Abstract
Free full text

The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP sensitive potassium channels: implications for ischemic preconditioning
Abstract
Ischemic preconditioning (IPC) affords cardioprotection against ischemia-reperfusion (IR) injury, and while the molecular mechanisms of IPC are debated, the mitochondrial ATP sensitive K+ channel (mKATP) has emerged as a candidate effector for several IPC signaling pathways. The molecular identity of this channel is unknown, but significant pharmacologic overlap exists between mKATP and mitochondrial respiratory complex II (succinate dehydrogenase). In this investigation, we utilized isolated cardiac mitochondria, Langendorff perfused hearts, and a variety of biochemical methods, to make the following observations: (i) The competitive complex II inhibitor malonate is formed in mitochondria under conditions resembling IPC. (ii) IPC leads to a reversible inhibition of complex II that has likely been missed in previous investigations due to the use of saturating concentrations of succinate. (iii) Malonate opens mKATP channels even when mitochondria are respiring on complex I-linked substrates, suggesting an effect of this inhibitor on the mKATP channel independent of complex II inhibition. Together, these observations suggest that complex II inhibition by endogenously formed malonate may represent an important activation pathway for mKATP channels during IPC.
Introduction
The acute pathology of cardiac ischemia-reperfusion (IR) injury is largely mediated through a series of key events at the mitochondrial level, including mitochondrial Ca2+ overload and the overproduction of reactive oxygen species (ROS) [11,63,69], which trigger opening of the mitochondrial permeability transition (PT) pore at reperfusion [29,41], leading to cytochrome c release and cell death. In contrast, despite being a site of damage, mitochondria are also implicated in the endogenous cardioprotective mechanism of ischemic preconditioning (IPC), in which short non-lethal periods of IR protect the heart from subsequent prolonged IR injury [48]. Preservation of mitochondrial function is critical to post-ischemic functional recovery [1,36,43,57,63,68], and the cardioprotective effect of IPC can be mimicked by various pharmacological agents which act at the mitochondrial level [6,14,35,56,60,75]. Most notable among these agents are activators of mitochondrial ATP-sensitive potassium channels (mKATP) [16,23] and reversible inhibitors of the respiratory chain complexes [13,14,49,58,62]. To date, an overlap in the mechanism of protection afforded by these mitochondrial targets has not been fully elucidated.
Extensive pharmacological evidence implicates the mKATP channel in IPC [4,30,47]. Diazoxide (DZX), a pharmacological agonist of mKATP mimics IPC [28,45], while mKATP antagonists prevent both IPC and DZX-mediated cardioprotection [5,37,66]. Initially, the pharmacological effects of KATP modulators were attributed to classic surface KATP channels, causing a shortening of action potentials and thereby depressing contractility [51]. However, the protective effect of KATP modulators remains in non-contracting cardiomyocytes [44,45]. The detection of a KATP channel in the inner mitochondrial membrane [8,38] correlated the pharmacological evidence, but despite extensive investigation the mechanisms of endogenous upstream regulation of mKATP activity during IPC remain elusive. The mechanism of protection downstream of mKATP opening also remains unclear [16], but may involve mild uncoupling [17,42] resulting in the inhibition of Ca2+ overload and ROS generation [42]. Alternatively, mKATP-mediated K+ influx may result in water influx, causing mild matrix swelling [21] that may improve coupling of Ox-Phos or interfere with PT pore assembly [16,17].
The pharmacological evidence implicating mKATP in IPC also alludes to a role for complex II (succinate dehydrogenase, SDH) in IPC [3]. Complex II is a trans-membrane protein of the mitochondrial respiratory chain, and also an enzyme of the TCA cycle, transferring electrons from succinate oxidation onto ubiquinone. Interestingly, IPC is known to trigger endogenous mechanisms that reversibly inhibit the respiratory chain [13,49,58]. Furthermore, the commonly used mKATP agonist DZX [15] inhibits complex II [20,65], while complex II inhibitors such as 3-NP [53] and HNO [25,55,67] have been shown to mimic IPC and protect both the heart [53] and brain against IR injury [61]. Genetic overlap between complex II subunit C and the sulfonylurea receptor of KATP channels has also been reported [74]. These findings led to the hypothesis that respiratory chain inhibition rather than mKATP channel activity may underlie IPC-mediated protection, and generated some doubt regarding the existence of mKATP [3,18]. The lack of a molecular identity for mKATP compounded these doubts, and led to proposals that the channel may be composed of preexisting mitochondrial proteins, including complex II, mitochondrial ATP-binding cassette protein-1, adenine nucleotide translocator, ATP synthase, and the phosphate carrier [3]. In parallel, complex II inhibitors such as malonate and 3-NP also trigger K+ transport, suggesting that complex II may be a component of, or important regulator of, the mKATP channel [3].
Another important characteristic of the mKATP channel appears to be its complex interactions with mitochondrial ROS generation. It has been reported that ROS reside either upstream [22] or downstream [2] of mKATP within the signaling cascade of IPC. However, the molecular mechanisms by which ROS may regulate channel activity are unclear. In this regard, it has been recently shown that several metabolic α-keto acids of the TCA cycle can react with H2O2 to undergo non-enzymatic decarboxylation [24], to generate molecules capable of regulating the respiratory chain. Among the most biologically relevant of such reactions are the conversions of pyruvate → acetate, α-ketoglutarate → succinate, and oxaloacetate (OAA) → malonate [24]. Notably, both OAA and malonate are competitive inhibitors of complex II, but with very different inhibitory properties. Thus, we hypothesized that OAA may be converted to malonate during IPC, leading to reversible complex II inhibition. In addition we hypothesized that malonate inhibition of complex II would regulate mKATP channel activity. In this investigation, isolated cardiac mitochondria were studied to elucidate a potential novel mechanism of mKATP channel regulation in the context of IPC.
