Abstract
Free full text

BMP Signaling Dynamics in Embryonic Orofacial Tissue
Abstract
The bone morphogenetic protein (BMP) family represents a class of signaling molecules, that plays key roles in morphogenesis, cell proliferation, survival and differentiation during normal development. Members of this family are essential for the development of the mammalian orofacial region where they regulate cell proliferation, extracellular matrix synthesis, and cellular differentiation. Perturbation of any of these processes results in orofacial clefting. Embryonic orofacial tissue expresses BMP mRNAs, their cognate proteins, and BMP-specific receptors in unique temporo-spatial patterns, suggesting functional roles in orofacial development. However, specific genes that function as downstream mediators of BMP action during orofacial ontogenesis have not been well defined. In the current study, elements of the Smad component of the BMP intracellular signaling system were identified and characterized in embryonic orofacial tissue and functional activation of the Smad pathway by BMP2 and BMP4 was demonstrated. BMP2 and BMP4-initiated Smad signaling in cells derived from embryonic orofacial tissue was found to result in: (1) phosphorylation of Smads 1 and 5; (2) nuclear translocation of Smads 1, 4, and 5; (3) binding of Smads 1, 4, and 5 to a consensus Smad binding element (SBE)-containing oligonucleotide; (4) transactivation of transfected reporter constructs, containing BMP-inducible Smad response elements; and (5) increased expression at transcriptional as well as translational levels of Id3 (endogenous gene containing BMP receptor-specific Smad response elements). Collectively, these data document the existence of a functional Smad-mediated BMP signaling system in cells of the developing murine orofacial region.
The bone morphogenetic proteins (BMPs) are multifunctional morphogens belonging to the transforming growth factor β (TGFβ) superfamily of signaling molecules. This family regulates a wide variety of developmental processes crucial for gastrulation, organogenesis, and pre- and post-natal growth (Jones et al., 1991; Hogan, 1996a; Anderson et al., 2002; Petryk et al., 2004). The BMPs were originally identified and isolated from demineralized bone matrix and characterized by their ability to induce ectopic bone formation in vivo (Wozney et al., 1988; Lyons et al., 1990; Rosen and Thies, 1992). Subsequently, these growth factors were found to be widely expressed in the vertebrate embryo and fetus (Lyons et al., 1990, 1991; Rosen and Thies, 1992) where they were shown to regulate diverse elements of development including patterning of mesoderm, neurogenesis, ossification, organogenesis, and tissue growth (Hogan, 1996a; Kishigami et al., 2004).
Members of the BMP family are known as either osteogenic proteins (OP), cartilage derived morphogenetic proteins (CDMP), or growth and differentiation factors (GDF). BMPs are further divided into subfamilies based on phylogenetic analysis and sequence homology (Hogan, 1996b). To date, over 20 known members of the BMP family have been identified (Kawabata et al., 1998). BMP signaling is initiated via growth factor interaction with the type I (ALK2, ALK3 or BMPRIA and ALK6 or BMPRIB) and type II (BRII, ActRIIA and ActRIIB) receptors (Nishitoh et al., 1996; von Bubnoff and Cho, 2001; Nohe et al., 2004). Genes encoding each of these receptor types are expressed in developing orofacial tissue (Mukhopadhyay et al., 2006a). The epithelia and mesenchyme of murine embryonic orofacial tissue express genes encoding BMP2, BMP3, BMP4, and BMP5 during secondary palate morphogenesis (Lu et al., 2000). Throughout this process, expression of these BMPs is highly dynamic and is maintained by developmental regulation along the anterior–posterior axis (Nie, 2005). Additionally, reduction in expression of BMP2, 3, 4, and 5 mRNAs (Lu et al., 2000), deletion of genes encoding BMP receptors such as Alk2 or BmprIa (Alk3) (Dudas et al., 2004; Liu et al., 2005), and deficiency of the type II BMP receptor, ActRII (Matzuk et al., 1995), are all associated with palatal clefting, signifying the importance of properly coordinated BMP signaling during orofacial development. BMP2-mediated mesenchymal cell proliferation appears to be crucial for normal palatal ontogenesis. Indeed, Nie (2005) demonstrated strong expression of Bmp2 in the mesenchyme adjacent to the palatal midline epithelium during palatal fusion. Mcpherron et al. (1999), reported abnormal palate development in mutant mice homozygous for the targeted deletion of Gdf11—implicating a critical role for GDF11 (BMP11) in palatogenesis. Further, expression of Bmp4 and Bmp2 in developing palatal mesenchyme requires the expression of the Msx1 homeobox gene (Zhang et al., 2002), a gene, that when mutated, is associated with cleft palate and tooth agenesis in humans (van den Boogaard et al., 2000). Indeed, transgenic expression of Bmp4 in Msx1−/− murine embryonic palatal mesenchyme rescues the cleft palate phenotype (Zhang et al., 2002). Facial clefting and exencephaly have also been reported in transgenic mice overexpressing the BMP-target gene Msx2 (Winograd et al., 1997), and in embryos in which the expression of Msx2 had been ectopically activated by BMP2 and 4 (Barlow and Francis-West, 1997). Collectively, these experimental findings emphasize the key roles played by members of the BMP subfamily in orofacial morphogenesis during development.
Upon activation by the appropriate BMP ligand, the type I and type II receptors interact to form hetero-oligomers, which initiate Smad-mediated intracellular signaling cascades regulating the transcription and expression of various target genes. BMP-induced receptor activation initiates two types of signaling pathways: (a) the canonical pathway involving activation of receptor-specific Smad 1, 5, and 8 (R-Smads), followed by formation of a complex including the common Smad, Smad 4, which translocates into the nucleus to regulate transcription of BMP target genes (Yamamoto et al., 1997; Hollnagel et al., 1999; Nohe et al., 2004) and (b) mitogen-activated protein kinase (MAPK) pathways including p38, c-jun-N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) pathways (Gallea et al., 2001; Zhao et al., 2002; Guicheux et al., 2003). In addition, BMPs are also known to activate PI3 kinase and PKC pathways (Kishigami and Mishina, 2005).
Once in the nucleus, BMP type I receptor-specific Smads (BR-Smads), Smad 1, 5, and 8, interact with DNA-binding proteins which, bind specific sequences, known as Smad-binding elements (SBEs; AGAC or GTCT sequence), in the promoters of BMP target genes (Ogata et al., 1993; Korchynskyi et al., 2003; for reviews Miyazawa et al., 2002; Nishimura et al., 2003; Miyazono et al., 2005). Promoters of the BMP target genes Xvent2B and Smad6, contain GCAT or GCCG motifs respectively, to which BMP R-Smads can only weakly bind and mutations in these SBEs lead to diminished transcriptional activity (Henningfeld et al., 2000; Ishida et al., 2000). The BMP-responsive region in the Id1 promoter possesses two critical motifs, that is, SBEs and GC-rich boxes (Katagiri et al., 2002; Korchynskyi and ten Dijke, 2002; Lopez-Rovira et al., 2002). Using these BMP-responsive sequences from the Id1 promoter, and cloning these upstream of a minimal promoter reporter, Korchynskyi and ten Dijke (2002) generated a BMP-specific transcriptional reporter (BRE-luc) that can be dose-dependently activated following BMP treatment (Korchynskyi and ten Dijke, 2002), and that was utilized in the present study. In this study, elements of the Smad component of the BMP intracellular signaling system were identified and characterized in cells derived from the embryonic orofacial region and functional activation of the BR-Smad pathway in these cells was demonstrated. BMP2- and BMP4-initiated Smad signaling was found to result in: (1) phosphorylation of Smads 1 and 5; (2) nuclear translocation of the Smads 1, 4, and 5 protein complex; (3) binding of Smads 1, 4, and 5 to a consensus SBE-containing oligonucleotide; (4) activation of a transfected reporter gene containing a BMP-inducible SBE; and (5) stimulation of Id3 (endogenous gene containing BMP-responsive SBEs).
