Abstract
Background & aims
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer worldwide and the third most lethal. Dysregulation of alternative splicing underlies a number of human diseases, yet its contribution to liver cancer has not been explored fully. The Krüppel-like factor 6 (KLF6) gene is a zinc finger transcription factor that inhibits cellular growth in part by transcriptional activation of p21. KLF6 function is abrogated in human cancers owing to increased alternative splicing that yields a dominant-negative isoform, KLF6 splice variant 1 (SV1), which antagonizes full-length KLF6-mediated growth suppression. The molecular basis for stimulation of KLF6 splicing is unknown.Methods
In human HCC samples and cell lines, we functionally link oncogenic Ras signaling to increased alternative splicing of KLF6 through signaling by phosphatidylinositol-3 kinase and Akt, mediated by the splice regulatory protein ASF/SF2.Results
In 67 human HCCs, there is a significant correlation between activated Ras signaling and increased KLF6 alternative splicing. In cultured cells, Ras signaling increases the expression of KLF6 SV1, relative to full-length KLF6, thereby enhancing proliferation. Abrogation of oncogenic Ras signaling by small interfering RNA (siRNA) or a farnesyl-transferase inhibitor decreases KLF6 SV1 and suppresses growth. Growth inhibition by farnesyl-transferase inhibitor in transformed cell lines is overcome by ectopic expression of KLF6 SV1. Down-regulation of the splice factor ASF/SF2 by siRNA increases KLF6 SV1 messenger RNA levels. KLF6 alternative splicing is not coupled to its transcriptional regulation.Conclusions
Our findings expand the role of Ras in human HCC by identifying a novel mechanism of tumor-suppressor inactivation through increased alternative splicing mediated by an oncogenic signaling cascade.Free full text

Ras Promotes Growth by Alternative Splicing-Mediated Inactivation of the KLF6 Tumor Suppressor in Hepatocellular Carcinoma
Abstract
Background & Aims
HCC is the 5th most prevalent cancer worldwide and the 3rd most lethal. Dysregulation of alternative splicing underlies a number of human diseases, yet its contribution to liver cancer has not been fully explored. The KLF6 gene is a zinc finger transcription factor that inhibits cellular growth in part by transcriptional activation of p21. KLF6 function is abrogated in human cancers due to increased alternative splicing that yields a dominant negative isoform, ‘KLF6 SV1’, which antagonizes full-length KLF6 (‘KLF6Full’)-mediated growth suppression. The molecular basis for stimulation of KLF6 splicing is unknown.
Methods
In human HCC samples and cell lines, we functionally link oncogenic Ras signaling to increased alternative splicing of KLF6 through signaling by PI-3 kinase and Akt, mediated by the splice regulatory protein ASF/SF2.
Results
In 67 human HCCs, there is a significant correlation between activated Ras signaling and increased KLF6 alternative splicing. In cultured cells, Ras signaling increases the expression of KLF6 SV1, relative to KLF6Full, thereby enhancing proliferation. Abrogation of oncogenic Ras-signaling by siRNA or a farnesyl-transferase inhibitor (FTS) decreases KLF6 SV1 and suppresses growth. Growth inhibition by FTS in transformed cell lines is overcome by ectopic expression of KLF6 SV1. Down-regulation of the splice factor ASF/SF2 by siRNA increases KLF6 SV1 mRNA levels. KLF6 alternative splicing is not coupled to its transcriptional regulation.
Conlcusions
Our findings expand the role of Ras in human HCC by identifying a novel mechanism of tumor suppressor inactivation through increased alternative splicing mediated by an oncogenic signaling cascade.
Introduction
Alternative splicing is a nuclear process that contributes to expanded protein diversity from a limited number of genes. Dysregulation of alternative splicing may contribute to degenerative, developmental, and malignant diseases1–3.
Knowledge about alternative splicing in human hepatocellular carcinoma is incomplete. Human cancers display alternatively spliced gene products in the absence of genomic mutations, pinpointing the splicing machinery as a cause of disease1–3. In general, affected proteins include transcription factors, cell signal transducers, and components of the extracellular matrix. Other examples where alternatively spliced transcripts are increased in human cancers include LKB1 (premature termination codon), KIT (aberrant splicing leading to constitutively activated receptor tyrosine kinase), CDH17 (unknown mechanism), and BRCA1 (exon skipping)4.
While the role of alternative splicing in cancer is not well characterized, in normal tissues alternative splicing is regulated by specific signaling pathways, yielding temporally and spatially appropriate patterns of gene expression5–8. For example, pre-mRNA alternative splicing mediated by Sam68, a member of the STAR (signal transduction and activation of RNA) family of proteins, has been directly linked to Ras signaling. Specifically, Ras-mediated phosphorylation of Sam68 activates alternative splicing of CD44 pre-mRNA, a cell surface molecule and hyaluronic acid receptor, inducing expression of CD44v5 in an ERK-dependent manner7. Although the normal function of this isoform is currently unknown, CD44v5 can contribute to carcinogenesis by enhancing tumor cell invasion in a SRm160-dependent manner9. Additionally, monoclonal Ab-mediated neutralization of CD44 eliminates the oncogenic potential of leukemic stem cells10, 11.
