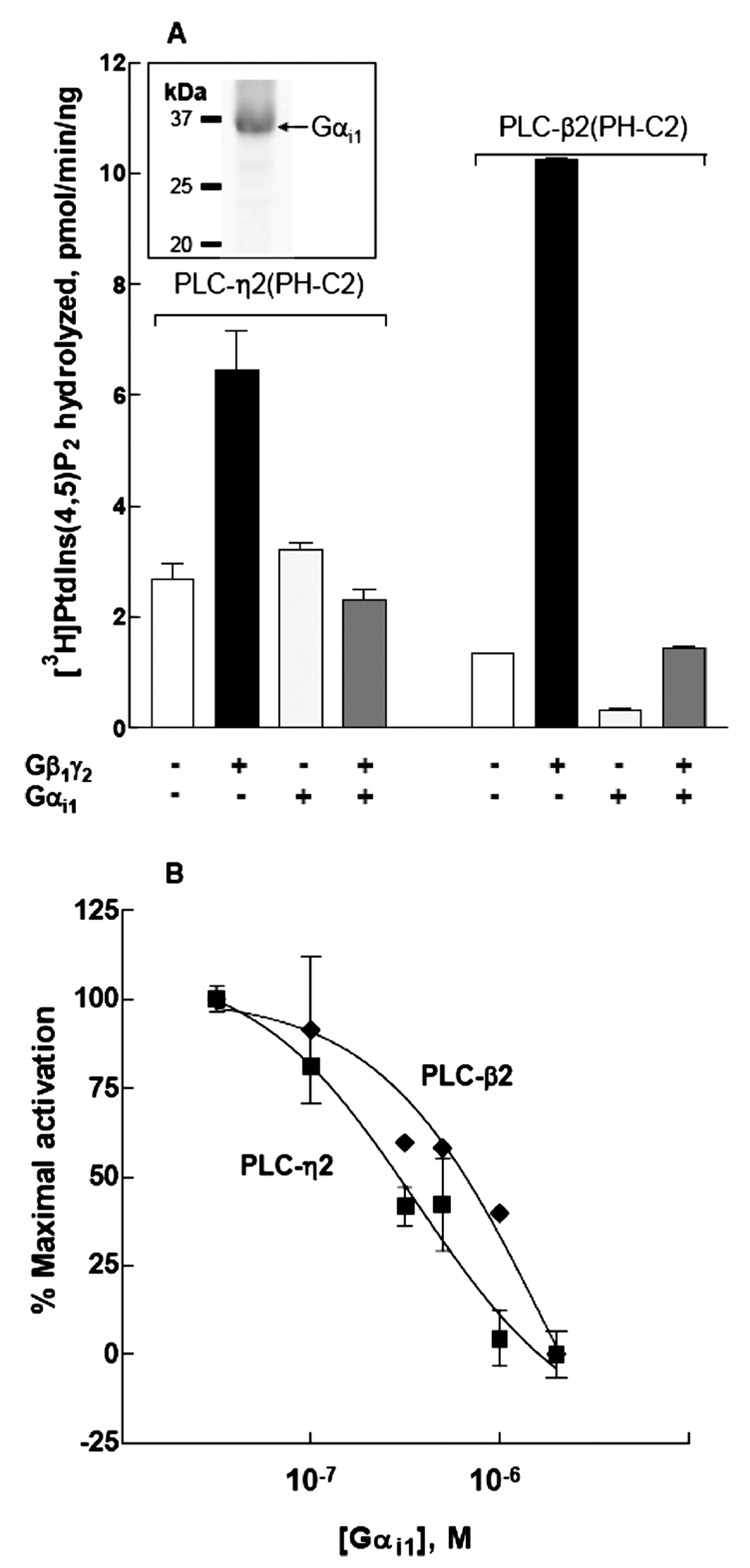
Gαi1-dependent inhibition of Gβ1γ2-promoted activation of PLC-η2(PH-C2). Recombinant Gαi1 at 2 µM (A) or various concentrations (B) was reconstituted with 1 µM Gβ1γ2 in phospholipid vesicles as described under Materials and Methods. PLC-η2(PH-C2) (1 ng) or PLC-β2(PH-C2) (5 ng) was added, and the assay was performed at 32 °C for 10 min. The data from multiple experiments were transformed and pooled by subtracting basal activities (2–4 pmol min−1 ng−1 for PLC-η2 and 0.5–1.0 pmol min−1 ng−1 for PLC-β2) from activities (6–9 pmol min−1 ng−1 for PLC-η2 and 12–15 pmol min−1 ng−1 for PLC-β2) observed in the presence of 1 ηMGβ1γ2 to provide values for maximal activation. Identically calculated values were obtained for each combination of 1 µMGβ1γ2 with the indicated concentrations, and these values were divided by that for maximal activation to provide a value for percent maximal activation. The results presented are the means ± the standard error of three separate experiments. The inset shows recombinant Gαi1 purified from detergent-extracted HighFive insect cell membranes, resolved by SDS–PAGE, and stained with Coomassie blue.