Materials and Methods
Male Sprague-Dawley rats of 200-225 g body mass were purchased from Harlan (Indianapolis, IN) and housed on a 12 hr. light/dark cycle with food and water available ad libitum. All procedures were performed in accordance with the US National Institutes of Health “Guide for the care and use of laboratory animals”. Unless otherwise stated, all chemicals were of the highest grade obtainable, from Sigma (St. Louis MO).
Isolated rat hearts were retrograde perfused using the Langendorff method, in constant-flow mode (12 ml/min), as previously described [10,71]. Hearts were subjected to one of the following global ischemia or IPC protocols: 1) Control: 1 hr. normoxic perfusion. 2) Ischemia: 25 min. normoxic perfusion plus 25 min. ischemia. 3) IPC alone: 3 cycles of 5 min. ischemia followed by 5 min. reperfusion. 4) IPC + ischemia: IPC as in protocol 3, followed by 25 min. ischemia.
Mitochondria were isolated either from fresh rat hearts or following the above protocols by differential centrifugation in sucrose-based buffer as previously described [13,50,71], with slight modifications to ensure rapid isolation (<45 min. total), to preserve mKATP activity which deteriorated rapidly, despite storing mitochondria on ice (see below). Protein was determined using the Folin-phenol method [46]. Sub-mitochondrial particles (SMPs) were prepared from frozen isolated mitochondria by diluting to 15mg/ml in SMP buffer (20 mM MOPS, pH 7.2, 4°C), followed by 7 sonication cycles of 30 s. on / 30 s. off. The suspension was centrifuged at 16,000 × g for 10 min, the pellet discarded, the centrifugation repeated, and the resulting supernatant then centrifuged at 100,000 × g for 45 min. The SMP pellet was resuspended in 0.5ml buffer [13].
Complex II enzymatic activity was determined spectrophotometrically as the rate of succinate-driven, co-enzyme Q2-linked reduction of dichlorophenolindophenol (DCPIP) [71]. Mitochondria or SMPs were incubated in phosphate buffer (pH 7.4) containing 40 μM DCPIP, 1 mM KCN, 10 μM rotenone, and 50 μM co-enzyme Q2. The rate of reduction of DCPIP to DCPIPH2 was followed at 600 nM (ε = 21,000 M-1). Varying amounts of succinate and inhibitors were used, as detailed in the figures and legends. At the end of each run thenoyltrifluoroacetone (1 mM) was added and the residual TTFA-insensitive rate subtracted.
HPLC analysis of α-keto acids and TCA cycle intermediates was performed essentially as described [24]. Heart mitochondria (5 mg/ml) were set to respire on 5 mM malate and 10 mM succinate, in 125 mM KCl, 10 mM HEPES, 1.5 mM phosphate, pH 7.4, 37°C, with continuous stirring in open cuvets for 1 hr. Additions of H2O2 or glutathione (GSH) were made during the incubations, then mitochondria were added to HClO4 (3% final) and pelleted by centrifugation. Supernatants were analyzed using two Aminex HPX-87H columns (300 × 7.8 mm; Bio-Rad) in series, at a flow rate of 0.7 ml/min. with 10 mM H2SO4 mobile phase, at 35°C, with detection at 210 nm. Calibration curves were constructed using standard solutions of authentic acids, and internal standards were spiked into all samples.
mKATP channel activity was measured by the commonly used swelling/light-scatter assay [7,26,27,33,42,59], at 520 nm using a Beckman DU800 spectrophotometer. There has been much debate regarding the existence of mKATP, and several laboratories have used a light-scatter/swelling assay to characterize this channel [7,22,26,42,59]. However, other groups have not been able to repeat these experiments [18]. During the course of initiating these studies, we found that a critical factor determining the ability to measure mKATP channel activity is freshness of mitochondria. Typical mitochondrial isolation protocols consist of either 3 high speed centrifugation steps (7 – 10,000 × g), or gradients of Percoll™, all followed by ~1 hr. incubation of the final mitochondrial pellet on ice, during which time the protein assay is performed. We found that such mitochondria were devoid of mKATP channel activity in the swelling assay. Modifications to our standard method determined via empirical practice, led to our ability to measure this channel. Firstly, we omitted one high-speed spin from the mitochondrial isolation method, thereby reducing the total time by ~15 min., and we compensated for any effect of this on mitochondrial purity by discarding more material at each step and sacrificing mitochondrial yield. Secondly, we performed a very fast protein assay based on optical density, and began to measure mKATP channel activity as soon as the mitochondria were ready, and then returned at a later time point to perform the standard quantitative protein assay [46]. Despite these modifications, we routinely found that mKATP channel activity deteriorated within 1.5 hr. of beginning experiments.