Whereas signals that increase splicing of CD44 may lead to tumor enhancement, there are no examples where a signaling pathway leads to functional inactivation of tumor suppressor genes in human cancers through dysregulated alternative splicing. Moreover, although alternative splicing of tumor suppressor genes such as BRCA112, 13, WT114, NF115, Men116, p5317 and PTEN18 has been described, the signals that result in their dysregulated splicing are not yet defined.
Krüppel-like Factor 6 (KLF6) is a ubiquitously expressed zinc finger transcription factor and tumor suppressor gene that is inactivated in several human cancers, including HCC, by loss of heterozygosity and/or inactivating mutations19–22. KLF6 can suppress growth by p53-independent up-regulation of p2121, sequestration of cyclin D123, and/or antagonism of the c-jun proto-oncogene24.
We have recently characterized three alternative splice variants of KLF6 that encode truncated isoforms (Supplementary Fig 1), at least one of which, KLF6 SV1, acts as a dominant negative protein that antagonizes full length KLF6 (‘KLF6Full’)25. Significant over-expression of SV1 has been identified in several human cancers including prostate26, ovarian27, and hepatocellular carcinomas28. Over-expression of KLF6 SV1 in human tumors correlates with poorer outcome and reduced survival27. In contrast, targeted inhibition of KLF6 SV1 suppresses prostate and ovarian cancer cell growth26, 27, 29 both in vivo and in vitro. These studies indicate that the net activity of KLF6 can be regulated by the relative expression of KLF6Full compared to SV1, such that either a relative increase in SV1 and/or decrease in KLF6Full can promote cellular growth26, 27, 29. While the functional outcomes of KLF6 alternative splicing have become clearer, the pathways regulating KLF6 alternative splicing remain obscure.
With the recognition that Ras regulates pre-mRNA alternative splicing in normal cells (e.g., Sam68), we explored a potential functional link between enhanced tumor suppressor alternative splicing and Ras dysregulation in human HCC. Specifically, oncogenic Ras signaling via PI3-K/Akt leads to increased alternative splicing of KLF6, and furthermore, KLF6 alternative splicing partly mediates Ras’s growth-promoting effects. These data link oncogene-mediated alternative splicing of a tumor suppressor gene to its functional inactivation in hepatocellular carcinoma.
Materials and Methods
Patients and Samples
Human HCCs were obtained as described recently30, 31. A total of 67 fresh-frozen HCC samples from HCV-positive patients were analyzed in the study. Patients with HBV-positive markers or a background of alcohol consumption, nonalcoholic steatohepatitis, hemochromatosis, or other causes of chronic liver disease were excluded. Lesions previously treated by percutaneous ablation or chemoembolization/lipidolization were also excluded. The liver sample set included the following histological groups: HCC patient samples including early, advanced HCC (large HCC with microvascular invasion or satellites and/or poorly differentiated tumors) and very advanced HCC (with evidence of macrovascular invasion or extrahepatic metastases).
DNA mutation analysis
Genomic DNA samples were amplified using Clontech Advantage PCR plates. Primers are available upon request. Genewiz, Inc. (Paramus, NJ) purified then directly sequenced PCR products. Data were analyzed using Sequencher (Gene Codes, MI).
Cell culture, transient transfection, and stable infection
Cell lines were obtained from ATCC (MD). Live cells and mRNA from HCT116, DLD-1, and their daughter cell lines (except Hke-3) were obtained from the UK; live Hke-3 cells were never obtained due to x-radiation during shipment. Chemicals used in studies included: Akt Inhibitor V, Triciribine, LY 294002 in Solution, JNK Inhibitor II in Solution, U0126, Akt Inhibitor VII, EGFR inhibitor, Phorbol-12-myristate-13-acetate (all from Calbiochem, Germany), and Rapamycin (Sigma, MO). Modified siRNA duplexes were purchased from Invitrogen.
Transfections were performed using Lipofectamine 2000 reagent (Invitrogen, CA). Polyclonal pools of stable cell lines were generated by retroviral infection of pBabe-Ctrl, pBabe-KLF6, and pBabe-SV1 plasmids. Infected cells were selected with 2 μg/mL of puromycin. For each construct, at least two independent polyclonal pools of stable cell lines were generated and analyzed.