Materials and Methods
Establishment of primary cultures of murine embryonic maxillary mesenchymal (MEMM) cells
ICR mice (Harlan Laboratories, Indianapolis, IN) were housed in a climate-controlled room at a temperature of 22°C with an alternating 12-h dark–light cycle and were provided access to food and water ad libitum. Mature male and female mice were mated overnight and the presence of a vaginal plug the following morning (day 0 of gestation) was considered as evidence of mating. Pregnant mice were euthanized on gestation day 13, embryos removed from pregnant dams and embryonic maxillary tissue including secondary palates was microdissected from the developing orofacial region in sterile, cold phosphate-buffered saline (PBS), as described earlier (Mukhopadhyay et al., 2006a). Tissue was dissociated with trypsin, and cells plated at a density of approximately 2 × 105 cells/100 mm culture dish in Opti-MEM (Invitrogen Life Technologies, Inc., Carlsbad, CA), and maintained at 37°C in an atmosphere of 95% air/5% CO2, with medium replaced every 48 h.
TaqMan® quantitative real-time PCR (QRT-PCR)
Embryonic orofacial tissue was dissected from gestational day (GD) 12, 13, or 14 embryos and total RNA isolated using the RNeasy Protect Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s recommendations. Total RNA from BMP2- or BMP4- or vehicle-treated MEMM cells was isolated as noted above. BMP2- or BMP4 treatment of MEMM cells was performed as described below under “BMP treatment of MEMM cells and determination of nuclear translocation of BR-Smad proteins.” The quality and quantity of extracted total RNAs were assessed by formaldehyde agarose gel electrophoresis and spectrophotometric UV absorbance at 260/280 nm, respectively. RNA was treated with DNase I in the presence of RNaseOUT (Invitrogen Life Technologies, Inc.) to remove contaminating DNA before cDNA synthesis. cDNA was synthesized from total RNA using random hexamer primers and Superscript II reverse transcriptase (Invitrogen Life Technologies, Inc.). QRT-PCR analysis was performed on a TaqMan® ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Primers and their corresponding fluorescence probes (Assays-on-Demand) were purchased from Applied Biosystems. For each gene analyzed, both forward and reverse primers were used at a concentration of 900 nM and the final fluorescent probe concentration was 200 nM. The PCR reaction was performed in a total volume of 25 μl containing 0.2 mM each of dATP, dCTP, and dGTP, 0.4 mM dUTP, 0.625 unit of Amplitaq Gold (Applied Biosystems) and 2 μl (8.0 ng) of cDNA template. Cycling parameters were: 50°C for 2 min for probe and primer activation, 95°C for 10 min for denaturation of DNA strands, 40 cycles of denaturation at 95°C for 15 sec, and primer extension at 60°C for 1 min. For each reaction, a parallel reaction lacking template served as a negative control. Raw data were acquired and processed with ABI Sequence Detection System software, version 1.0 (Applied Biosystems, Foster city, CA). mRNA amounts for each gene were normalized to GAPDH mRNA present in each sample.
Western blot analysis of Smad and Id proteins
Total protein was extracted from embryonic orofacial tissue from gestational day (GD) 12, 13, or 14 embryos and steady state levels of Smad proteins determined by Western (immuno) blotting as previously described (Kusek et al., 2000). Protein was denatured by boiling in 2X Laemmle sample buffer (Laemmle, 1970) and separated (50 μg/lane) by SDS–PAGE electrophoresis at 125 V using 8% polyacrylamide Tris–glycine gels (Novex, San Diego, CA) followed by electrophoretic transfer (30 V for 2 h) to PVDF membranes. To visualize proteins, and ensure the efficiency of transfer, gels were stained with Coomassie blue (Sasse and Gallagher, 1991) and membranes with 0.1% fast green, respectively. Blots were blocked by incubation in 5% non-fat dry milk in TBST buffer (50 mM Tris, pH 7.6; 150 mM NaCl; 0.1% Tween-20) for 1 h at room temperature. Antibodies (see below) were prepared by diluting in blocking solution and blots incubated with primary antibody for 2 h at room temperature, washed extensively, and then incubated for 0.5 h at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibody. Immune complexes were detected using the ECL-Plus™ chemiluminescent detection system (Amersham Pharmacia Biotech, Piscataway, NJ) according to manufacturer’s instructions. Molecular weights of proteins on each gel were estimated by reference to a MagicMark™ (20–200 kDa) protein ladder (Invitrogen Life Technologies, Inc.). A methodological control, wherein buffer was utilized in place of primary antibody, was utilized to distinguish between specific immunoreactive bands and non-specific bands due to interaction of the secondary antibody with endogenous proteins. Immunoblots of embryonic orofacial tissue were replicated with similar results utilizing a minimum of three independent sets of tissue samples. To confirm equal loading of proteins, blots were stripped and reprobed for β-actin, a housekeeping protein. Commercially available primary antibodies that were utilized included: rabbit polyclonal Smad 1 IgG1, (Upstate Biotechnology, Lake Placid, NY; catalog #06-653) and Smad 5 IgG (Zymed Laboratories, San Francisco, CA; catalog #51–3700); rabbit polyclonal anti-phospho-Smad 1 IgG (Upstate Biotechnology; catalog #06-702) and anti-phospho-Smad 1/5 IgG (Zymed Laboratories; catalog #40-3300); rabbit polyclonal anti-Smad 4 IgG (Upstate Biotechnology; catalog #06–693); mouse monoclonal anti-actin IgG1 (Santa Cruz Biotechnology, Santa Cruz, CA; catalog #sc-8432); mouse monoclonal anti-Id3 IgG1 (BD Biosciences, San Diego, CA; catalog #556524). Secondary antibodies that were utilized included: horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology; catalog #sc-2004) or horseradish peroxidase-conjugated rabbit anti-mouse IgG1 (Zymed Laboratories; catalog #61–0120).
Densitometric analysis
Densitometric analyses of Smad, phospho-Smad, Id3 and β-actin protein bands were performed with Image J (version 1.38) software (Abramoff et al., 2004). The blots were scanned, analyzed by densitometry and the intensities of the β-actin bands were recorded and used as an internal control to correct for differences in sample loading. Densitometric data for each protein band was normalized to that of β-actin in that lane by subtracting the intensity value for the protein band from the corresponding intensity value for the β-actin band for each sample.
BMP treatment of MEMM cells and determination of nuclear translocation of BR-Smad proteins
MEMM cells were plated in 60 mm tissue culture dishes at an initial density of 1.2 × 105 cells/dish. Cells were grown to 75% confluence in complete medium, washed and equilibrated in serum-free medium (DME/F12 supplemented with 2 mM glutamine and antibiotics [100 units/ml penicillin G, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml Amphotericin B]), and treated with vehicle or recombinant BMP2 or BMP4 (R&D Systems, Minneapolis, MN) at a final concentration of 0.1, 1.0, 10.0, or 100.0 ng/ml for 2 h. Each experiment was conducted using cells derived from embryonic maxillary tissue from the developing orofacial region dissected from a single litter of embryos. A nuclear-enriched protein extract was prepared from MEMM cells using the NE-PER kit (Pierce, Rockford, IL) following the manufacturer’s recommendations. Steady state levels of BR-Smad proteins as well as that of the BMP target protein, Id3, were determined by Western blotting as described above.