Mitochondria (0.25 mg/ml) were rapidly added to a stirred cuvette containing mKATP buffer (100 mM KCl, 10 mM HEPES, 2 mM succinate, 2 mM MgCl2, 2 mM KH2PO4, 1 μg/ml oligomycin, pH 7.2 at 37°C). In some experiments, as indicated by figure legends, K+ in the buffer was replaced with Na+, or complex-I linked substrates (2 mM glutamate + 2 mM malate) were used instead of succinate.
Results
While it has previously been shown that complex II linked respiration is somewhat decreased following IPC [58], it was not known if this was due to a de-facto inhibition of complex II itself, or possibly due to effects on substrate transport into mitochondria. In Figure 1A, mitochondria were rapidly isolated from hearts that underwent control perfusion or IPC alone protocol (3 × 5 min. ischemia interspersed with 5 min. reperfusion), freeze-thawed, and then subjected to complex II activity assays. The data show that IPC mitochondria exhibited a small (12%) but significant decrease in complex II activity, which thus cannot be attributed to inhibition of substrate transport into mitochondria. In Figure 1B, control mitochondria were incubated in the standard complex II activity assay (see methods), in the absence or presence of 1 mM ATP. This concentration of ATP, which is typically used to inhibit mKATP channel activity, caused a stimulation of complex II activity. This result is consistent with previous reports that ATP can allosterically activate complex II [32], although it was felt to be important to reconstitute this finding in the current model system (rat heart mitochondria).
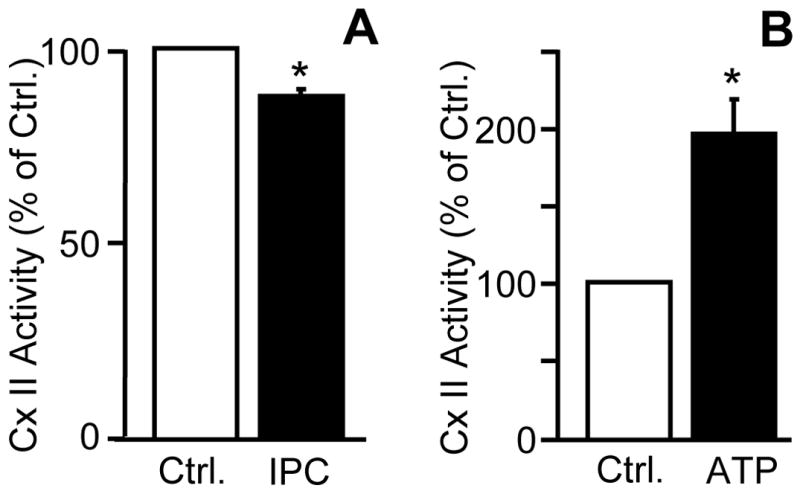
(A): Hearts were subjected to Langendorff perfusion protocols as detailed in methods and mitochondria were isolated from the perfused hearts. Freeze-thawed mitochondria were and subjected to a complex II activity assay which was initiated by the addition of 20 mM succinate. Control activity was 87 ± 3.6 nmol/min/unit citrate synthase (B): Intact, isolated mitochondria were subjected to a complex II activity assay, in the absence or presence of 1mM ATP. Control activity was 82 ± 12.2 nmol/min/unit citrate synthase. Data are normalized to percent of control. All data are means ± SEM, N≥3. *p<0.05 vs control.
The mechanism of complex II inhibition in IPC mitochondria is not known, but since IPC leads to the generation of ROS such as H2O2 [72,73] and it is known that H2O2 can non-enzymatically decarboxylate metabolic α-keto acids [24], we hypothesized that such reactions may occur in IPC, thereby generating molecules capable of regulating complex II activity. However, initial experiments investigating metabolic acid profiles in mitochondria isolated from IPC hearts were not successful (due to possible redistribution of metabolite pools during the isolation process). Similarly, whole-heart extracts were not compatible with the HPLC method (too many additional species absorbing in the UV range). Thus, a model system was established, in which isolated mitochondria were incubated with substrates, and various concentrations of oxidants and antioxidants were added, to mimic IPC. The data in Figure 2A show that the HPLC method developed is capable of detecting malonate in the mitochondrial matrix. While the formation of malonate from the oxidative decarboxylation of OAA has previously been shown only in free solution, and hypothesized to occur inside mitochondria [24], these data show for the first time that this reaction actually does occur inside mitochondria. While OAA was not reliably detectable by HPLC (owing to its rapid decomposition to pyruvate in free solution, unpublished observations), we could clearly observe that under oxidative conditions (100 or 300 μM H2O2) the levels of another α-keto acid, α-ketoglutarate, were decreased (Figure 2B). Furthermore, addition of 1 mM GSH to the incubations abrogated the effect of H2O2. In addition, malonate levels (presumably from the oxidative decarboxylation of OAA) increased in the presence of H2O2, and this effect was also blocked by GSH. Notably, the baseline level of malonate (i.e. no H2O2 added) was also decreased by a small but significant amount in the presence of GSH, suggesting that malonate may be generated under normal mitochondrial functional conditions, possibly driven by endogenously generated H2O2 from the respiratory chain.