Analysis of proliferation
Proliferation was determined as previously described21. Proliferation was determined by assaying [3H]-thymidine incorporation. Cells were plated at a density of 50,000 cells per well in 12-well dishes. At appropriate time points after plating, 1 μCi/mL [3H]-thymidine (Amersham, Piscataway, NJ) was added. After 2 hours, cells were washed three times with ice-cold PBS and fixed in methanol for 30 minutes at 4°C. After methanol removal and cell drying, cells were solubilized in 0.25% sodium hydroxide/0.25% SDS. Following neutralization with hydrochloric acid (1 N), disintegrations per minute were estimated by liquid scintillation counting.
RNA and quantitative real-time PCR analysis
RNA from cell lines and tumors was extracted using the RNeasy Mini and Midi kit (Qiagen, CA). RNA was treated with DNase (Roche, Switzerland). One μg of total RNA was reverse-transcribed per reaction using first-strand complementary DNA synthesis with random primers (Clontech, Carpenteria, CA). Quantitative real-time PCR was done on a Roche LightCycler 480. Experiments were performed in triplicate three independent times. All values were normalized to GAPDH and β-actin. KLF6 primer sequences and protocols have been previously described25–28;
KRas-F – GCTGGTGGCGTAGGCAAGAG
KRas-R – CTCCTCTTGACCTGCTGTGTCG
HRas-F – AGGAGACCCTGTAGGAGGA
HRas-R – CGCTAGGCTCACCTCTATAGTG
PI3K-F – CTGTGTGGGACTTATTGAGGTGGTGC
PI3K-R – GGCATGCTGTCGAATAGCTAGATAAGC.
Statistical significance was determined by ANOVA.
Protein Extracts and Western Blots
Cells were lysed in Lysis M (Roche) buffer, and lysates were sonicated and pelleted. Supernatants were denatured, and 30 μg of protein was separated by PAGE (Invitrogen), then transferred to nitrocellulose membranes (Invitrogen). The following antibodies were used: Akt (Cell Signaling, MA), phospho-Akt (Cell Signaling), β-actin (Santa Cruz, CA), and secondary anti-goat (Santa Cruz) and -rabbit (Amersham) antibodies. ECL images were analyzed and quantified with a BIOQUANT NOVA. Values were normalized to control and expressed as relative fold changes.
Luciferase reporter assays
293T cells were transfected with pGL3-KLF6-promoter luciferase constructs (1 μg) and a K-RasV12 expression plasmid or K-Ras siRNA oligonucleotides (Invitrogen) as indicated in Results, together with the p21WAF1/Cip1 promoter-luciferase construct. Five ng of pRL-TK plasmid (Promega, WI) were co-transfected as a control for transfection efficiency. Twenty-four hours after tranfection, cells were washed with cold PBS and lysates prepared using Dual-luciferase Reporter Assay system (Promega). Luciferase activity in 10 μL of lysate was determined on a luminometer (Dynex Technologies, UK).
The p21 promoter construct consists of 181 bp upstream of the transcriptional start site21. Within these 181 bp are two known transcription factor binding motifs: a CCAAT box, which binds p53, and a GC box, which binds KLF6 and other KLFs.
Results
Ras Pathway Dysregulation is Associated with Increased KLF6 Alternative Splicing in Human HCC
To test for an association between Ras and KLF6 in vivo, we examined sixty-seven advanced and very advanced HCC samples for activated Ras signaling and KLF6 alternative splicing. Activated Ras signaling was assessed by two methods: sequencing for GTPase-inhibiting K-Ras point mutations in codons 12, 13, and 61 that result in constitutive protein function; and real-time qPCR analysis of H-Ras mRNA expression. KLF6 alternative splicing was measured by real-time qPCR with isoform specific primers. A large proportion (50 of 67, 75%) of these advanced and very advanced HCCs displayed increased H-Ras mRNA, and were classified as high H-Ras samples (Table 1), whereas only 1 of 67 samples harbored a mutant K-Ras allele (data not shown). Three independent H-Ras primer sets were used to measure and verify over-expression (Supplementary Table 1). Increased KLF6 alternative splicing, expressed as a ratio of KLF6 SV1 to KLF6Full, was present in 51 of 67 (76%) HCC samples (Table 1). When the level of H-Ras was compared against KLF6 splicing by non-parametric chi-square analysis, there was a significant correlation between elevated H-Ras expression and increased KLF6 alternative splicing, supporting an association between Ras activation and KLF6 splicing (Table 1).
