Abstract
Free full text

“Outside-to-Inside” (and Now Back to “Outside”) Pathogenic Mechanisms in Atopic Dermatitis
Abstract
The pathogenesis of atopic dermatitis (AD) has been attributed largely to abnormalities in the adaptive immune system, with key roles played by T-helper 1(Th1)/Th2 cell dysregulation, IgE production, dendritic cell signaling, and mast-cell hyperactivity, resulting in the pruritic, inflammatory dermatosis that characterizes AD (Leung et al., 2004). Accordingly, therapy has been focused on ameliorating Th2-mediated inflammation and pruritus (e.g., Leung, 2000). Indeed, there is emerging evidence that inflammation in AD results first from inherited and acquired insults that converge to alter epidermal structure and function, followed by immune system activation, which in turn has negative consequences for skin-barrier homeostasis. This cycle comprises an “outside–inside–outside” model of AD pathogenesis (Elias et al., in press).
The epidermis generates an impressive set of critical defensive functions (Elias, 2005; Elias and Choi, 2005) that include the permeability barrier, allowing survival in a potentially desiccating external environment, and an antimicrobial barrier, which simultaneously encourages colonization by nonpathogenic “normal” flora while resisting growth of microbial pathogens (Elias, 2007). The permeability barrier resides in the stratum corneum (SC), where multiple layers of anucleate corneocytes are embedded in an extracellular matrix, enriched in ceramides (Cer), cholesterol, and free fatty acids, arranged as planar lamellar sheets (Elias and Menon, 1991). These lipids, as well as an assortment of hydrolases important for lipid processing and desquamation and at least two key antimicrobial peptides (Aberg et al., 2007, and references cited therein), are delivered to the extracellular matrix through secretion of epidermal lamellar body contents. Whereas lamellar body–derived proteases and their inhibitors orchestrate the orderly digestion of corneodesmosomes, allowing cells to shed invisibly at the skin surface (Brattsand et al., 2005; Stefansson et al., 2008), the lipid-processing enzymes (β-glucocerebrosidase, acidic sphingomyelinase, and secretory phospholipase A2) generate the Cer and free fatty acids that, along with cholesterol, form the extracellular lamellar membrane (Elias and Menon, 1991). Thus, proteases as well as extracellular enzymes may ultimately be involved in the pathophysiology of eczema and pruritus.
Based primarily on the strong association of inherited abnormalities in filaggrin (FLG) expression, atopic dermatitis (AD), at least in Euro-Americans, is now increasingly considered a primary disorder of SC structure and function (Hudson, 2006; Irvine and McLean, 2006; Palmer et al., 2006; Smith et al., 2006; Weidinger et al., 2006). Thus, AD can be considered a disease of primary barrier failure, characterized by both a defective permeability (Proksch et al., 2006, and references therein) and antimicrobial function (Baker, 2006; Boguniewicz and Leung, 2006). Although both of these abnormalities are well-recognized features of AD, they have been widely assumed to reflect downstream consequences of a primary immunologic abnormality (the historical inside–outside view of AD pathogenesis). We and others have long proposed that the permeability-barrier abnormality in AD is not merely an epiphenomenon but rather the “driver” of disease activity (i.e., the reverse, outside–inside view of disease pathogenesis) (Elias and Feingold, 2001), because (1) the extent of the permeability-barrier abnormality parallels severity of disease phenotype in AD, (2) both clinically uninvolved skin sites and skin cleared of inflammation for as long as 5 years continue to display significant barrier abnormalities, (3) emollient therapy comprises effective ancillary therapy, and (4) specific replacement therapy, which targets the prominent lipid abnormalities that account for the barrier abnormality in AD, not only corrects the permeability-barrier abnormality but also comprises effective anti-inflammatory therapy for AD (Chamlin et al., 2002; Figure 1).

AD, atopic dermatitis; AMP, adenosine monophosphate; Cer, ceramide; FLG, filaggrin; LEKTI, lymphoepithelial Kazal-type related trypsin inhibitor; PS, psychological stress; RH, relative humidity; Th1, T-helper 1; Th2, T-helper 2 (Modified from Elias et al., in press.)