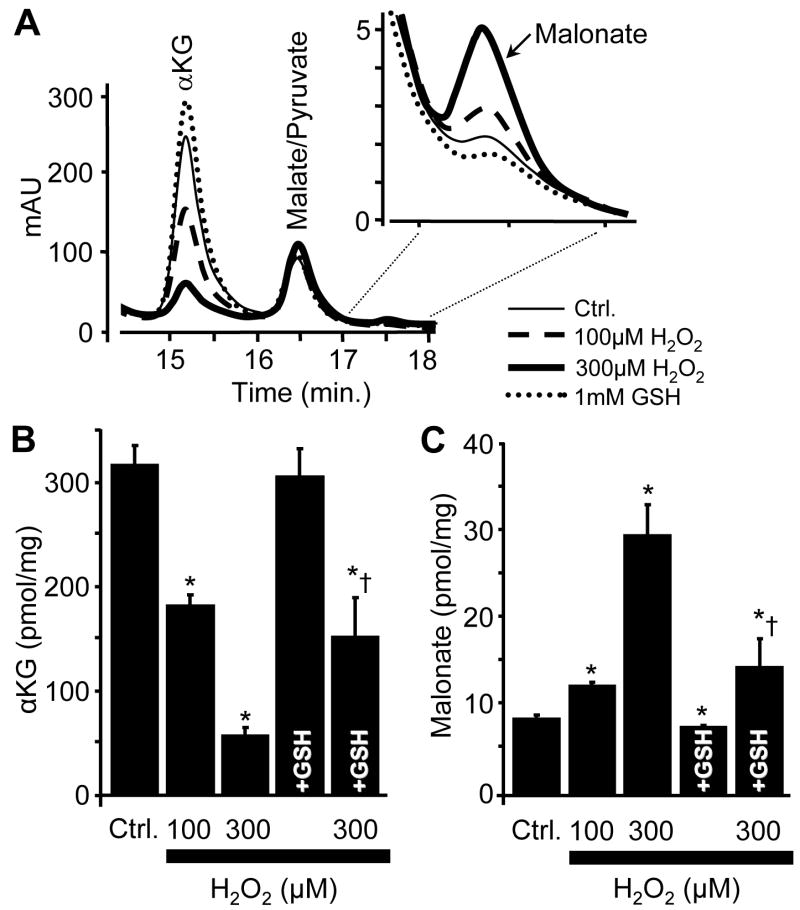
(A): The HPLC elution profiles (A210) of TCA cycle intermediates in mitochondria from 15-18 min. in the presence of 0 mM H2O2 (ctrl.), 100 μM H2O2, 300 μM H2O2, or 1 mM GSH. Peaks correspond to the following: α-KG, 14.8 min.; malate/pyruvate, 16.1 min.; malonate, 17.3 min. (B & C): Quantification of the elution profiles using a standard curve and an internal standard for α-KG and malonate. All data are means ± SEM, N≥4. *p<0.05 vs ctrl. †p<0.05 vs 300 μM H2O2.
Although both OAA and malonate are inhibitors of complex II [19,31,40], they exhibit very different kinetic effects on the enzyme, and thus it is possible that the ROS-driven inter-conversion of OAA to malonate may change the profile of complex II regulation during IPC. Furthermore, it is known that malonate and OAA may compete for binding on complex II, to the effect that malonate (the weaker inhibitor) may be able to displace OAA (the stronger) [40]. Thus, it was decided to investigate the interactions of various ROS and TCA-cycle acids with complex II activity. SMPs were used as a model system since they are free of matrix enzymes and transport functions, and we have found that dose responses to OAA were significantly affected by uptake of the inhibitor across the mitochondrial membrane (data not shown). In Figure 3A, SMPs were incubated with 2.5 mM succinate to drive complex II, followed by addition of either, malonate, OAA, H2O2, or OAA plus H2O2. The data show that H2O2 alone only caused mild inhibition of complex II activity, and was without any significant effect at concentrations below 35μM. By contrast, OAA inhibited complex II with an IC50 of ~18 μM. Malonate was not as potent as OAA, inhibiting complex II with an IC50 of ~40 μM. Most notably though, is the effect of adding OAA and H2O2 together. Far from the expected result (an additive inhibitory effect), this combination of reagents led to a weaker level of inhibition than seen for OAA alone. In-fact, the profile of complex II inhibition seen in the presence of OAA plus H2O2 resembles that of malonate more than it does that of OAA alone. These data suggest that H2O2 is driving the oxidative decarboxylation of OAA to malonate, resulting in a malonate-like inhibition profile.