Table 1
In vivo relationship linking activated Ras signaling to increased KLF6 alternative splicing. Chi square analysis results indicating significant correlation between H-Ras expression and KLF6 alternative splicing in human HCC tumors. Median curves were implemented to determine high (for H-Ras, >3 fold over-expression; for KLF6 alternative splicing, >5 fold over-expression) and low expression.
| H-Ras Expression | KLF6 Alternative Splicing | |||
| Low | High | Total | ||
| Low | 1 | 16 | 17 | |
| High | 15 | 35 | 50 | |
| Total | 16 | 51 | 67 | |
Degrees of freedom: 1
Chi-square = 4.05
p is less than 0.05
KLF6 Alternative Splicing is Regulated by Ras Signaling
To further elucidate the signaling cascades that regulate KLF6 alternative splicing, several cell lines were incubated with TPA (phorbol myristate acetate), a potent and immediate activator of Protein Kinase C (PKC), Ras, and its downstream effectors, the MAP kinases and PI3-K. Addition of TPA to the 293T human fibroblast cell line, which expresses wild-type Ras, significantly increased KLF6 SV1 mRNA at 24 hrs, as determined by splice form-specific real-time qPCR (Fig 1A). KLF6 alternative splicing increased as early as 3 hours after TPA treatment (data not shown). These results were verified using standard RT-PCR and agarose gel electrophoresis (data not shown). In PC3M cells, a metastatic prostate cancer line with constitutively active Ras signaling, KLF6 SV1 mRNA was also significantly increased by TPA, but to a much lesser extent (Fig 1A). Specifically, compared to 293T cells, PC3M cells, which are known to harbor highly increased Ras signaling, displayed elevated baseline levels of KLF6 SV1 mRNA (data not shown) suggesting that Ras–dependent splicing may already be activated in this line. Similar to 293T cells, increases in SV1 mRNA following TPA treatment were also observed in the human HCC cell lines Hep3B and Huh7, as well as the human hepatoblastoma cell line HepG2 (Fig 1B).
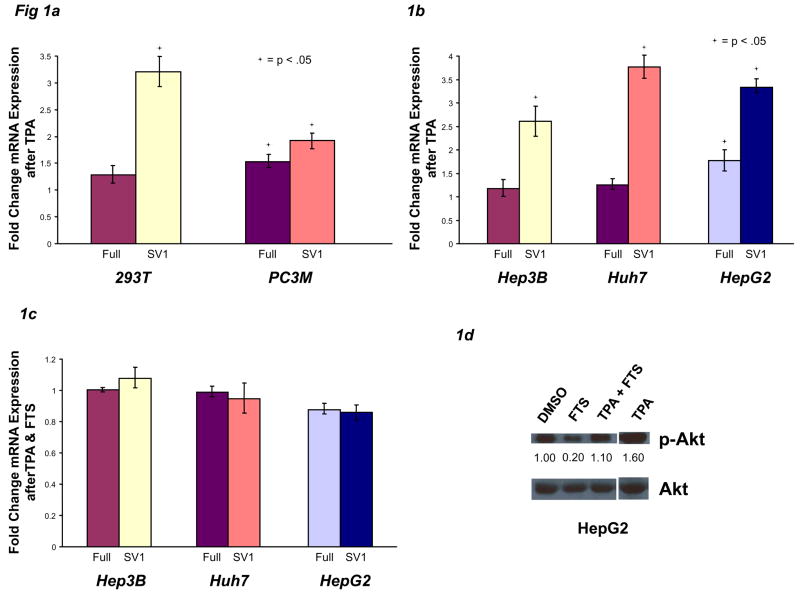

Oncogenic Ras signaling increases KLF6 alternative splicing. A–B) 293T, PC3M, Hep3B, Huh7, and HepG2 cells were plated at sub-confluent densities, treated with 40 ng/ml TPA (PMA) for 24 hours, then harvested for mRNA and protein. DMSO solvent served as vehicle control. All KLF6Full and KLF6 SV1 data are expressed as a fold change (ratio) of the experimental condition compared to vehicle control. For all western blots, densitography values are shown beneath each condition. C–D) Hep3B, Huh7, and HepG2 cells were treated with 40 ng/ml TPA and/or 100 μM FTS for 24 hours, then harvested for mRNA and protein. E) 293T & Hep3B cells were treated as in A–B. F–G) Hep3B, Huh7, & HepG2 cells were treated with 100 μM FTS, then harvested for mRNA and protein.
We next examined whether FTS, a farnesyl-transferase inhibitor (FTI) that prevents Ras proteins from localizing to the inner cell membrane, and hence prevents signal transduction32, blocked TPA-induced KLF6 alternative splicing. Indeed, when the cell lines Hep3B, Huh7, and HepG2 were incubated with TPA and FTS together, there were no significant changes in KLF6Full or SV1 expression (Fig 1C), suggesting that Ras signaling was necessary during TPA-induced KLF6 splicing. Furthermore, western analyses of these experiments (Fig 1D) confirmed Ras-dependent Akt phosphorylation in this cellular context, validating the use of p-Akt as a marker of Ras-activity. These results also implicated Ras-dependent Akt as a potential KLF6 splicing regulator, a hypothesis we tested below.
Activated Ras signaling and Ras-dependent Akt phosphorylation for the experiments in Figures 1A and 1B were confirmed by increased phosphorylation of Akt in 293T and Hep3B cell lines (Fig 1E, results are representative of all cell lines).