DNA affinity purification assay (DAPA)
A DNA affinity purification assay, developed by Glass et al. (1987), was employed to detect protein–DNA interactions in cell extracts of MEMM cells. 5′-Biotinylated, double-stranded oligonucleotides (200 pmol) containing two copies of a consensus BR-Smad-binding, BMP responsive element (BRE) that contains Smad-binding elements (AGAC) and other critical sequence elements such as those containing a GGCGCC palindromic sequence flanked by two CAGC (GCTG) and two CGCC motifs [5′-CTCAGACCGTTAGACGCCAGGACGGGCTGTCAGGCTG GCGCCG -3′] (Korchynskyi and ten Dijke, 2002; Monteiro et al., 2004), were mixed for 3 h at 4°C with 100–200 μg of a cell extract (prepared as previously described in Kusek et al., 2000) of vehicle-, BMP2-, or BMP4-treated MEMM cells. A mutant sequence, 5′-CTCACAGCGTTACAGGGCAGGACGGCCTCTCAG CCTCGGGGCG-3′, in which the second and fourth nucleotide of each Smad-binding element and some more nucleotides in other critical Smad-binding regions (as noted above) were changed (underlined), was used to demonstrate specificity of Smad binding. Neutravidin–agarose (10 μl packed beads; UltraLink Immobilized Neutravidin Plus, Pierce) that was precleared via incubation with cell extract to reduce non-specific binding, was added and the incubation continued with mixing for an additional 3 h at 4°C. The beads were collected via centrifugation, and washed four times with ice-cold PBS containing 0.5% Triton X-100 and 5 mM EDTA. Proteins were eluted from the beads by the addition of 2X SDS sample loading buffer (Laemmle, 1970) followed by boiling for 5 min. Eluted proteins were analyzed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting using various BR-Smad antibodies as detailed above.
Transfection of MEMM cells with reporter constructs containing BMP-inducible, BR-Smad-binding elements
Primary cultures of MEMM cells were established at a density of 7 × 104 cells in 35 mm tissue culture dishes and grown for 4–5 days in complete medium to approximately 75% confluence. Cells were then transfected by the “Effectene Transfection Method” (Qiagen) with 1.0 μg of the BMP-responsive plasmid BRE-luc (Korchynskyi and ten Dijke, 2002) and 0.1 μg of the control plasmid pRLCMV (Promega Corp., Madison, WI). Cells were incubated in complete medium containing the transfection mixture for 24 h. The transfection mixture was removed, cells were washed, and then treated with either 25, 50, or 100 ng/ml BMP2 or BMP4, or 2 ng/ml TGFβ1 (all growth factors from R&D Systems) in complete medium for 24 h. Cells were then extracted in “Passive Lysis Buffer” provided with the “Dual Luciferase Reporter Assay System” (Promega Corp.) according to the method detailed by the manufacturer. Firefly luciferase activity (from BRE-luc and p3TP-lux) was assayed in cell extracts and expressed relative to Renilla luciferase activity, in order to control for variability in transfection efficiencies. The transient transfection experiment was performed three times with independent samples, and for each experiment every condition was assayed in triplicate with comparable results.
Results
Identification of BR-Smad mRNAs in extracts derived from murine embryonic orofacial tissue
Total RNA from murine embryonic orofacial tissue (days 12, 13, and 14 of gestation) was analyzed by TaqMan® QRT-PCR and found to contain significant levels of Smads 1 and 5 mRNA on each day of gestation examined (Table 1). The presence of Smad 4 mRNA in developing embryonic orofacial tissue has been previously reported (Greene et al., 2003). Comparison of the Ct values (Gibson et al., 1996) for each gene across the gestational period examined failed to reveal any statistically significant temporal changes in either Smad 1 or Smad 5 gene expression (Table 1). Ct value is representative of no less than three separate assays of unique RNA extracts from embryonic orofacial tissue from each of GD 12, 13, and 14.
TABLE 1
TaqMan® QRT-PCR analysis of Smad 1 and Smad 5 mRNA expression during murine orofacial morphogenesis
| Developmental stagea | Gene | Mean Ctb,c,d | Fold change (vs. GD 12)d,e |
|---|---|---|---|
| GD 12 | Smad 1 | 26.19 ± 0.03 | — |
| GD 13 | Smad 1 | 26.07 ± 0.02 | +1.04 ± 0.05 |
| GD 14 | Smad 1 | 26.64 ± 0.05 | +1.60 ± 0.03 |
| GD 12 | Smad 5 | 26.35 ± 0.01 | — |
| GD 13 | Smad 5 | 26.15 ± 0.03 | +1.10 ± 0.02 |
| GD 14 | Smad 5 | 26.70 ± 0.02 | −1.12 ± 0.03 |
Identification of BR-Smad proteins in extracts derived from murine embryonic orofacial tissue
Embryonic orofacial tissue (days 12, 13, and 14 of gestation) was examined for the steady state expression of Smad 1 and Smad 5 proteins by immunoblotting using Smad 1 (Upstate Biotechnology) and Smad 5 (Zymed Laboratories) polyclonal antibodies. Multiple (3) bands in the molecular weight range of 55–60 kDa were detected for Smad 1, and a single major band at ~58 kDa and a minor band at 60 kDa were detected for Smad 5 on immunoblots of embryonic orofacial tissue extracts from GD 12, 13, and 14 embryos (Fig. 1a,b). The presence of multiple bands for Smads 1 and 5 may be explained by the existence of various known, modified forms (e.g., sumoylated, phosphorylated, etc.) of Smad proteins. Phosphorylated forms of Smad 1 and Smad 5 proteins were also detected on immunoblots of orofacial tissue extracts from GD 12, 13, and 14 embryos (Fig. 1c,d), using rabbit polyclonal anti-phospho-Smad 1 (Zymed Laboratories) and anti-phospho-Smad 1/5 (Upstate Biotechnology) antibodies. Both of these antibodies detected a major 58 kDa band and a minor 60 kDa band corresponding to phospho-Smad 1 and phospho-Smad 5 proteins, respectively. To confirm equal loading of proteins, blots were stripped and reprobed for β-actin, a housekeeping protein. As shown in Figure 1e (lower part), the abundance of β-actin in the three lanes of the blot was similar. These results indicate that both Smad 1 and Smad 5, as well as their phosphorylated forms, are expressed in embryonic orofacial tissue in vivo during gestational days 12–14, the critical period of palatal ontogenesis.
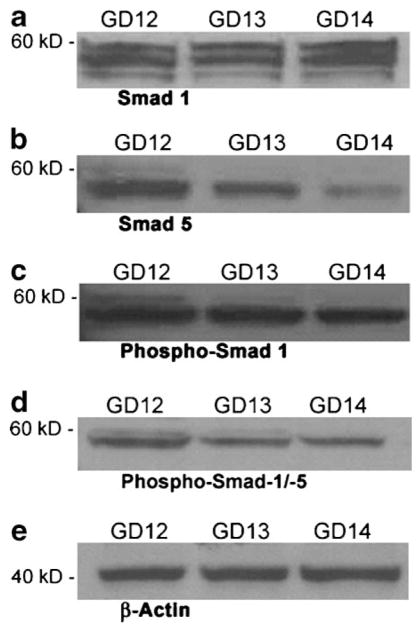
Immunoblot analysis of steady state levels of Smad 1 (a), Smad 5 (b), phospho-Smad 1 (c), and phospho-Smad 1/5 (d) proteins in cell extracts of murine embryonic orofacial tissue from gestational days (GD) 12, 13, and 14 of development. Equal amounts of protein (50 μg) were resolved by SDS–PAGE on 8% polyacrylamide Tris/glycine gels, transferred to PVDF membranes, probed with Smad-specific antibodies and immunoreactive species detected by chemiluminescence, as detailed in the Materials and Methods Section. Molecular weights of the marker proteins are indicated to the left of each part. Each immunoblot is representative of no less than three independent blots of unique tissue extracts from embryonic orofacial tissue from each of GD 12, 13, and 14. Lower part (e) shows one representative western blot of the loading control β-actin.