To further confirm the selectivity of Gβγ activation, GRK2-ct (amino acids 495–689) was employed in the reconstitution assay as another protein that binds Gβγ and, thereby, potentially inhibits activation of effector enzymes that is dependent on the presence of free Gβγ. As shown in Figure 6, the addition of GRK2-ct reversed the activation of both PLC-η2 and PLC-β2 by Gβ1γ2 to basal levels, and it did so in a concentration-dependent manner. Thus, GRK2-ct sequesters free Gβγ, and therefore, Gβγ-mediated activation of PLC-η2 is lost.
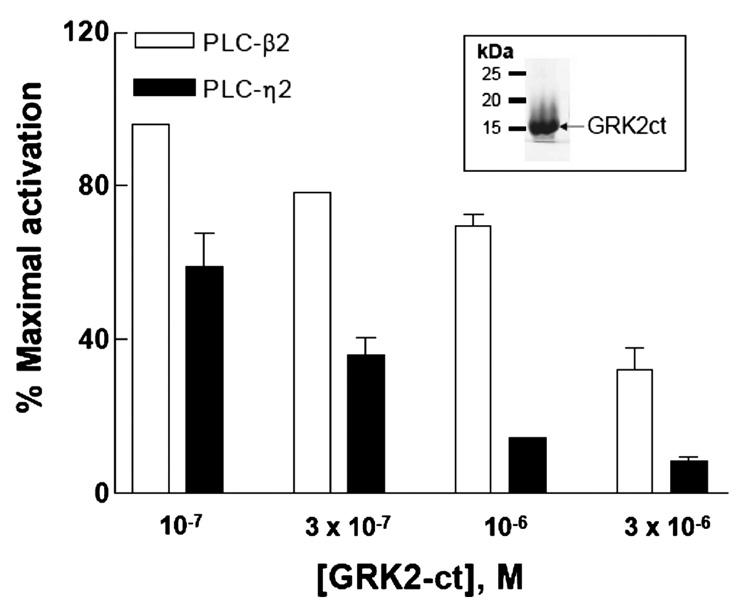
GRK2-ct-mediated reversal of Gβ1γ2-promoted activation of PLC-η2(PH-C2). Increasing concentrations of GRK2-ct were reconstituted with 1 µMGβ1γ2 in phospholipid vesicles as described in Materials and Methods. PLC-η2(PH-C2) (5 ng) or PLC-β2(PHC2) (5 ng) was added, and the assay was performed at 32 °C for 10 min. The data from multiple experiments were transformed and pooled by subtracting basal activities (2–4 pmol min−1 ng−1 for PLC-η2 and 0.5–1.0 pmol min−1 ng−1 for PLC-β2) from activities (6–9 pmol min−1 ng−1 for PLC-η2 and 12–15 pmol min−1 ng−1 for PLC-β2) observed in the presence of 1 µM Gβ1γ2 to provide values for maximal activation. Identically calculated values were obtained for each combination of 1 µM Gβ1γ2 with the indicated concentrations, and these values were divided by that for maximal activation to provide a value for percent maximal activation. The results presented are the means ± the standard error of three separate experiments.
Previous studies with PLC-β2 have implicated the PH domain in activation of this isozyme by Gβγ (19–21). Therefore, a construct of PLC-η2 that eliminates this conserved domain and encompasses the EF-hand repeats through the C2 domain [amino acids 168–872 (Figure 1A)] was produced, and PLC-η2(EF-C2) was purified to homogeneity (Figure 3A inset) as described in Materials and Methods. Kinetic analyses using addition of different amounts of PtdIns(4,5)P2 substrate in the bulk medium revealed that the lipase activity (Vmax = 7.8 µmol min−1 mg−1) of purified monomeric PLC-η2(EF-C2) was similar to that of PLC-η2(PH-C2), although the observed Km (approximately 50 µM) for PtdIns(4,5)P2 was higher (data not shown). As illustrated in Figure 7, Gβ1γ2 also activated this PLC-η2 lacking the PH domain, and therefore, we conclude that activation by this G protein heterodimer occurs at least in part through interaction with the catalytic core of the isozyme.