Still, how loss of FLG (an intracellular protein) provokes a permeability-barrier abnormality is not known. In addition, it is not clear what drives barrier dysfunction in the skin of AD patients without a FLG mutation. Although it has been hypothesized that loss of FLG could cause corneocyte deformation, the barrier abnormality more likely is linked to lack of FLG as a substrate for proteolytic processing and further deimination into polycarboxylic acids, such as pyrrolidine carboxylic acid and trans-urocanic acid. These metabolites normally act as osmolytes (natural moisturizing factors), drawing water into corneocytes, partially accounting for corneocyte hydration. Hence, the most immediate result of FLG deficiency is decreased SC hydration, a well-recognized feature of AD. A steeper water gradient would inexorably accelerate transcutaneous water loss, particularly if a paucity of extracellular lipids results in decreased water-retaining ability. However, either corneocyte flattening or decreased SC hydration can suffice to enhance antigen penetration. We suspect that another mechanism is operative, because another inevitable consequence of decreased downstream production of polycarboxylic acid metabolites is an increase in the SC pH (Krien and Kermici, 2000), sufficient to increase the activities of the multiple serine proteases in SC, which all exhibit neutral-to-alkaline pH optima (Brattsand et al., 2005; Stefansson et al., 2008; Steinhoff et al., 2005). Increased serine protease activity could generate the active forms of the primary cytokines IL-1α and IL-1β from their 33-kDa pro-forms, which are stored in large quantities in the cytosol of corneocytes (Hachem et al., 2002; Nylander-Lundqvist et al., 1996), the first step in the cytokine cascade that we propose initiates inflammation in AD (Figure 2).
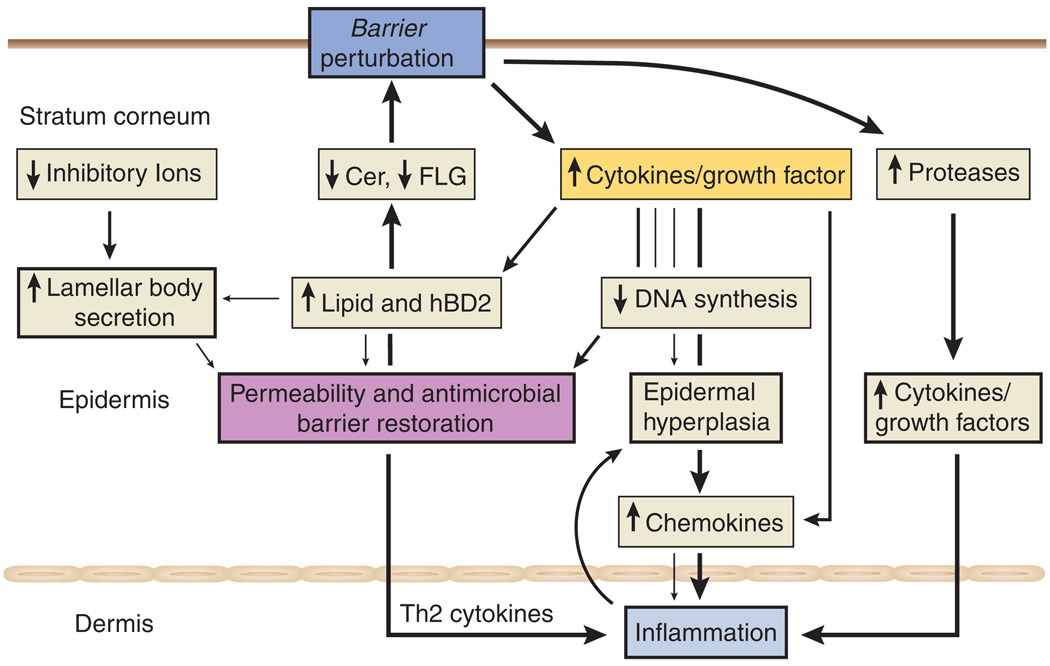
Cer, ceramide; FLG, filaggrin; hBD2, human β-defensin-2; Th2, T-helper 2. (From Figure 2. Cer, ceramide; FLG, filaggrin; hBD2, human β-defensin-2; Th2, T-helper 2. (From Steinhoff et al., 2005; modified from Elias et al., in press.)