BMP-induced nuclear translocation of Smad 1, Smad 5, and Smad 4 in MEMM cells
Treatment of MEMM cells with BMP2 or BMP4 (0.1–100 ng/ml) for 2 h resulted in a dose-dependent increase in the nuclear translocation of phospho-Smad 1 and phospho-Smad 5 proteins as revealed by Western (immuno) blotting and densitometric analyses (Fig. 2c,d; Table 2 [BMP2 treatment] and Fig. 3c,d; Table 3 [BMP4 treatment]). Immunoblotting with a Smad 1- or Smad 5-specific polyclonal antibody revealed a doublet of approximately 60 kDa in response to BMP4 treatment (Fig. 3a,b), whereas only a single 58 kDa Smad 1 or Smad 5 band was seen in response to BMP2 treatment (Fig. 2a,b). However, no dose-dependent increase in the nuclear steady state levels of Smad 1 or Smad 5 protein was detected following treatment of MEMM cells with either BMP2 and BMP4 (Figs. 2a,b and 3a,b; Tables 2 and and3).3). Both BMP2 and BMP4 are thus able to induce phosphorylation (i.e., activation) and nuclear translocation of Smad 1 and Smad 5 proteins in MEMM cells in a dose-dependent manner (Figs. 2c,d and 3c,d; Tables 2 and and3).3). A dose-dependent increase in nuclear Smad 4 level in MEMM cells was observed following exposure to BMP2 but not BMP4 (Figs. 2e and and3e;3e; Tables 2 and and3).3). Blots were stripped and reprobed for β-actin for confirmation of equal protein loading. Figures 2f and and3f3f (lower parts), demonstrate that the abundance of β-actin in the three lanes of the blot was similar.
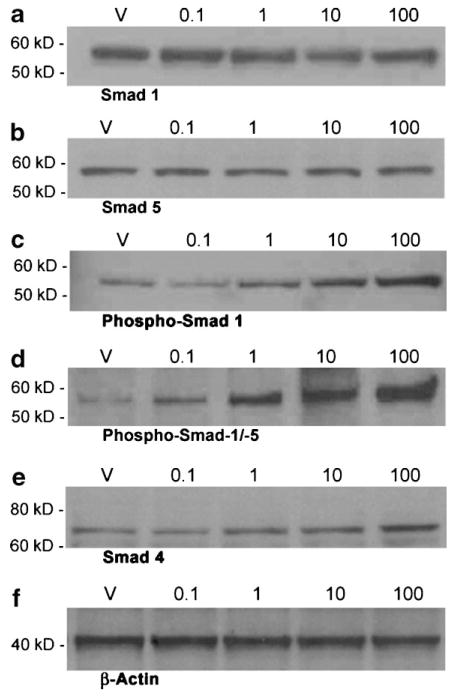
Immunoblot analysis of steady state levels of Smad 1 (a), Smad 5 (b), phospho-Smad 1 (c), phospho-Smad 1/5 (d), and Smad 4 (e) proteins in nuclear extracts of MEMM cells treated with 0.1–100 ng/ml BMP2. Proteins were separated and immunoreactive species detected as described in Figure 1. Each immunoblot is representative of no less than three independent blots of unique extracts from BMP2-treated MEMM cells. Lower part (f) shows one representative western blot of the loading control β-actin.

Immunoblot analysis of steady state levels of Smad 1 (a), Smad 5 (b), phospho-Smad 1 (c), phospho-Smad 1/5 (d), and Smad 4 (e) proteins in nuclear extracts of MEMM cells treated with 0.1–100 ng/ml BMP4. Proteins were separated and immunoreactive species detected as described in Figure 1. Each immunoblot is representative of no less than three independent blots of unique extracts from BMP4-treated MEMM cells. Lower part (f) shows one representative western blot of the loading control β-actin.
TABLE 2
Densitometric analysis of steady state levels of Smad and phospho-Smad proteins in nuclear extracts of MEMM cells treated with BMP2
| Proteina | Treatment with BMP2 (ng/ml) | Fold change in protein level (vs. vehicle-treated samples)b |
|---|---|---|
| Smad 1 | 0.1 | +1.09 ± 0.15 |
| 1.0 | −1.07 ± 0.03 | |
| 10.0 | −1.23 ± 0.05 | |
| 100.0 | +1.03 ± 0.03 | |
| Smad 5 | 0.1 | +1.05 ± 0.11 |
| 1.0 | −1.05 ± 0.09 | |
| 10.0 | +1.05 ± 0.03 | |
| 100.0 | +1.05 ± 0.13 | |
| Phospho-Smad 1 | 0.1 | −1.33 ± 0.02 |
| 1.0 | +1.67 ± 0.04 | |
| 10.0 | +2.58 ± 0.09 | |
| 100.0 | +4.17 ± 0.11 | |
| Phospho-Smad 1/5 | 0.1 | +2.10 ± 0.02 |
| 1.0 | +4.67 ± 0.07 | |
| 10.0 | +6.78 ± 0.12 | |
| 100.0 | +10.33 ± 0.01 | |
| Smad 4 | 0.1 | −1.15 ± 0.15 |
| 1.0 | +1.33 ± 0.04 | |
| 10.0 | +1.67 ± 0.01 | |
| 100.0 | +2.53 ± 0.11 |
TABLE 3
Densitometric analysis of steady state levels of Smad and phospho-Smad proteins in nuclear extracts of MEMM cells treated with BMP4
| Proteina | Treatment with BMP4 (ng/ml) | Fold change in protein level (vs. vehicle-treated samples)b |
|---|---|---|
| Smad 1 (upper band) | 0.1 | −1.33 ± 0.09 |
| 1.0 | −1.04 ± 0.04 | |
| 10.0 | −1.50 ± 0.13 | |
| 100.0 | −1.41 ± 0.03 | |
| Smad 1 (lower band) | 0.1 | −1.45 ± 0.14 |
| 1.0 | +1.20 ± 0.11 | |
| 10.0 | −1.70 ± 0.06 | |
| 100.0 | −1.60 ± 0.06 | |
| Smad 5 (upper band) | 0.1 | +1.00 ± 0.05 |
| 1.0 | +1.06 ± 0.15 | |
| 10.0 | +1.10 ± 0.11 | |
| 100.0 | −1.14 ± 0.07 | |
| Smad 5 (lower band) | 0.1 | +1.35 ± 0.11 |
| 1.0 | +1.37 ± 0.04 | |
| 10.0 | +1.11 ± 0.13 | |
| 100.0 | −1.15 ± 0.03 | |
| Phospho-Smad 1 | 0.1 | +4.00 ± 0.25 |
| 1.0 | +2.33 ± 0.02 | |
| 10.0 | +10.33 ± 0.03 | |
| 100.0 | +20.33 ± 0.03 | |
| Phospho-Smad 1/5 | 0.1 | +1.51 ± 0.18 |
| 1.0 | +1.48 ± 0.05 | |
| 10.0 | +3.00 ± 0.03 | |
| 100.0 | +5.22 ± 0.06 | |
| Smad 4 | 0.1 | −1.45 ± 0.03 |
| 1.0 | −1.23 ± 0.03 | |
| 10.0 | −1.07 ± 0.11 | |
| 100.0 | −1.23 ± 0.01 |
Binding of nuclear BR-Smad proteins to BMP responsive Smad-binding element (BRE) DNA
Smad 1, Smad 4, and Smad 5 directly and specifically bind to a DNA sequence (BRE) derived from the Id1 promoter (Korchynskyi and ten Dijke, 2002). This sequence contains two SBEs and other critical elements necessary for BR-Smad binding. Binding of BR-Smads to such promoter BREs has been shown to transactivate gene expression (Korchynskyi and ten Dijke, 2002; Monteiro et al., 2004). Extracts of BMP2- or BMP4-treated MEMM cells (0.1–10.0 ng/ml) were examined for binding of Smad 1, 4, and 5 proteins to a 5′-biotinylated, double-stranded oligonucleotide containing two copies of a consensus BR-Smad binding BMP responsive element (BRE). Immunoblot analysis of affinity purified BRE-containing protein/DNA complexes revealed binding of phospho-Smad 1, phospho-Smad 5 and Smad 4 (Fig. 4; Table 4). Specificity of Smad binding to the BR-SBE was demonstrated by the absence of binding when a mutant oligo, differing at several nucleotide positions in each of the two BREs, was utilized in the DAPA/immunoblot assay (Fig. 4, lane “mO”).