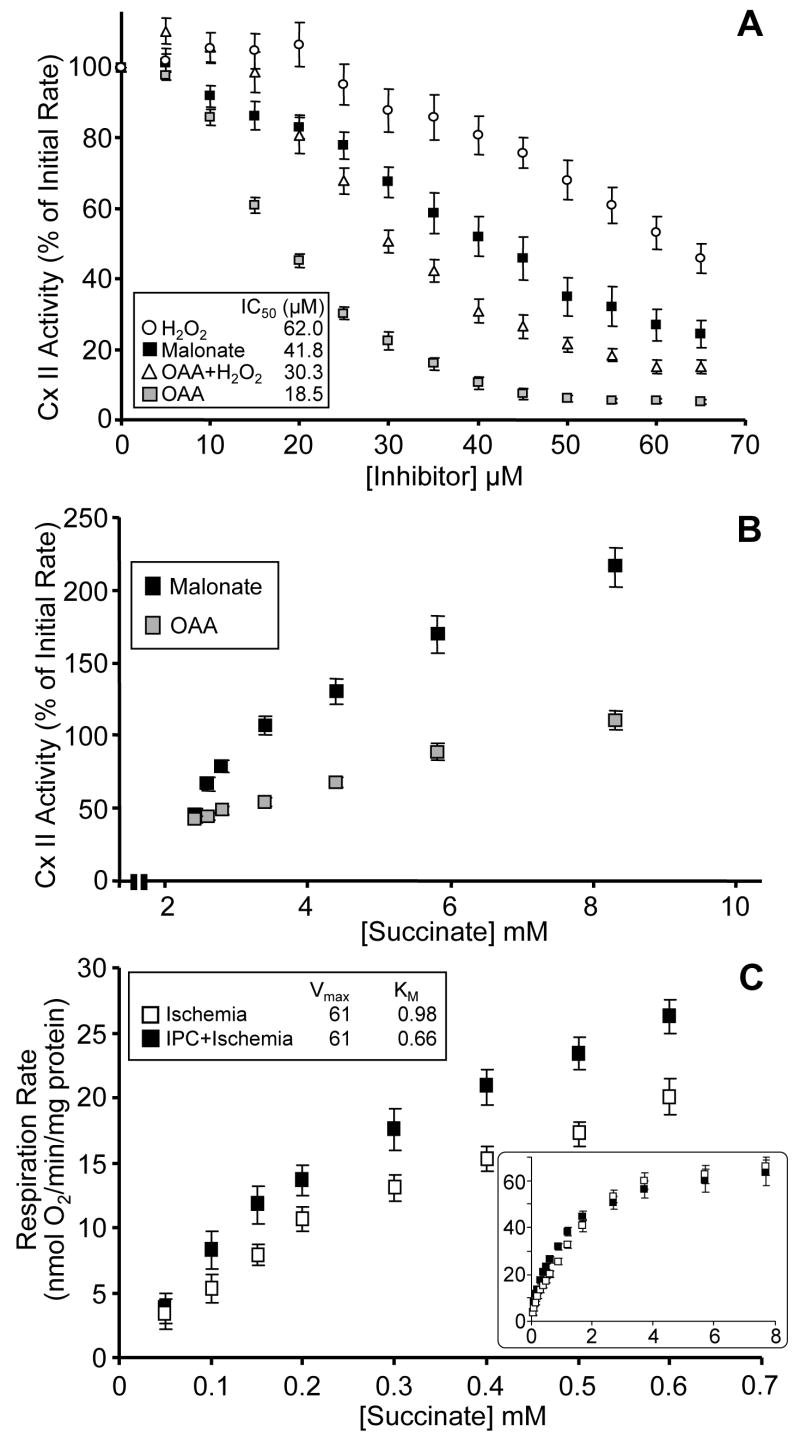
(A): Titration of complex II activity was determined using SMPs. The initial rate (100%) was set by adding 2.5 mM succinate (apparent KM, determined elsewhere). Gray squares, OAA; open triangles, OAA + H2O2; black squares, malonate; open circles, H2O2. Inset shows values of IC50 for each inhibitor. (B): The inhibition and recovery of complex II activity. Initial rate (100%) of complex II activity in SMPs, was inhibited to ~50% with either malonate (black squares) or OAA (gray squares). Activity was then recovered by titrating back in succinate, to remove the competitive inhibitors. (C): Oxygen consumption (nmols O2/min/mg protein) utilizing succinate, measured using a Clark-type oxygen electrode, was determined in mitochondria isolated from Langendorff perfused hearts subjected to either ischemia alone (open squares), or ischemia with prior IPC (filled squares). Upper inset shows values of Vmax (nmols O2/min/mg) and KM (mM succinate). Lower inset is the concentration of succinate from 0 to 8 mM. All data are means ± SEM; N≥4.
In the next series of experiments, SMPs were incubated with 2.5 mM succinate, followed by titrating in OAA or malonate to concentrations that elicited 50% inhibition of complex II activity (i.e. their IC50 values). Succinate was then titrated back in, up to 8 mM, and the reversal of complex II inhibition observed. Consistent with OAA being a more tight-binding inhibitor than malonate, the OAA-inhibited complex II did not recover as fully as malonate-inhibited complex II (Figure 3B). The apparent recovery of malonate-inhibited complex II activity to a value greater than 100% is due to the use of higher concentrations of succinate in recovery titrations than present during initial inhibition titrations.
Following ischemia, the recovery of respiratory chain activity upon reperfusion is essential to allow Ox-Phos to generate ATP for contraction of the heart. Thus, it could be hypothesized that the mode of complex II inhibition (i.e. OAA- or malonate-mediated) could impact on the recovery of complex II activity during reperfusion. To test this hypothesis, mitochondria were isolated from hearts subjected to ischemia, or ischemia with prior IPC, and their succinate-titration profiles were determined. The data in Figure 3C show that at low concentrations of succinate (<1 mM), mitochondria from the hearts subjected to IPC exhibit a faster respiration rate. Put differently, the apparent kM for succinate of complex II-linked respiration, is left shifted (decreased) in the IPC hearts. Complex II is inhibited during ischemia by OAA, which accumulates due to stoppage of the TCA cycle at citrate synthase (no entry of substrates at acetyl-CoA). Thus, the left-shifted succinate titration curve in the IPC hearts suggests the mode of inhibition may be shifted to a more malonate-like profile. It is postulated that this malonate generation could be driven by H2O2 generated during IPC. Notably, in contrast with previous findings [58], higher concentrations of succinate elicited almost identical respiration rates in mitochondria from ischemia vs. IPC + ischemia hearts. It is postulated that differences in succinate titration and delivery may be responsible for these contrasting results. Indeed, most mitochondrial studies use bolus high concentrations of substrates (e.g. in oxygen electrode incubations) and it is suggested that subtle variations in complex activities such as seen here may have been overlooked. These data highlight the importance of substrate availability in determining mitochondrial function, and suggest that adding saturating concentrations of substrates to isolated mitochondrial incubations may mask a variety of critical information.