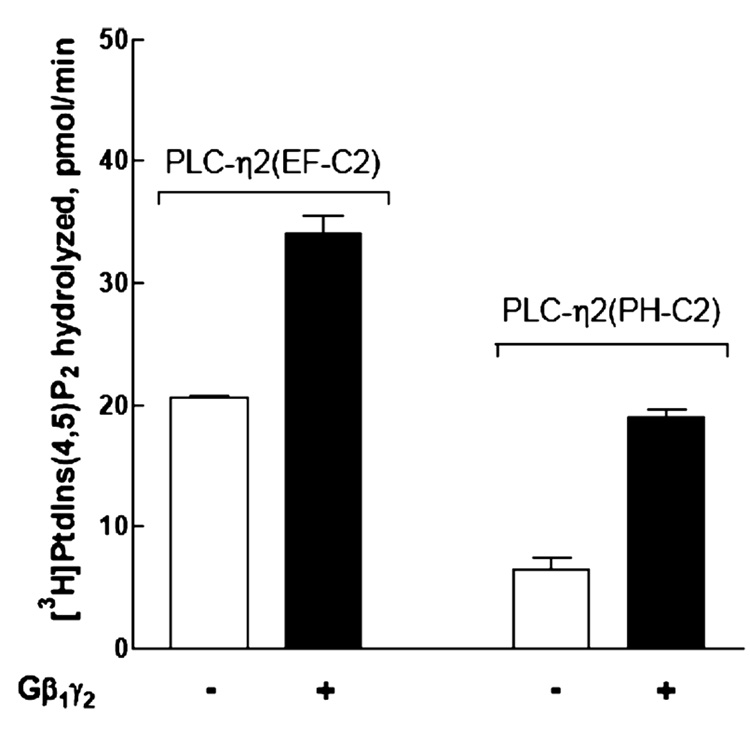
Retention of Gβ1γ2-mediated activation in a purified construct of PLC-η2 lacking the PH domain. Recombinant Gβ1γ2 at 500 nM was reconstituted in phospholipid vesicles with PLC-η2(PH-C2) or PLC-η2(EF-C2). The assay was performed at 32 °C for 10 min. The results presented are the means ± the standard error of three separate experiments.
DISCUSSION
The work described here illustrates direct, concentration-dependent stimulation of purified PLC-η2 in phospholipid vesicles reconstituted with purified Gβ1γ2. Gβγ-dependent activation of PLC-η2 also is observed in intact cells with multiple forms of the heterotrimeric subunit. Thus, we have identified a novel effector of Gβγ and have implicated this PLC isozyme in signaling responses downstream of heterotrimeric G protein-coupled receptors.
The involvement of multiple G proteins in receptor-promoted regulation of inositol lipid signaling was first realized in studies of the PLC-β isozymes. Gα subunits of the Gq family initially were shown to mediate guanine nucleotide-dependent activation (12–15), but subsequent studies revealed that PLC-β2 and PLC-β3 also are activated by Gβγ subunits of heterotrimeric G proteins (16–18). The apparent complexity of G protein-dependent regulation of inositol lipid signaling was further increased by realization that the small GTPase Rac binds and activates PLC-β2 (19–21). Moreover, the most divergent member of the PLC isozyme family, PLC-ε, was initially discovered as a Ras-binding protein in Caenorhabditis elegans, and the mammalian isoform is dually activated by Ras (22–24) and Rho (26, 27) through direct binding of these GTPases to two different regions of the isozyme. Although PLC-γ isozymes are activated by phosphorylation by receptor and other tyrosine kinases, PLC-γ2 is additionally activated by Rac (11). Transient expression studies in intact COS-7 cells implicated activation of PLC-η2 by Gβγ subunits, and the results presented here illustrate that this novel PLC isozyme is a direct effector of Gβγ.