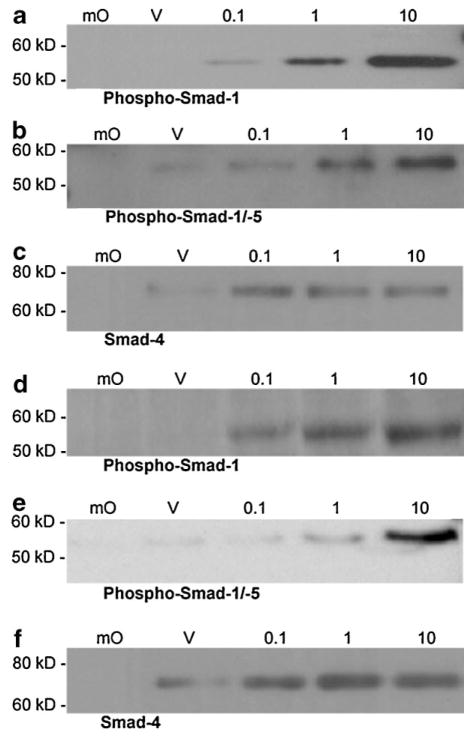
Combined DNA affinity purification and immunoblot analysis of phospho-Smad 1 (a and d), phospho-Smad 1/5 (b and e), and Smad 4 (c and f), binding to a BR-Smad binding element (BRE)-containing oligonucleotide in MEMM cells in response to various concentrations of BMP2 (a–c) and BMP4 (d–f). Cell extracts derived from BMP2-, BMP4-, or vehicle-treated MEMM cells were incubated with a biotinylated BR-SBE oligonucleotide. Protein–oligonucleotide complexes were retrieved on streptavidin–agarose beads, resolved by SDS–PAGE, and subjected to immunoblotting. Specificity of Smad binding to the BR-SBE is demonstrated by the absence of binding when a mutant oligonucleotide was utilized that differed at several nucleotide positions within the BR-Smad binding response element. BMP-treated (10 ng/ml) utilizing mutated BR-SBE (mO); Vehicle control utilizing wild-type BR-SBE (V); BMP2- or BMP4-treated (0.1–10 ng/ml) utilizing wild-type BR-SBE (0.1, 1, 10). Each immunoblot is representative of no less than three independent blots from three unique sets of DAPA-purified MEMM cell extracts.
TABLE 4
Densitometric analysis of Smad binding to a BR-Smad binding element (BRE)-containing oligonucleotide in MEMM cells in response to BMP2 and BMP4
| Proteina | Treatment with BMP2 or BMP4 (ng/ml) | Fold change in protein level (vs. vehicle-treated samples)b |
|---|---|---|
| BMP2 | ||
| Phospho-Smad 1 | 0.1 | 2.01 ± 0.03 |
| 1.0 | 9.13 ± 0.06 | |
| 10.0 | 30.05 ± 0.12 | |
| Phospho-Smad 1/5 | 0.1 | 1.58 ± 0.04 |
| 1.0 | 7.67 ± 0.04 | |
| 10.0 | 31.83 ± 0.03 | |
| Smad 4 | 0.1 | 4.50 ± 0.15 |
| 1.0 | 3.75 ± 0.21 | |
| 10.0 | 3.25 ± 0.05 | |
| BMP4 | ||
| Phospho-Smad 1 | 0.1 | 5.50 ± 0.04 |
| 1.0 | 12.00 ± 0.13 | |
| 10.0 | 31.05 ± 0.03 | |
| Phospho-Smad 1/5 | 0.1 | 1.00 ± 0.02 |
| 1.0 | 4.60 ± 0.08 | |
| 10.0 | 34.50 ± 0.15 | |
| Smad 4 | 0.1 | 2.40 ± 0.04 |
| 1.0 | 3.00 ± 0.02 | |
| 10.0 | 2.50 ± 0.12 | |
Transcriptional activation of BMP-inducible, BRE-containing promoters in MEMM cells
A well-characterized BMP-inducible, BRE-containing promoter, transcriptional reporter construct (BRE-luc, Korchynskyi and ten Dijke, 2002), was utilized to examine induction of transcriptional activity by BMP2 and BMP4 in primary cultures of MEMM cells. MEMM cells were transfected for 24 h with BRE-luc and pRL-CMV (the latter to control for possible differences in transfection efficiency), treated with 25, 50, or 100 ng/ml BMP2 or BMP4 or 2.0 ng/ml of TGFβ1 (negative control) for 24 h, and firefly and Renilla luciferase activities measured and normalized using a “Dual Luciferase Reporter Assay System.” Both BMP isoforms stimulated transcriptional activation of luciferase (reporter) expression in cells of the embryonic orofacial region in a dose-dependent, statistically significant manner (Fig. 5). Specificity of the BRE-luc reporter construct for detecting BMP-mediated transcriptional activation of the reporter gene was demonstrated by the inability of TGFβ1 to activate reporter gene transcription (Fig. 5).