As discussed in the introduction, a substantial pharmacologic overlap exists between the mKATP channel and complex II, and thus our next series of experiments sought to characterize the possible link between the endogenous complex II inhibitor malonate and the mKATP channel, in IPC.
The data in Figure 4A demonstrate the successful reconstitution of the mKATP channel activity swelling assay in this laboratory. Mitochondrial swelling in K+ based buffer [22] was inhibited by ATP, and this inhibition was reversed by DZX (Figure 4B, filled bars). Notably, only 10 μM DZX was required; a concentration that does not inhibit complex II [20,65]. The effect of DZX was reversed by the mKATP channel inhibitor 5-hydroxydecanoate (5HD), and furthermore the repetition of these incubations in a medium in which all K+ was replaced with Na+, resulted in loss of all pharmacologic effects (Figure 4B, open bars). The non-specific KATP channel blocker glyburide gave the same effects as DZX. Also, it is known that the mKATP channel is redox-sensitive [22], and its opening can be inhibited by antioxidants such as MPG. The mKATP channel activity indicated herein was also inhibited by MPG (Figure 4B). While the specificity of some individual mKATP channel modulators (e.g. DZX, 5HD) has recently been questioned [34], we believe that this complex combination of pharmacologic agents (ATP/DZX/Glyburide/5HD/MPG) is a reliable assay of mKATP channel function. Cromakalim and pinacidil also modulated mKATP channel activity in this assay (data not shown).
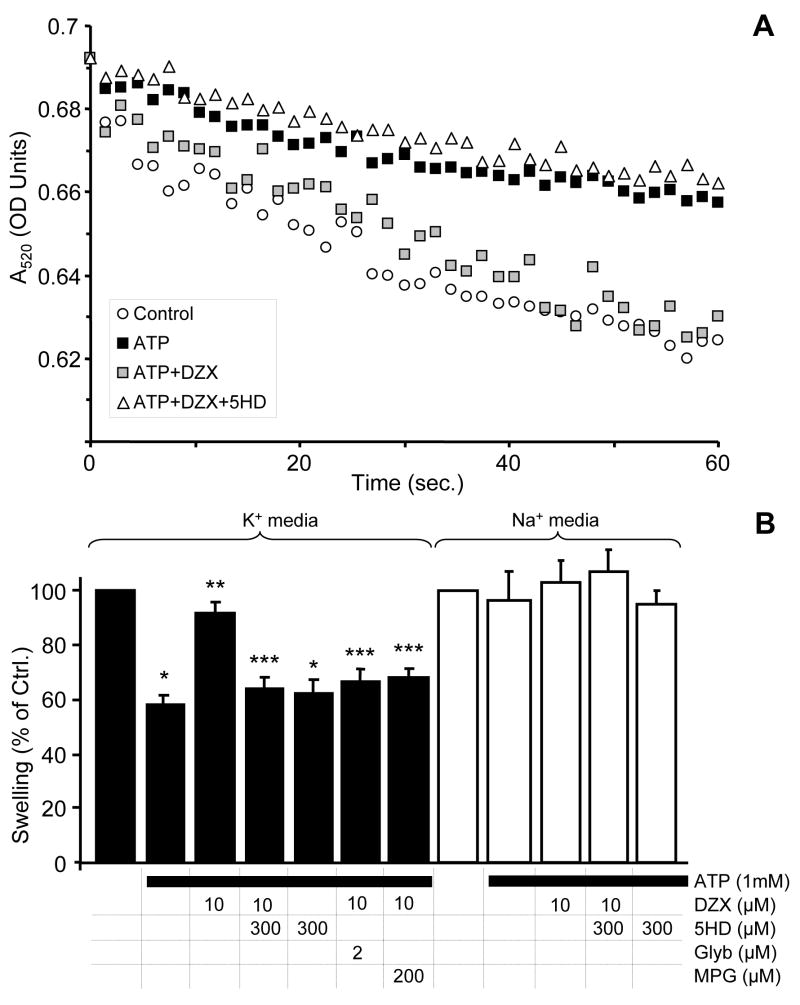
(A): Osmotic swelling assay for determination of the mKATP channel activity. Representative swelling traces of mitochondria (~0.25 mg/ml) in K+ media supplied with 2 mM succinate. Where indicated, 1 mM ATP, 10 μM DZX, or 300 μM 5HD were present in the media. (B): Magnitude of swelling, relative to control, as determined by a decrease in absorbance after 0.2 min. incubation in K+ media (filled bars) or Na+ media (open bars). Control swelling after 0.2 min resulted in a decrease in A520 of 0.0210 ± 0.001 OD units in K+ media and 0.0200 ± 0.002 OD units in Na+ media. Experimental conditions are listed below the x-axis. Data are means ± SEM *p<0.05 vs. control, **p<0.05 vs. ATP, ***p<0.05 vs. ATP+DZX; N≥4.