We previously screened all of the heterotrimeric Gα subunits and many Ras superfamily GTPases for stimulation of PLC-η2 after coexpression in COS-7 cells, and only Gβγ was identified as a potential activator of the isozyme (31). Additional comprehensive analyses are needed, but within the limitations of our screens to date, PLC-η2 is the only PLC isozyme that is solely regulated by Gβγ. Thus, our data are consistent with the idea that G protein-coupled receptors activate PLC-η2 by release of Gβγ from heterotrimeric G proteins. The broad expression of PLC-η2 in the brain together with the high levels of neuronal Gαo and other members of the Gi subfamily suggests that this PLC isozyme may in part function downstream of Gi-coupled receptors in the neuronal signaling pathways. For example, PLC-η2 is strongly expressed in hippocampus, cerebral cortex, and olfactory bulb in mouse brain (30). Expression of PLC-η2 was detected in early developmental stages in mouse brain and was proposed to be important in formation and/or maintenance of the neuronal networks in postnatal brain (30). The exact roles of G protein-mediated regulation of PLC-η2 in brain development await further exploration.
Gβ1γ2 was the only G protein dimer tested as an activator of purified PLC-η2. However, the relative effects of Gβγ subunits of various subunit composition on PLC-η2-versus PLC-β2-promoted activity were very similar in transfection studies, and therefore, it is unlikely that remarkable differences exist in the selectivity of regulation of these two PLC isozymes by certain Gβγ subunits. Given its similarity in sequence with PLC-η2, we also tested whether Gβ1γ2 activates PLC-η1. Multiple coexpression experiments with COS-7 cells carried out under a variety of conditions failed to reveal any Gβγ-dependent activation of PLC-η1. Thus, within the limitations of these transfection experiments, we conclude that Gβγ differentially regulates the two PLC-η isozymes. Whether PLC-η1 is regulated by small GTPases or by other heterotrimeric G protein subunits remains unreported.
The last three exons of the PLC-η2 gene are alternatively spliced in both mice and humans to produce at least four splice variants containing markedly variable C-terminal domains beyond the C2 domain (31). The physiological relevance of these different forms of PLC-η2 is not yet known, but as illustrated here, removal of the 545 C-terminal amino acids does not demonstrably affect its regulation by Gβ1γ2. Previous studies have suggested that Gβγ interacts with multiple sites in PLC-β2, and both the catalytic and PH domains have been implicated in Gβγ-dependent activation of the isozyme (19, 35–37). A construct of PLC-η2 that encompassed the PH through C2 domain retained activation by Gβ1γ2 both in vivo and in vitro, but observation of similar levels of activation of a fragment of PLC-η2 lacking the PH domain indicates that in contrast to PLC-β2, the PH domain of PLC-η2 appears to be relatively unimportant in Gβ1γ2-mediated regulation. However, Nakahara and co-workers (30) illustrated that PLC-η2 is predominantly associated with the plasma membrane in mouse brain and reported that the PH domain is necessary for membrane localization.
The mechanism by which Gβγ activates PLC-β isozymes, and now PLC-η2, remains to be defined. Activation could follow from simple Gβγ-dependent recruitment to phosphoinositide substrate-containing membranes, or activation may involve G protein-promoted conformational changes in the catalytic core of the enzyme. Mechanistic insight into the activation of PLC isozymes has accrued from our recent structural and biochemical studies illustrating that PLC-β2 and, ostensibly, PLC-δ and PLC-ε are autoinhibited by the unstructured X–Y linker region of the isozymes (ref 21 and unpublished observations of S. Hicks et al., 2007). These studies strongly suggest that the active site-occluding X–Y linker moves as a consequence of dense negative charges in the linker encountering negatively charged membrane surfaces; autoinhibition is relieved, and access to the PtdIns(4,5)P2 substrate is promoted. Thus, our structures of PLC-β2 alone and PLC-β2 in a GTP-dependent complex with Rac1 illustrate that Rac-mediated activation of PLC-β2 follows from simple recruitment of the enzyme to membrane surfaces. In contrast, biochemical studies of Gβγ- dependent activation of PLC-β2 suggest a more complex mode of regulation involving the catalytic core of the enzyme, and therefore, the mechanism(s) by which Gβγ activates PLC-β2 and other PLC isozymes remains unclear. The Gβγ-binding interface has not yet been structurally identified in any PLC isozyme, and it will be important to establish unambiguously whether Gβγ binds to PLC-η2 and PLC-β2 through similar or different determinants.