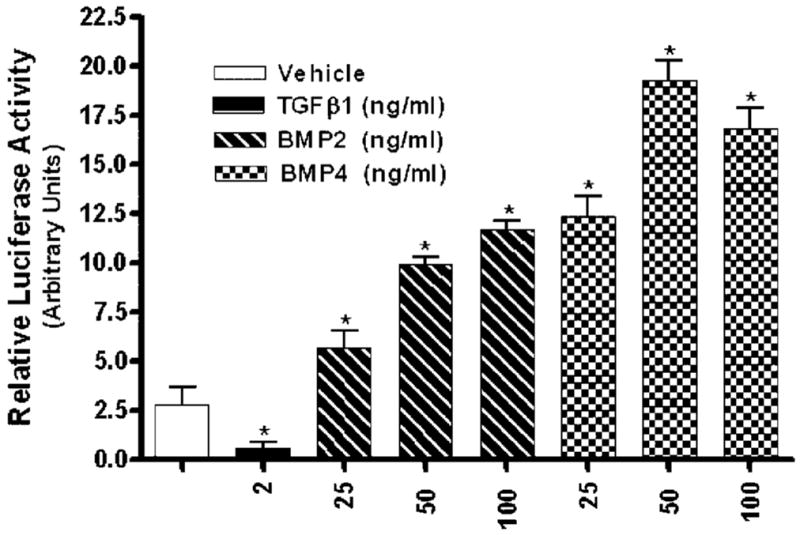
Transcriptional activation of a BMP-inducible luciferase reporter plasmid in MEMM cells. Primary cultures of MEMM cells were co-transfected by the Effectene transfection method with 1.0 μg of the BMP-responsive plasmid BRE-luc and 0.1 μg pRL-CMV for 24 h followed by 24 h exposure to either vehicle, 2.0 ng/ml TGFβ1, or 25, 50, 100 ng/ml BMP2 or BMP4. Cells were extracted and luciferase activity was measured as described in Materials and Methods Section. Firefly luciferase activity was assayed in cell extracts and expressed relative to Renilla luciferase activity to control for variability in transfection efficiencies. Mean luciferase activities and standard errors were calculated from triplicate cell culture samples, and treatments compared by analysis of variance (ANOVA), followed by Student’s t-test. P values <0.05 were considered statistically significant. *Indicates luciferase activity significantly different from control.
Expression of an endogenous gene containing BMP receptor-specific Smad response elements
The ability of BMPs to induce expression of functional, endogenous, and physiologically relevant Id proteins (e.g., Id3), whose expression appears to be crucial for orofacial development (see Discussion Section), and whose gene promoters contain BRE motifs was examined. MEMM cells were treated with 10 ng/ml BMP2 or BMP4 for 2 h and induction of Id3 mRNA and protein expression was determined by TaqMan® QRT-PCR and immunoblotting of MEMM cell nuclear extracts, respectively. Treatment of MEMM cells with BMP2 and BMP4 resulted in significant induction (11.2- and 8.2-fold, respectively) of the gene encoding Id3 and a significant increase (3.21- and 3.93-fold, respectively) in nuclear expression of Id3 protein (Table 5).
TABLE 5
Induction of Id3 mRNA and protein transcription in BMP2 and BMP4-treated MEMM cells
| Treatmenta,b | Gene/protein | Mean Ctc,d,e | Fold increase in Id3 mRNA level (vs. vehicle-treated samples)f | Fold increase in Id3 protein level (vs. vehicle-treated samples)g |
|---|---|---|---|---|
| Vehicle | Id3 | 28.30 ± 0.02 | — | — |
| BMP2 | Id3 | 24.18 ± 0.05 | 11.20 ± 0.04 | 3.21 ± 0.05 |
| BMP4 | Id3 | 24.77 ± 0.01 | 8.20 ± 0.03 | 3.93 ± 0.03 |
Discussion
BMPs are multifunctional morphogens of the TGFβ superfamily executing pivotal roles in nearly all morphogenetic processes and operating as regulators of cell growth, differentiation, and apoptosis (Hogan, 1996b). Numerous studies indicate that BMP signaling plays a critical role in development of the orofacial region (Bennett et al., 1995; Lu et al., 2000; Gong and Guo, 2003; Liu et al., 2005). Similar to the TGFβs, BMP signals are transduced intracellularly by Smad proteins. BMPs, however, utilize three distinct Smad proteins (Smad 1, Smad 5, and Smad 8), referred to as BR-Smads, to transduce their signals. Functional activation of the Smads is achieved through a cascade of events initiated via BMP binding to its cell surface type I and type II receptors, followed by activation of Smads by phosphorylation, translocation to the nucleus and Smad-mediated activation of specific target genes (Chen et al., 2006; Tateishi et al., 2007). Data from the current study documents the existence of a functional BMP–Smad signal transducing system in the developing murine orofacial region. Elucidation of downstream effector pathways activated by the BMPs in cells derived from the embryonic orofacial region enables in depth investigation of molecular mechanisms adopted by the BMP signal transduction pathway to regulate the intricacies of orofacial morphogenesis. Any perturbation of these BMP-dependent signaling mechanisms could result in orofacial defects.
In the current study, elements of the Smad component of the BMP intracellular signaling system were identified in cells of the embryonic orofacial region. Murine embryonic mesenchymal (MEMM) cells responded to both BMP2 and BMP4 with functional activation of the BR-Smad signaling pathway, substantiating the presence of type I and type II receptors (e.g., Alk-2, -3, -6 and BMPRII, ActRIIa and ActRIIb) that act to initiate the signaling cascade. Genes encoding each of these receptors are expressed in developing murine orofacial tissue on gestational days 12–14 (Mukhopadhyay et al., 2006a). Moreover, the genes encoding Alk2 and BMPRII demonstrate significant upregulation in MEMM cells following BMP2 or BMP4 treatment (Mukhopadhyay et al., 2006b). Immunoblot analysis of nuclear extracts from MEMM cells treated with BMP2 or BMP4 revealed dose-dependent nuclear translocation as well as phosphorylation of Smad 1 and Smad 5. Under similar conditions there was a dose-dependent increase in the nuclear levels of the common Smad, Smad 4 in MEMM cells following BMP2 but not BMP4 treatment. Phosphorylation of the BR-Smads (e.g., Smad 1 and 5) by the BMP receptor complex, and subsequent translocation of phosphorylated Smads to the nucleus, are essential steps in the BMP signal transduction cascade (Noth et al., 2003; Zuzarte-Luis et al., 2004).
A DNA affinity purification assay, using Smad isoform-specific antibodies, was employed in the current study to detect Smad 1, Smad 5, and Smad 4 as components of a BMP responsive Smad binding element (BRE)-containing protein complex of proteins in cell extracts of BMP-treated MEMM cells. Specific binding of BR-Smads (Smad 1, 5, and 8) to this BRE (derived from the promoter of Id1—a BMP target gene) has been demonstrated using reporter transfection and EMSA studies and such binding has been reported to activate specific gene expression (Korchynskyi and ten Dijke, 2002; Monteiro et al., 2004). The present study is the first to demonstrate successful utilization of the BRE sequence as a probe in a DAPA.
For characterization of the transcriptional response to BMP2 and BMP4, MEMM cells were transfected with a well-characterized BMP-responsive construct, BRE-luc, containing the BMP responsive Smad binding elements (AGAC) linked to a luciferase reporter (Korchynskyi and ten Dijke, 2002). In addition to the SBEs, this reporter construct contains other critical sequence motifs (e.g., a GGCGCC palindromic sequence flanked by two CAGC and CGCC motifs) from the mouse Id1 promoter. BR-Smads such as Smad 1, Smad 5, Smad 8 and the co-Smad, Smad 4, can bind to this BRE and induce the BRE-luc reporter activity. TGFβ-specific Smads (AR-Smads) such as Smad 2 and Smad 3, on the other hand, fail to activate this reporter (Korchynskyi and ten Dijke, 2002; 73; Monteiro et al., 2004). Data from the present study indicate that in MEMM cells, both BMP2 and BMP4 effectively induced luciferase expression from the BRE-luc reporter. TGFβ1 failed to induce the BRE-luc reporter activity verifying the BMP-specificity of this reporter construct.