These findings supported the prospect that induction of Ras by TPA contributed to enhanced alternative splicing of KLF6 and generation of KLF6 SV1. To specifically address this possibility, 293T, BPH1 (a benign prostatic hyperplasia cell line characterized by an intermediate level of Ras signaling39), and PC3M cells were transiently transfected with an oncogenic H-Ras expression vector, and expression of KLF6 SV1 was quantified. H-Ras transfection significantly increased KLF6 SV1 mRNA in 293T and BPH1 cells, but not PC3M (Supplementary Fig 2A). Activated Ras signaling was confirmed by increased phospho-Akt in 293T cells (Supplementary Fig 2B). Interestingly, the expression pattern of KLF6 SV1 in these cells parallels the relative activity of Ras. For example, in PC3M cells, where Ras signaling is constitutively active, ectopic expression of oncogenic Ras did not further increase KLF6 SV1 mRNA (Supplementary Fig 2A).
We next explored the potential role of K-Ras in KLF6 alternative splicing, since this Ras isoform is expressed more ubiquitously than H-Ras in human tissues. Similar to experiments with H-Ras, transient transfection of oncogenic K-Ras into BPH1 and PC3M cells resulted in significantly increased KLF6 SV1 mRNA only in BPH1 cells (Supplementary Fig 2C), indicating that splicing was enhanced by Ras, but primarily in cells not already expressing an activated isoform.
To further link Ras to KLF6 alternative splicing, cells were incubated with FTS. Incubation of Hep3B, Huh7, and HepG2 cells with FTI significantly decreased KLF6 SV1 mRNA in all three lines, while KLF6Full mRNA expression remained unchanged (Fig 1F upper panel). FTS-mediated inhibition of Ras signaling was confirmed by decreased phospho-Akt in Huh7 and HepG2 cells (Fig 1F, lower panel).
Deletion of Endogenous Oncogenic Ras Decreases KLF6 Alternative Splicing
Rather than relying solely on exogenous manipulation of human cancer cell lines, we employed a somatic cell system using two sublines of HCT116 (Hkh-2 and Hke-3) and DLD-1 (Dko-4 and Dks-8) colon cancer cells in which one K-Ras allele has been genetically deleted through homologous recombination. The Hkh-2 and Hke-3 lines express ~50% of the levels of K-Ras mRNA expressed by their wild-type counterpart (Supplementary Fig 3A), consistent with loss of heterozygosity for K-Ras. Hkh-2, Dko-4, and Dks-8 daughter cell lines contain less phospho-Akt than their normal counterparts (Supplementary Fig 3B), confirming attenuated Ras signaling. As predicted, these cells with deletion of one oncogenic K-Ras allele expressed significantly less KLF6 SV1 mRNA (Fig 2A). Reconstitution of K-Ras via transient transfection in Hkh-2 daughter cells significantly induced SV1 expression compared to the parental HCT116 line, without affecting KLF6Full (Fig 2B). Increased levels of p-Akt confirmed that Ras activity was increased following K-Ras transfection (Fig 2C). Conversely, when K-Ras expression was knocked down by siRNA40 in Hkh-2, Dko-4, & Dks-8 cell lines (Supplementary Fig 3C), KLF6 SV1 mRNA, but not KLF6Full, was significantly decreased in all three lines (Fig 2D, relative expression of each isoform in the presence of siK-Ras vs siLuc), suggesting that elimination of Ras signaling largely abrogated alternative splicing of KLF6.
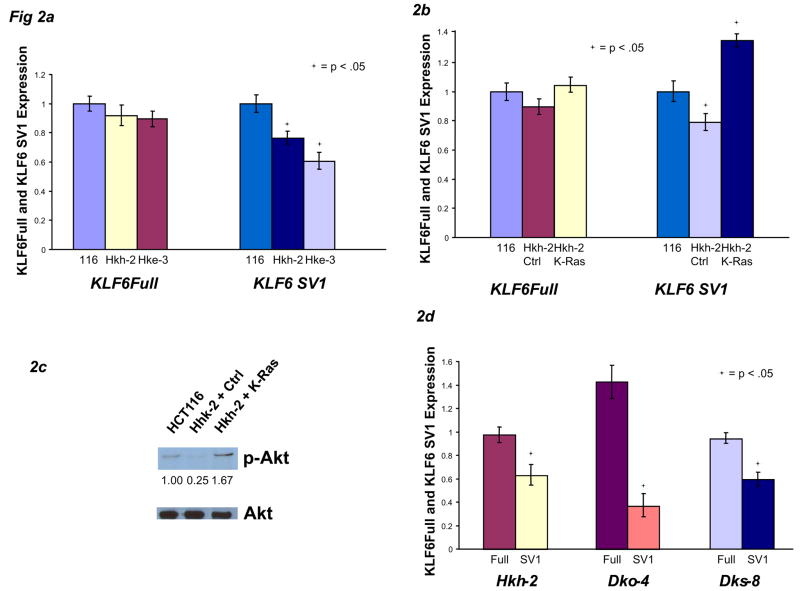
Abrogation of oncogenic Ras signaling decreases KLF6 alternative splicing. A) mRNA from HCT116 cells and the two daughter cell lines, Hkh-2 and Hke-3, were analyzed by qRT-PCR. B-C) HCT116 cells were transfected with control plasmid, Hkh-2 cells were transfected with 1 μg pcDNA-K-Ras, and mRNA and protein were analyzed. Data are normalized to their respective empty-vector controls. D) Hkh-2, Dko-4, and Dks-8 cell lines were plated at 50,000 cells per well, transiently transfected with either 1 μg of pSuper-Luc control vector or pSuper-K-Ras siRNA vector for 48 hours, then harvested for mRNA.