Another cause of inflammation in AD doubtless includes sustained antigen ingress through a defective barrier, leading to a T-helper 2 (Th2)-dominant infiltrate (Hudson, 2006). Yet FLG mutations alone do not provoke AD, as demonstrated in ichthyosis vulgaris, in which the same single- or double-allele FLG mutations reduce FLG content, but inflammation (i.e., AD) does not inevitably occur (Sandilands et al., 2007). We and others have suggested that certain acquired stressors could elicit disease by aggravating the barrier abnormality. Indeed, a barrier-dependent increase in pH (and serine protease activity) likely accounts for the precipitation of AD following the use of neutral-to-alkaline soaps (Figure 1) (Cork et al., 2006). Likewise, prolonged exposure to reduced environmental humidity, a well-known risk factor for AD, likely accelerates transcutaneous water loss rates across defective SC in AD, further aggravating the barrier abnormality. Finally, psychological stress, which aggravates permeability-barrier function in humans (Altemus et al., 2001), is both a well-known precipitant of AD and a cause of resistance to therapy. Moreover, a dysregulation of proteases, highly expressed by keratinocytes, protease inhibitors, and protease-activated receptors, may be important for neuronal–epidermal communication during barrier dysfunction (Demerjian et al., 2008; Hachem et al., 2006; Steinhoff et al., 2000, 2005).
Despite accumulating evidence in support of a barrier-initiated pathogenesis of AD, recent studies suggest specific mechanisms whereby Th2-generated cytokines could also further aggravate AD. As described in this issue by Kurahashi et al. (2008), exogenous applications of the Th2 cytokine, IL-4, impede permeability-barrier recovery after acute perturbations. The basis for the negative effects of IL-4 could include the prior observation by these authors that exogenous IL-4 inhibits Cer synthesis (Hatano et al., 2005). But IL-4 also inhibits expression of both FLG (Howell et al., 2007) and desmoglein 3 (Kobayashi et al., 2004), which also could further compromise barrier function, completing a potential outside–inside–outside pathogenic loop in AD (Figure 2). Furthermore, nerves may be activated by “barrier stressors” such as UV light, toxic or allergic agents, and microbial agents. Therefore, nerve-derived mediators could also modulate enzyme production, antimicrobial peptide (defensin) generation, pH changes, or SC humidity, thereby influencing keratinocyte function (Paus et al., 2006; Roosterman et al., 2006; Steinhoff et al., 2006).
Together, the converging pathogenic features described above create a strong rationale for the deployment of specific strategies to restore barrier function in AD. When used under nursing supervision, moisturizers have been shown to reduce topical steroid usage (Cork et al., 2003). Of various barrier-repair approaches, a mixture of the three SC lipids in a Cer-dominant formulation (TriCeram, Osmotics Cosmeceuticals) demonstrated improved clinical status, permeability-barrier function, and SC integrity when this technology was substituted for standard moisturizers in children with severe, recalcitrant AD (Chamlin et al., 2002). More recently, a higher-strength, FDA cleared formulation (EpiCeram cream, Ceragenix Pharmaceuticals) demonstrated efficacy that was comparable to that of a mid-potency steroid (fluticasone; Cutivate cream) in an investigator-blinded, multicenter clinical trial of pediatric patients with moderate to severe AD (Sugarman and Parish, in press). These studies, although still preliminary, suggest that correction of the lipid biochemical abnormality that is responsible for the barrier defect in AD could also downregulate the further insideto-outside alterations provoked by IL-4 and could comprise a new or ancillary approach to the therapy of AD.
ACKNOWLEDGMENTS
We gratefully acknowledge the superb editorial assistance of Joan Wakefield and Jerelyn Magnusson, including preparation of the graphics. This work was supported by National Institutes of Health grant AR19098, Department of Defense grant W81XWH-05-2-0094, and the Medical Research Service, Department of Veterans Affairs, grants DFG (STE 1014/2-2) (M.S.) and SFB 293 (M.S.). No company provided support for this article.