Functionality of the BR-Smad-mediated BMP signaling pathway in embryonic maxillary mesenchymal cells was further demonstrated by BMP induction of Id3 mRNA and protein. A member of the inhibitors of differentiation (Id) family of helix-loop-helix (HLH) factors, Id3 is well documented as a BMP target gene (Ogata et al., 1993; Hollnagel et al., 1999). Ids function as dominant negative inhibitors of basic HLH (bHLH) protein-dependent transcription (Zebedee and Hara, 2001; Ghil et al., 2002). In various cell types and tissues, Id and bHLH proteins antagonistically modulate cell proliferation and differentiation (Chaudhary et al., 2001; Zebedee and Hara, 2001). In a recent gene expression profiling study utilizing murine high-density GeneChip® arrays, Mukhopadhyay et al. (2006b) demonstrated a substantial, statistically significant upregulation of Id1, Id2, and Id3 transcripts by BMP2 or BMP4 in MEMM cells. BMP2 and 4 have also been shown to modulate Id1, Id2, and Id4 protein levels in MEMM cells (Mukhopadhyay et al., unpublished work). These findings underscore potential BMP-regulated functions of the Id family of HLH factors during orofacial development. Indeed, experimental findings from Rice et al. (2005) suggested Id1 as a critical effector of BMP and FGF signaling cross-talk during development of the secondary palate. BMPs are thus able to induce expression of functional, endogenous, physiologically and developmentally relevant transcription factors (i.e., Id3) both at transcriptional and translational levels, in cells derived from the embryonic orofacial region. This suggests the possibility that, inasmuch as Id proteins play an important role in the regulation of apoptosis, proliferation and differentiation (Tanaka et al., 1998; Norton, 2000), all processes essential for normal orofacial development (for review Greene and Pisano, 2004; Greene and Pisano, 2005), these proteins may subserve a pivotal role in orchestrating these cellular processes during orofacial ontogenesis.
Acknowledgments
Contract grant sponsors: NIH; COBRE Program of the National Center for Research Resources.
Contract grant numbers: DE05550, HD053509, P20 RR017702.
We thank Dr. Peter ten Dijke, Leiden University Medical Center, Leiden, Netherlands for the BRE2-luc reporter. This research was supported in part by NIH grants DE05550, HD053509, and P20 RR017702 from the COBRE Program of the National Center for Research Resources.
Literature Cited
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with image. J Biophotons Int. 2004;11:36–42. [Google Scholar]
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. [Abstract] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. [Abstract] [Google Scholar]
- Bennett JH, Hunt P, Thorogood P. Bone morphogenetic protein-2 and -4 expression during murine orofacial development. Arch Oral Biol. 1995;40:847–854. [Abstract] [Google Scholar]
- Chaudhary J, Johnson J, Kim G, Skinner MK. Hormonal regulation and differential actions of the helix-loop-helix transcriptional inhibitors of differentiation (Id1, Id2, Id3, and Id4) in Sertoli cells. Endocrinology. 2001;142:1727–1736. [Abstract] [Google Scholar]
- Chen X, Zankl A, Niroomand F, Liu Z, Katus HA, Jahn L, Tiefenbacher C. Upregulation of ID protein by growth and differentiation factor 5 (GDF5) through a smad-dependent and MAPK-independent pathway in HUVSM. J Mol Cell Cardiol. 2006;41:26–33. [Abstract] [Google Scholar]
- Dudas M, Sridurongrit S, Nagy A, Okazaki K, Kaartinen V. Craniofacial defects in mice lacking BMP type I receptor alk2 in neural crest cells. Mech Dev. 2004;121:173–182. [Abstract] [Google Scholar]
- Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001;28:491–498. [Abstract] [Google Scholar]
- Ghil SH, Jeon YJ, Suh-Kim H. Inhibition of BETA2/NeuroD by Id2. Exp Mol Med. 2002;34:367–373. [Abstract] [Google Scholar]
- Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. [Abstract] [Google Scholar]
- Glass C, Franco R, Weinberger C, Albert V, Evans R, Rosenfeld M. A c-erb-A binding site in rat growth hormone gene mediates trans-activation by thyroid hormone. Nature. 1987;329:738–741. [Abstract] [Google Scholar]
- Gong SG, Guo C. BMP4 gene is expressed at the putative site of fusion in the midfacial region. Differentiation. 2003;71:228–236. [Abstract] [Google Scholar]
- Greene RM, Pisano MM. Perspectives on growth factors and orofacial development. Curr Pharm Des. 2004;10:2701–2717. [Abstract] [Google Scholar]
- Greene RM, Pisano MM. Recent advances in understanding transforming growth factor beta regulation of orofacial development. Hum Exp Toxicol. 2005;24:1–12. [Abstract] [Google Scholar]
- Greene RM, Nugent P, Mukhopadhyay P, Warner DR, Pisano MM. Intracellular dynamics of Smad-mediated TGFβ signaling. J Cell Physiol. 2003;197:261–271. [Abstract] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–2068. [Abstract] [Google Scholar]
- Henningfeld KA, Rastegar S, Adler G, Knochel W. Smad1 and Smad4 are components of the bone morphogenetic protein-4 (BMP-4)-induced transcription complex of the Xvent-2B promoter. J Biol Chem. 2000;275:21827–21835. [Abstract] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996a;6:432–438. [Abstract] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: Multifunctional regulators of vertebrate development. Genes Dev. 1996b;10:1580–1594. [Abstract] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. [Abstract] [Google Scholar]
- Ishida W, Hamamoto T, Kusanagi K, Yagi K, Kawabata M, Takehara K, Sampath TK, Kato M, Miyazono K. Smad6 is a Smad1/5-induced smad inhibitor. Characterization of bone morphogenetic protein-responsive element in the mouse Smad6 promoter. J Biol Chem. 2000;275:6075–6079. [Abstract] [Google Scholar]
- Jones CM, Lyons KM, Hogan BL. Expression of TGF-beta-related genes during mouse embryo whisker morphogenesis. Ann NY Acad Sci. 1991;642:339–344. [Abstract] [Google Scholar]
- Katagiri T, Imada M, Yanai T, Suda T, Takahashi N, Kamijo R. Identification of a BMP-responsive element in Id1, the gene for inhibition of myogenesis. Genes Cells. 2002;7:949–960. [Abstract] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. [Abstract] [Google Scholar]
- Kishigami S, Mishina Y. Bmp signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. [Abstract] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–193. [Abstract] [Google Scholar]
- Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J Biol Chem. 2002;277:4883–4891. [Abstract] [Google Scholar]
- Korchynskyi O, Dechering KJ, Sijbers AM, Olijve W, ten Dijke P. Gene array analysis of bone morphogenetic protein type I receptor-induced osteoblast differentiation. J Bone Miner Res. 2003;18:1177–1185. [Abstract] [Google Scholar]
- Kusek J, Greene R, Nugent P, Pisano M. Expression of the E2F family of transcription factors during murine development. Int J Dev Biol. 2000;44:267–277. [Abstract] [Google Scholar]
- Laemmle UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. [Abstract] [Google Scholar]
- Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for BMP signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. [Abstract] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. [Abstract] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Massague J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem. 2002;277:3176–3185. [Abstract] [Google Scholar]