Reconstitution of KLF6 SV1 Restores a Ras-Transformed Phenotype
Similar to earlier results in prostate cancer cell lines26, stable over-expression of KLF6 in DLD-1 colon cancer cell lines resulted in significant growth inhibition (Fig 3A). Conversely, when KLF6 SV1 was stably over-expressed in HCT116 cells, their respective growth rates were significantly increased (Fig 3B). However, stable SV1 over-expression in Hkh-2 cells only partially restored the daughter cell line’s proliferative capacity compared to its parental counterpart, suggesting that, as expected, KLF6 SV1 is only partly responsible for Ras’s growth-promoting effects (data not shown).
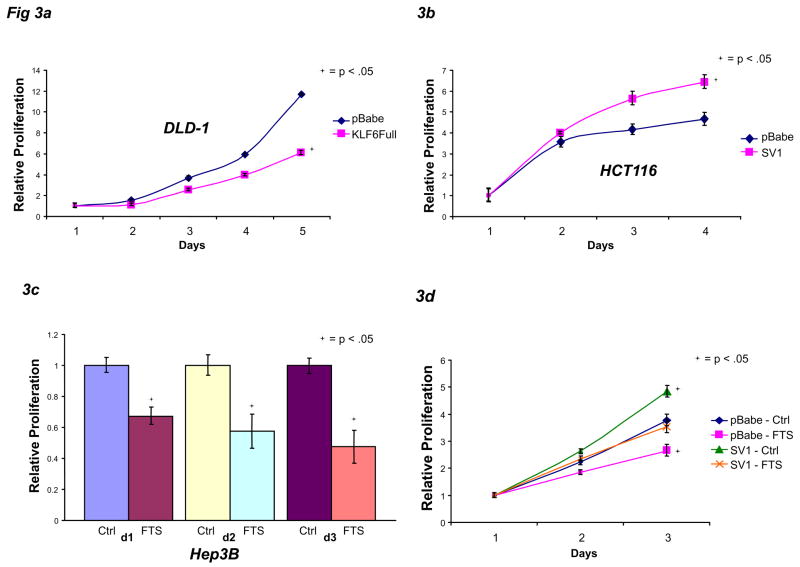
KLF6 SV1 can partially restore decreased cellular proliferation caused by Ras inhibition. A) DLD-1 cells stably over-expressing pBabe control vector or pBabe-KLF6Full were plated at 25,000 cells per well and allowed to grow in media for 5 days. B) HCT116 stably over-expressing pBabe control vector or pBabe-KLF6 SV1 were plated at 25,000 cells per well and allowed to grow in media for 4 days. C) Hep3B cells were plated at 25,000 cells per well and treated with DMSO vehicle control or 100 μM FTS for 3 days. D) Hep3B cells stably over-expressing pBabe control vector or pBabe-KLF6 SV1 were plated at 50,000 cells per well, then treated with DMSO vehicle control or 100 μM FTS for 3 days. Proliferation assays were performed at daily intervals.
We next examined whether KLF6 SV1 could overcome the growth inhibition conferred by blocking Ras. As predicted, incubation of parental Hep3B, HepG2, and PC3M cells with the Ras inhibitor FTS led to significantly decreased proliferation for 72 hours (Fig 3C, Supplementary Fig 4A-B). Next, we chose a representative Hep3B cell line in which we stably over-expressed KLF6 SV1 then assayed for the effects of FTS treatment. Also as predicted, the Hep3B cells stably over-expressing SV1 grew faster than vector-expressing cells, and FTS treatment of empty vector-expressing Hep3B cells greatly reduced their proliferation rates (Fig 3D). However, in Hep3B cells stably transduced with KLF6 SV1, the FTS-mediated reduction in growth rate was partially abrogated (Fig 3D).
In Hep3B cells stably over-expressing SV1, there was no change in KLF6Full expression while SV1 mRNA was increased approximately 12 fold (Supplementary Fig 4C). In the empty-vector transduced Hep3B cells treated with FTS, KLF6 SV1 was significantly decreased (Supplementary Fig 4D), mirroring previous results.