A notable difference between PLC-η2 and PLC-β2 is that the overall charge of PLC-η2 is much more positive than that of PLC-β2, and the X–Y linker of PLC-η2 is essentially devoid of sequence containing dense negative charge and overall is positively charged. Thus, we anticipate that PLC-η2 exhibits less autoinhibition and greater electrostatic force-dependent attraction to phosphoinositide substrate-containing membrane surfaces. Such properties may explain the high basal activity of PLC-η2, and given these differences in PLC-β2 and PLC-η2, it is perhaps not surprising that the fold activation of PLC-β2 by Gβ1γ2 was greater than that observed with PLC-η2. Nomikos and co-workers (38) recently concluded that a dense positive region in the X–Y linker of PLC-ζ also is important for membrane association and activity of this sperm-specific isozyme.
In conclusion, we have demonstrated that Gβ1γ2 directly activates PLC-η2. Future experiments will delineate the region of Gβ1γ2 interaction and reveal the mechanism of activation. The physiological role of Gβ1γ2-mediated activation of PLC-η2 is under investigation.
ACKNOWLEDGMENT
We are indebted to Rob Nicholas, Gary Waldo, Jason Seifert, and Stephanie Hicks for advice and helpful discussions and Mark Jezyk for the production of GRK2-ct.
Footnotes
†This work was supported by U.S. Public Health Service Grants GM57391 and GM65533.
1Abbreviations: PLC, phospholipase C; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; Ins(1,4,5)P3, inositol 1,4,5-trisphosphate; PH, pleckstrin homology; EF, elongation factor; TIM, triosephosphate isomerase; RA, Ras-associating; GRK2-ct, G protein-coupled receptor kinase 2 C tail; SEM, standard error of the mean.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1021/bi800044n
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2649797?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1021/bi800044n
Article citations
Activation Mechanisms and Diverse Functions of Mammalian Phospholipase C.
Biomolecules, 13(6):915, 31 May 2023
Cited by: 5 articles | PMID: 37371495 | PMCID: PMC10296670
Review Free full text in Europe PMC
Cryptic mutations of PLC family members in brain disorders: recent discoveries and a deep-learning-based approach.
Brain, 146(4):1267-1280, 01 Apr 2023
Cited by: 2 articles | PMID: 36448305 | PMCID: PMC10115239
Review Free full text in Europe PMC
Dissociation of the G protein βγ from the Gq-PLCβ complex partially attenuates PIP2 hydrolysis.
J Biol Chem, 296:100702, 01 Jan 2021
Cited by: 13 articles | PMID: 33901492 | PMCID: PMC8138763
Phospholipase C-η2 interacts with nuclear and cytoplasmic LIMK-1 during retinoic acid-stimulated neurite growth.
Histochem Cell Biol, 145(2):163-173, 15 Dec 2015
Cited by: 2 articles | PMID: 26671787 | PMCID: PMC4735258
Phospholipase Cη2 Activation Redirects Vesicle Trafficking by Regulating F-actin.
J Biol Chem, 290(48):29010-29021, 02 Oct 2015
Cited by: 3 articles | PMID: 26432644 | PMCID: PMC4661413
Go to all (24) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (2)
- (1 citation) ENA - DQ176850
- (1 citation) ENA - BC055005
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Activation of phospholipase C-epsilon by heterotrimeric G protein betagamma-subunits.
J Biol Chem, 276(51):48257-48261, 18 Oct 2001
Cited by: 58 articles | PMID: 11641393
G protein βγ subunits directly interact with and activate phospholipase Cϵ.
J Biol Chem, 293(17):6387-6397, 13 Mar 2018
Cited by: 21 articles | PMID: 29535186 | PMCID: PMC5925795
RhoA activates purified phospholipase C-epsilon by a guanine nucleotide-dependent mechanism.
J Biol Chem, 279(46):47992-47997, 18 Aug 2004
Cited by: 41 articles | PMID: 15322077
Subtype-dependent regulation of Gβγ signalling.
Cell Signal, 82:109947, 11 Feb 2021
Cited by: 19 articles | PMID: 33582184 | PMCID: PMC8026654
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (5)
Grant ID: GM65533
Grant ID: R01 GM057391
Grant ID: P01 GM065533
Grant ID: GM57391
Grant ID: R01 GM057391-10