Footnotes
CONFLICT OF INTEREST
Dr Elias is a co-inventor of Cer-dominant formulation (TriCeram, Osmotics Corp.), a patented technology, and an officer of Ceragenix Pharmaceuticals, the licensee of this technology. Dr Steinhoff states no conflict of interest.
REFERENCES
- Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. [Europe PMC free article] [Abstract] [Google Scholar]
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J Invest Dermatol. 2001;117:309–317. [Abstract] [Google Scholar]
- Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144:1–9. [Abstract] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–S480. [Abstract] [Google Scholar]
- Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198–203. [Abstract] [Google Scholar]
- Chamlin SL, Kao J, Frieden IJ, Sheu MY, Fowler AJ, Fluhr JW, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol. 2002;47:198–208. [Abstract] [Google Scholar]
- Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. Br J Dermatol. 2003;149:582–589. [Abstract] [Google Scholar]
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene–environment interactions. J Allergy Clin Immunol. 2006;118:3–21. quiz 22–3. [Abstract] [Google Scholar]
- Demerjian M, Hachem JP, Tschachler E, Denecker G, Declercq W, Vandenabeele P, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol. 2008;172:86–97. [Europe PMC free article] [Abstract] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. [Abstract] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. [Abstract] [Google Scholar]
- Elias PM, Choi EH. Interactions among stratum corneum defensive functions. Exp Dermatol. 2005;14:719–726. [Abstract] [Google Scholar]
- Elias PM, Feingold KR. Does the tail wag the dog? Role of the barrier in the pathogenesis of inflammatory dermatoses and therapeutic implications. Arch Dermatol. 2001;137:1079–1081. [Abstract] [Google Scholar]
- Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991;24:1–26. [Abstract] [Google Scholar]
- Elias P, Hatano Y, Williams M. Basis for the barrier abnormality in atopic dermatitis: “outside–inside–outside“ pathogenic mechanisms. J Allergy Clin Immunol. (in press) [Europe PMC free article] [Abstract] [Google Scholar]
- Hachem JP, Fowler A, Behne M, Fluhr J, Feingold KR, Elias PM. Increased stratum orneum pH promotes activation and release of primary cytokines from the stratum corneum attributable to activation of serine proteases. J Invest Dermatol. 2002;119:207–350. abstr 306. [Google Scholar]
- Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol. 2006;126:2074–2086. [Abstract] [Google Scholar]
- Hatano Y, Terashi H, Arakawa S, Katagiri K. Interleukin-4 suppresses the enhancement of ceramide synthesis and cutaneous permeability barrier functions induced by tumor necrosis factor-alpha and interferon-gamma in human epidermis. J Invest Dermatol. 2005;124:786–792. [Abstract] [Google Scholar]
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–155. [Europe PMC free article] [Abstract] [Google Scholar]
- Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006;38:399–400. [Abstract] [Google Scholar]
- Irvine AD, McLean WH. Breaking the (un)sound barrier: filaggrin is a major gene for atopic dermatitis. J Invest Dermatol. 2006;126:1200–1202. [Abstract] [Google Scholar]
- Kobayashi J, Inai T, Morita K, Moroi Y, Urabe K, Shibata Y, et al. Reciprocal regulation of permeability through a cultured keratinocyte sheet by IFN-gamma and IL-4. Cytokine. 2004;28:186–189. [Abstract] [Google Scholar]
- Krien PM, Kermici M. Evidence for the existence of a self-regulated enzymatic process within the human stratum corneum–an unexpected role for urocanic acid. J Invest Dermatol. 2000;115:414–420. [Abstract] [Google Scholar]
- Kurahashi R, Hatano Y, Katagiri K. IL-4 suppresses the recovery of cutaneous permeability barrier functions in vivo. J Invest Dermatol. 2008;128:1329–1331. [Abstract] [Google Scholar]
- Leung DY. Atopic dermatitis: new insights and opportunities for therapeutic intervention. J Allergy Clin Immunol. 2000;105:860–876. [Abstract] [Google Scholar]