The PI3-K/Akt Pathway Downstream of Ras Signaling Regulates KLF6 Alternative Splicing
Ras proteins transduce their signals through two major kinase cascades, the MAP kinases and PI3-K. Incubation of 293T, BPH1, PC3M, as well as liver cancer lines (Hep3B, Huh7, and HepG2) with MAP kinase inhibitors did not significantly affect KLF6 alternative splicing (data not shown). However, transient over-expression of a dominant negative PI3-K construct33 in PC3M cells significantly decreased SV1 mRNA (Supplementary Fig 5A). Moreover, when Hep3B, Huh7, and HepG2 cells were treated with the PI3-K small molecule inhibitor, LY294002, KLF6 SV1 mRNA was significantly decreased in all three lines (Fig 4A). PI3-K activity was maximally inhibited in HepG2 cells, as depicted by the absence of phospho-Akt upon LY294002 treatment (Supplementary Fig 5B). Because Akt/PKB and mTOR are downstream effectors of PI3-K signaling, we predicted that inhibition of these proteins would result in decreased KLF6 SV1 mRNA. Indeed, the Akt inhibitor, Triciribine, and the mTOR inhibitor, rapamycin, both significantly reduced SV1 expression (Fig 4B-C). Finally, because EGFR tyrosine kinase is among the most potent signals that activate Ras, the effect of EGFR inhibition on KLF6 splicing was assessed. Inhibition of this receptor reduced KLF6 SV1 mRNA expression (Fig 4D).
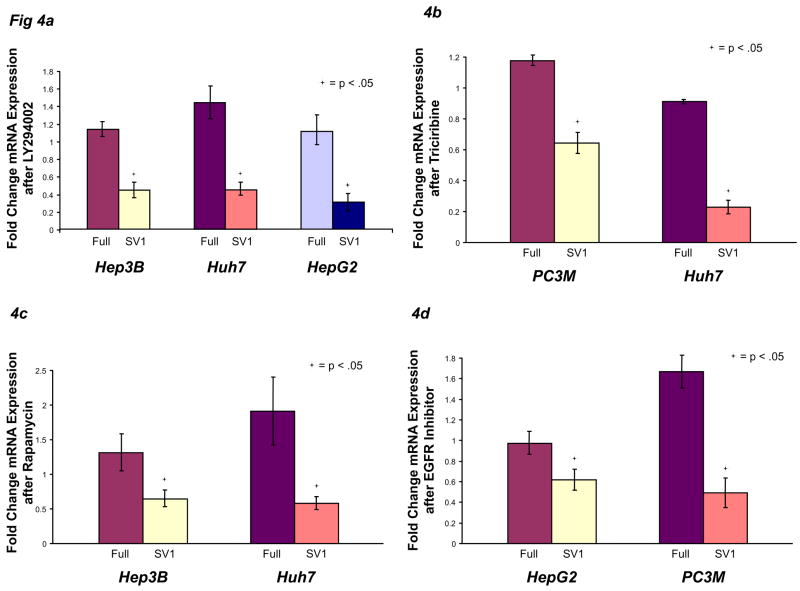
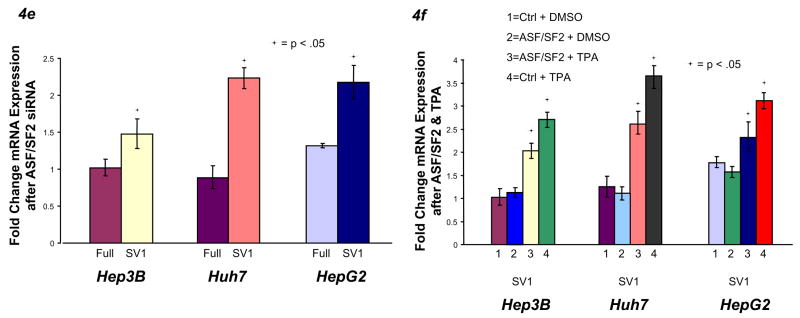
PI3-K/Akt signaling to ASF/SF2 regulates KLF6 alternative splicing. A) Hep3B, Huh7, and HepG2 cells were plated at 50,000 cells per well, treated with 10 μM LY294002 or vehicle control for 24 hours, then harvested for mRNA. B) PC3M and Huh7 cells were treated with 100 μM Triciribine or vehicle control for 24 hours, then harvested for mRNA. C) Hep3B and Huh7 cells were treated with 25 nM rapamycin or vehicle control for 24 hours, then harvested for mRNA. D) HepG2 and PC3M cells were treated with 2.5 μM EGF Receptor inhibitor or vehicle control, then harvested for mRNA. E) Hep3B, Huh7, and HepG2 cells were transfected with 5 nM control siRNA, ASF/SF2 siRNA, or 9G8 siRNA for 48 hours, then harvested for mRNA. F) Hep3B, Huh7, and HepG2 cells were transfected with 1 μg of ASF/SF2 or control plasmid for 24 hours and/or treated with 40 ng/ml TPA or vehicle control, then analyzed for mRNA.