Having established a reliable mKATP channel assay, we next sought to examine the effects of malonate on mKATP channel activity. The results in Figure 5A and 5B (filled bars) show that malonate (25 – 100 μM) was able to mimic and surpass the effect of DZX, leading to activation of swelling which was inhibited by 5HD. The pharmacological effects of malonate-induced mKATP channel activity were both inhibited by glyburide and MPG, and were lost in a Na+-based media (data not shown).

(A): Representative swelling traces of mitochondria (~0.25 mg/ml) in K+ media supplied with 2 mM succinate. Where indicated, 1 mM ATP, 100 μM malonate, or 300 μM 5HD were present in the media. (B): Magnitude of swelling, relative to control, as determined by a decrease in absorbance after 0.2 min. incubation in K+ media supplied with 2 mM succinate (filled bars) or 2 mM glutamate + 2 mM malate (open bars). Control swelling supplied with glutamate + malate resulted in a decrease in A520 of 0.0153 ± 0.001 OD units. Experimental conditions are listed below the x-axis. *p<0.05 vs. control, **p<0.05 vs. ATP, ***p<0.05 vs. ATP + DZX or ATP + malonate; N≥4.
Since malonate is a competitive inhibitor of complex II, the concentrations of succinate, malonate and complex II itself, determine the degree of inhibition. Utilizing the same conditions as in the mKATP swelling assay, the concentrations of malonate which activated mKATP activity, exhibited little or no effect on complex II activity (100 μM malonate ~20% inhibition, data not shown). Note that the inhibition profile for malonate seen in Figure 3A is different, as those data were obtained in SMPs.
Perhaps the most notable property of malonate stimulation of mKATP activity was that swelling also occurred in mitochondria respiring on complex I-linked substrates (glutamate plus malate, Figure 5B, open bars). These data indicate that malonate may have an effect on mKATP channel activity that is independent of effects complex II activity. This is in agreement with the mismatch in dose-responses to malonate, of mKATP channel opening vs. complex II inhibiton.
Discussion
In the current study, we sought to investigate in more detail the complex inter-relationships between complex II, the mKATP channel, and ROS, all within the context of IPC. Both complex II inhibitors [53,61] and mKATP agonists [4,30,47] are protective against IR injury, and the pharmacological overlap between complex II and the mKATP channel implicates complex II as a component or regulator of the channel. For example, the mKATP agonist DZX [15] inhibits complex II [20,65] and the administration of the complex II inhibitor malonate can mimic DZX protection and decrease ROS [54]. Furthermore, reconstitution experiments with complex II, along with at least four other mitochondrial proteins, reproduced the K+ transporting capabilities of mKATP [3]. These investigations suggest that the inhibition of complex II is a potential modulator of mKATP activity. Additionally, another link between complex II modulation and mKATP activity can be demonstrated by the use of flavoprotein fluorescence as a measure of channel activity, albeit under very specific conditions [45,64]. The main source of flavoprotein fluorescence in cells is attributed to the FAD associated with TCA cycle dehydrogenases, such as complex II [32]. Overall, the emerging consensus from this and other studies is that complex II and mKATP are inversely related – i.e. inhibition of complex II is correlated with channel opening, while activation of complex II is correlated with channel closing. The fact that we herein observed effects of mKATP channel regulators on complex II, in an experimental system without mKATP channel activity (SMPs and frozen mitochondria), suggests that complex II lies upstream of the channel, not vice-versa.
In this study, the detection of endogenous complex II inhibitors in conditions that mimic IPC (Figure 2) supports the concept of an overlap in cardioprotection afforded by complex II inhibition and mKATP opening. The IPC mediated inhibition of complex II activity is consistent with the results of Pasdois et al [58], who additionally showed that the IPC-mediated inhibition is reversed post-reperfusion. Investigations to date have supported the hypothesis that reversible inhibition of the respiratory chain is protective in IR injury [12,14] and this inhibition can be mediated by IPC [13,49,53,58,62]; however, the endogenous generation of complex II inhibitors in IPC has been overlooked. The cardioprotective effects of IPC-mediated complex I inhibition have been previously demonstrated [13,49], and we hypothesize that this effect could be complemented by complex II inhibition. Mitochondria are a source of ROS [11], and oxidative conditions associated with IPC [72] can non-enzymatically decarboxylate α-keto acids in TCA cycle [24], resulting in the formation of the complex II inhibitor, malonate (Figure 2). The malonate detected herein originated within the mitochondrial matrix and not in the external medium, since mitochondria were supplemented with only malate and succinate as substrates, and thus any malonate must have originated from OAA generated by the TCA cycle. Assuming a typical heart mitochondrial matrix volume of 0.65 μl per mg protein [50], 20 pmols malonate per mg equates to an intra-mitochondrial concentration of 30 μM. Thus, while the absolute amounts of malonate generated in these in-vitro incubations are low, they may still be of physiological relevance inside the mitochondrial matrix. The observation that malonate-mediated complex II inhibition is more easily reversed following reintroduction of succinate (Figs. 3B & 3C) is consistent with the importance of inhibition reversal, at reperfusion.