- Lu H, Jin Y, Tipoe GL. Alteration in the expression of bone morphogenetic protein-2,3,4,5 mRNA during pathogenesis of cleft palate in BALB/c mice. Arch Oral Biol. 2000;45:133–140. [Abstract] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. [Abstract] [Google Scholar]
- Lyons KM, Jones CM, Hogan BL. The DVR gene family in embryonic development. Trends Genet. 1991;7:408–412. [Abstract] [Google Scholar]
- Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374:356–360. [Abstract] [Google Scholar]
- Mcpherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. [Abstract] [Google Scholar]
- Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7:1191–1204. [Abstract] [Google Scholar]
- Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–263. [Abstract] [Google Scholar]
- Monteiro RM, de Sousa Lopes SM, Korchynskyi O, ten Dijke P, Mummery CL. Spatio-temporal activation of Smad1 and Smad5 in vivo: Monitoring transcriptional activity of Smad proteins. J Cell Sci. 2004;117:4653–4663. [Abstract] [Google Scholar]
- Mukhopadhyay P, Greene RM, Pisano MM. Expression profiling of transforming growth factor beta superfamily genes in developing orofacial tissue. Birth Defects Res A Clin Mol Teratol. 2006a;76:528–543. [Europe PMC free article] [Abstract] [Google Scholar]
- Mukhopadhyay P, Singh S, Greene RM, Pisano MM. Molecular fingerprinting of BMP2-and BMP4-treated embryonic maxillary mesenchymal cells. Orthod Craniofac Res. 2006b;9:93–110. [Abstract] [Google Scholar]
- Nie XG. Differential expression of bmp2, bmp4 and bmp3 in embryonic development of mouse anterior and posterior palate. Chin Med J (Engl) 2005;118:1710–1716. [Abstract] [Google Scholar]
- Nishimura R, Hata K, Ikeda F, Matsubara T, Yamashita K, Ichida F, Yoneda T. The role of Smads in BMP signaling. Front Biosci. 2003;8:s275–s284. [Abstract] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. [Abstract] [Google Scholar]
- Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291–299. [Abstract] [Google Scholar]
- Norton JD. ID helix–loop–helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113:3897–3905. [Abstract] [Google Scholar]
- Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291:201–211. [Abstract] [Google Scholar]
- Ogata T, Wozney JM, Benezra R, Noda M. Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix-loop-helix molecule in osteoblast-like cells. Proc Natl Acad Sci USA. 1993;90:9219–9222. [Europe PMC free article] [Abstract] [Google Scholar]
- Petryk A, Anderson RM, Jarcho MP, Leaf I, Carlson CS, Klingensmith J, Shawlot W, O’Connor MB. The mammalian twisted gastrulation gene functions in foregut and craniofacial development. Dev Biol. 2004;267:374–386. [Abstract] [Google Scholar]
- Rice R, Thesleff I, Rice DP. Regulation of Twist, Snail, and Id1 is conserved between the developing murine palate and tooth. Dev Dyn. 2005;234:28–35. [Abstract] [Google Scholar]
- Rosen V, Thies RS. The BMP proteins in bone formation and repair. Trends Genet. 1992;8:97–102. [Abstract] [Google Scholar]
- Sasse J, Gallagher SR. Staining protein gels. In: Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York: John Wiley and Sons; 1991. pp. 10.6.1–10.6.8. [Google Scholar]
- Tanaka K, Pracyk JB, Takeda K, Yu ZX, Ferrans VJ, Deshpande SS, Ozaki M, Hwang PM, Lowenstein CJ, Irani K, Finkel T. Expression of Id1 results in apoptosis of cardiac myocytes through a redoxdependent mechanism. J Biol Chem. 1998;273:25922–25928. [Abstract] [Google Scholar]
- Tateishi K, Higuchi C, Ando W, Nakata K, Hashimoto J, Hart DA, Yoshikawa H, Nakamura N. The immunosuppressant FK506 promotes development of the chondrogenic phenotype in human synovial stromal cells via modulation of the Smad signaling pathway. Osteoarthritis Cartilage. 2007;15:709–718. [Abstract] [Google Scholar]
- van den Boogaard MJ, Dorland M, Beemer FA, van Amstel HK. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet. 2000;24:342–343. [Abstract] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: Pathway or network? Dev Biol. 2001;239:1–14. [Abstract] [Google Scholar]
- Winograd J, Reilly MP, Roe R, Lutz J, Laughner E, Xu X, Hu L, Asakura T, vander Kolk C, Strandberg JD, Semenza GL. Perinatal lethality and multiple craniofacial malformations in MSX2 transgenic mice. Hum Mol Genet. 1997;6:369–379. [Abstract] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. [Abstract] [Google Scholar]
- Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. [Abstract] [Google Scholar]
- Zebedee Z, Hara E. Id proteins in cell cycle control and cellular senescence. Oncogene. 2001;20:8317–8325. [Abstract] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. [Abstract] [Google Scholar]
- Zhao M, Xiao G, Berry JE, Franceschi RT, Reddi A, Somerman MJ. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–1451. [Abstract] [Google Scholar]
- Zuzarte-Luis V, Montero JA, Rodriguez-Leon J, Merino R, Rodriguez-Rey JC, Hurle JM. A new role for BMP5 during limb development acting through the synergic activation of Smad and MAPK pathways. Dev Biol. 2004;272:39–52. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1002/jcp.21455
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2746655?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation.
Bone Res, 3:15005, 14 Apr 2015
Cited by: 292 articles | PMID: 26273537 | PMCID: PMC4472151
Review Free full text in Europe PMC
Alcohol modulates expression of DNA methyltranferases and methyl CpG-/CpG domain-binding proteins in murine embryonic fibroblasts.
Reprod Toxicol, 37:40-48, 06 Feb 2013
Cited by: 20 articles | PMID: 23395981 | PMCID: PMC4356550
Epigenetic regulation of Sox4 during palate development.
Epigenomics, 5(2):131-146, 01 Apr 2013
Cited by: 9 articles | PMID: 23566091 | PMCID: PMC3776497
Id2 controls chondrogenesis acting downstream of BMP signaling during maxillary morphogenesis.
Bone, 50(1):69-78, 01 Oct 2011
Cited by: 10 articles | PMID: 21985998
Null mutation of the transcription factor inhibitor of DNA binding 3 (id3) affects spermatozoal motility parameters and epididymal gene expression in mice.
Biol Reprod, 84(4):765-774, 08 Dec 2010
Cited by: 4 articles | PMID: 21148110 | PMCID: PMC4574637
Go to all (9) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Intracellular dynamics of Smad-mediated TGFbeta signaling.
J Cell Physiol, 197(2):261-271, 01 Nov 2003
Cited by: 34 articles | PMID: 14502566
DRAGON, a bone morphogenetic protein co-receptor.
J Biol Chem, 280(14):14122-14129, 25 Jan 2005
Cited by: 143 articles | PMID: 15671031
Temporal regulation of mRNAs for select bone morphogenetic proteins (BMP), BMP receptors and their associated SMAD proteins during bovine early embryonic development: effects of exogenous BMP2 on embryo developmental progression.
Reprod Biol Endocrinol, 12:67, 15 Jul 2014
Cited by: 17 articles | PMID: 25027287 | PMCID: PMC4110370
Bone morphogenetic protein receptor signal transduction in human disease.
J Pathol, 247(1):9-20, 27 Nov 2018
Cited by: 109 articles | PMID: 30246251 | PMCID: PMC6587955
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCRR NIH HHS (3)
Grant ID: P20 RR017702-07
Grant ID: P20 RR017702
Grant ID: P20 RR17702
NICHD NIH HHS (3)
Grant ID: R01 HD053509-01A2
Grant ID: HD053509
Grant ID: R01 HD053509
NIDCR NIH HHS (5)
Grant ID: R01 DE005550
Grant ID: R01 DE018215
Grant ID: DE05550
Grant ID: R01 DE005550-22
Grant ID: R01 DE018215-01A1