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. [Europe PMC free article] [Abstract] [Google Scholar]
- Nylander-Lundqvist E, Back O, Egelrud T. IL-1 beta activation in human epidermis. J Immunol. 1996;157:1699–1704. [Abstract] [Google Scholar]
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. [Abstract] [Google Scholar]
- Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest. 2006;116:1174–1186. [Europe PMC free article] [Abstract] [Google Scholar]
- Proksch E, Folster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006;43:159–169. [Abstract] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. [Abstract] [Google Scholar]
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. [Abstract] [Google Scholar]
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38:337–342. [Abstract] [Google Scholar]
- Stefansson K, Brattsand M, Roosterman D, Kempkes C, Bocheva G, Steinhoff M, et al. Activation of proteinase-activated receptor-2 by human kallikrein-related peptidases. J Invest Dermatol. 2008;128:18–25. [Abstract] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. [Abstract] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. [Abstract] [Google Scholar]
- Steinhoff M, Bienenstock J, Schmelz M, Maurer M, Wei E, Biro T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J Invest Dermatol. 2006;126:1705–1718. [Abstract] [Google Scholar]
- Sugarman J, Parish LJ. A topical lipid-based barrier repair formulation (EpiCeram) cream is high-effective monotherapy for moderate-to-severe pediatric atopic dermatitis. J Invest Dermatol. 2008;128 Suppl. 1:S54. [Abstract] [Abstract] [Google Scholar]
- Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–219. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1038/jid.2008.88
Read article for free, from open access legal sources, via Unpaywall:
http://www.jidonline.org/article/S0022202X1533863X/pdf
Citations & impact
Impact metrics
Article citations
Exploring causal correlations between circulating cytokines and atopic dermatitis: a bidirectional two-sample Mendelian randomization study.
Front Immunol, 15:1367958, 11 Jul 2024
Cited by: 0 articles | PMID: 39055710 | PMCID: PMC11269137
Study on mechanism of transdermal administration of eugenol for pain treatment by network pharmacology and molecular docking technology.
Heliyon, 10(8):e29722, 16 Apr 2024
Cited by: 0 articles | PMID: 38681628 | PMCID: PMC11046106
The role of the environment in allergic skin disease.
Curr Allergy Asthma Rep, 24(6):323-330, 11 May 2024
Cited by: 2 articles | PMID: 38733510
Review
Kahweol Inhibits Pro-Inflammatory Cytokines and Chemokines in Tumor Necrosis Factor-α/Interferon-γ-Stimulated Human Keratinocyte HaCaT Cells.
Curr Issues Mol Biol, 46(4):3470-3483, 18 Apr 2024
Cited by: 0 articles | PMID: 38666948 | PMCID: PMC11048935
Antioxidant, Antibacterial Properties of Novel Peptide CP by Enzymatic Hydrolysis of <i>Chromis notata</i> By-Products and Its Efficacy on Atopic Dermatitis.
Mar Drugs, 22(1):44, 12 Jan 2024
Cited by: 2 articles | PMID: 38248669 | PMCID: PMC10817315
Go to all (153) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms.
J Allergy Clin Immunol, 121(6):1337-1343, 07 Mar 2008
Cited by: 244 articles | PMID: 18329087 | PMCID: PMC2706021
Review Free full text in Europe PMC
Abnormal skin barrier in the etiopathogenesis of atopic dermatitis.
Curr Allergy Asthma Rep, 9(4):265-272, 01 Jul 2009
Cited by: 43 articles | PMID: 19656472
Review
Lipid abnormalities and lipid-based repair strategies in atopic dermatitis.
Biochim Biophys Acta, 1841(3):323-330, 12 Oct 2013
Cited by: 34 articles | PMID: 24128970 | PMCID: PMC3943491
Review Free full text in Europe PMC
Skin barrier function.
Curr Allergy Asthma Rep, 8(4):299-305, 01 Jul 2008
Cited by: 75 articles | PMID: 18606081 | PMCID: PMC2843412
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIAMS NIH HHS (3)
Grant ID: R01 AR019098
Grant ID: R01 AR019098-30
Grant ID: AR19098