The effects of malonate are not limited to complex II, and a swelling assay was used to investigate the effects of malonate on mKATP channel activity. Measuring the matrix volume as a means of monitoring ion transport in mitochondria is a commonly used technique [9] and has played a central role in the investigation of the mKATP channel [7,26,27,33,39,42,52,59,70]. Utilizing this technique, malonate caused K+-dependent swelling that was prevented by 5HD, glyburide, and MPG [22]. The uptake of K+ depends on membrane potential, and despite the use of succinate to energize mitochondria and generate the membrane potential, the complex II competitive inhibitor malonate increases swelling. Thus, the effect of malonate cannot be attributed to its effects on mitochondrial bioenergetic function.
The K+-dependent swelling was also still present when mitochondria were energized with complex I linked substrates (glutamate and malate), thus suggesting complex II does not need to be active to modulate the mKATP. This data supports the hypothesis that complex II is involved in the K+-mediated swelling of mitochondria, but the exact nature of this interaction (i.e. whether complex II is actually part of the channel, or just regulates the channel activity) is not known at this stage. Definitive conclusions can only be made once the components of the mKATP are known. It is possible that complex II may indirectly regulate the channel through changes in the mitochondrial redox state or simply the channel itself may contain a malonate binding site; however, the observation of K+-dependent swelling with complex I linked substrates (Figs. 5A & 5B) argues for a more direct role of complex II in the regulation of the mKATP.
The use of DZX to study the mKATP arises from the specificity of this compound for the mKATP, as opposed to the sarcolemmal KATP. This specificity might arise from the interaction with complex II, although it should be noted that the concentration of DZX used in these experiments (10 μM) is below that found to inhibit complex II [20,65]. Similarly, the concentrations of malonate which mimicked the ability of diazoxide to activate mKATP, also do not inhibit complex II activity. At a time when the identity of the channel remains unknown, a mechanistic and pharmacological link between complex II modulation and mKATP activation provides a novel means of activating the mKATP channel. Insight into the temporal relationship between mKATP channel opening and complex II inhibition is also provided, since both DZX and IPC mediated inhibition of complex II persisted in experimental systems in which mKATP channel activity was absent (i.e. frozen mitochondria and SMPs). Thus, mKATP channel opening does not appear to be a pre-requisite for complex II inhibition. The reverse may still be possible however.
In summary, we have herein identified that the endogenous complex II inhibitor malonate is generated under conditions mimicking IPC. The ability of malonate to open mKATP channels in a manner similar to DZX, and regardless of its effects on complex II activity, suggests the possible therapeutic use of strategies to augment malonate levels in cardiac mitochondria, to elicit cardioprotection.
Acknowledgments
We thank Sergiy Nadtochiy, Kurt Roser and Leif Olsen (University of Rochester) for their technical assistance, and Lindsay Burwell and David Hoffman (University of Rochester) for valuable discussions of the manuscript. This work was funded by a grant to PSB from the National Institutes of Health (RO1-HL071158).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bbabio.2008.03.025
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2507763?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.bbabio.2008.03.025
Article citations
Ion channel-mediated mitochondrial volume regulation and its relationship with mitochondrial dynamics.
Channels (Austin), 18(1):2335467, 28 Mar 2024
Cited by: 2 articles | PMID: 38546173 | PMCID: PMC10984129
Review Free full text in Europe PMC
Connexin 43 modulates reverse electron transfer in cardiac mitochondria from inducible knock-out Cx43<sup>Cre-ER(T)/fl</sup> mice by altering the coenzyme Q pool.
Basic Res Cardiol, 119(4):673-689, 09 May 2024
Cited by: 0 articles | PMID: 38724619
Metabolic Regulation of Copper Toxicity during Marine Mussel Embryogenesis.
Metabolites, 13(7):838, 11 Jul 2023
Cited by: 1 article | PMID: 37512545 | PMCID: PMC10385052
Targeting the Host Mitochondria as a Novel Human Cytomegalovirus Antiviral Strategy.
Viruses, 15(5):1083, 28 Apr 2023
Cited by: 2 articles | PMID: 37243170 | PMCID: PMC10223864
Review Free full text in Europe PMC
Mitochondrial function and Aβ in Alzheimer's disease postmortem brain.
Neurobiol Dis, 171:105781, 03 Jun 2022
Cited by: 13 articles | PMID: 35667615 | PMCID: PMC9329272
Go to all (71) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels.
Basic Res Cardiol, 104(2):121-129, 26 Feb 2009
Cited by: 76 articles | PMID: 19242645 | PMCID: PMC2776710
Is preconditioning by oxytocin administration mediated by iNOS and/or mitochondrial K(ATP) channel activation in the in vivo anesthetized rabbit heart?
Life Sci, 90(19-20):763-769, 14 Apr 2012
Cited by: 18 articles | PMID: 22525371
MitoK(ATP)-dependent changes in mitochondrial volume and in complex II activity during ischemic and pharmacological preconditioning of Langendorff-perfused rat heart.
J Bioenerg Biomembr, 38(2):101-112, 01 Apr 2006
Cited by: 13 articles | PMID: 17031549
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.
Biochem J, 474(12):2067-2094, 09 Jun 2017
Cited by: 23 articles | PMID: 28600454 | PMCID: PMC5568769
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (4)
Grant ID: R01 HL071158
Grant ID: R01-HL071158
Grant ID: R01 HL071158-05
Grant ID: R01 HL071158-06A1