Recently, Akt has been shown to directly phosphorylate and activate both the ASF/SF2 and 9G8 splice regulator proteins34. Combined with our data suggesting Akt-mediated regulation of KLF6 alternative splicing, we examined whether either of these molecules contributed to regulation of KLF6 alternative splicing. siRNA knockdown of ASF/SF234, but not 9G834 (data not shown), significantly increased KLF6 SV1 mRNA expression (Fig 4E, Supplementary Fig 5C), suggesting that this splice protein regulates KLF6 alternative splicing. Finally, although ectopic overexpression of ASF/SF2 alone in liver cancer cell lines did not alter KLF6 alternative splicing, exogenous ASF/SF2 partly reduced TPA-induced expression of SV1 (Fig 4F, Supplementary Fig 5D).
Ras-mediated Alterations in KLF6 Alternative Splicing are Not Due to Transcriptional Activation
A direct role for coupling of transcription with alternative splicing has been suggested by recent studies35. Hence, we investigated the possible effects of Ras signaling on KLF6 transcription through the use of KLF6 promoter-luciferase constructs (Fig 5A). Over-expression or knockdown of oncogenic K-Ras did not alter KLF6 promoter-luciferase activities (Fig 5B-C, Supplementary Fig 5F), suggesting that Ras does not transcriptionally regulate KLF6.
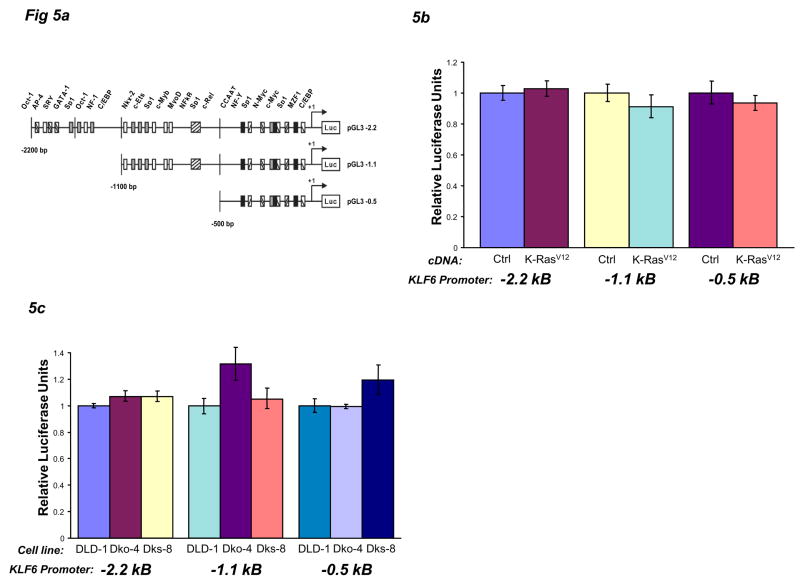
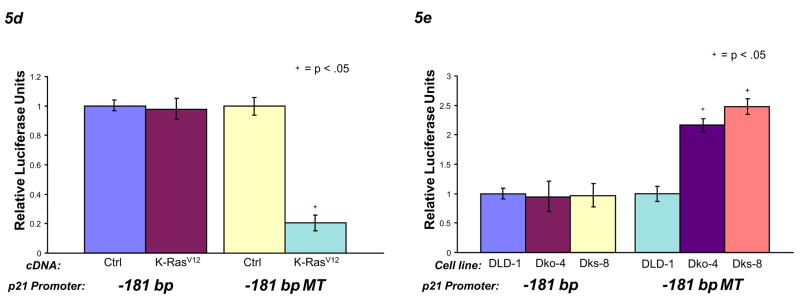
KLF6 alternative splicing is not coupled to transcription. A) A schematic diagram of three KLF6 promoter-luciferase constructs showing specific promoter binding elements. B) 293T cells were plated at 50,000 cells per well, co-transfected with pGL3-2.2 kb, pGL3-1.1 kb, or pGL3-0.5 kb promoter plasmids and pcDNA-K-RasV12 or control plasmid, then harvested 24 hours later for luciferase assay. All data are normalized to their empty-vector controls. C) DLD-1, Dko-4, and Dks-8 cells were plated at 50,000 cells per well, transfected with pGL3-2.2 kb, pGL3-1.1 kb, or pGL3-0.5 kb promoter plasmids, then harvested 24 hours later for luciferase assay. D) 293T cells were plated at 50,000 cells per well, co-transfected with pGL3-181 bp or pGL3-181 bp MT (mutated CCAAT box) promoter plasmids and pcDNA-K-RasV12, then harvested 24 hours later for luciferase assay. E) DLD-1, Dko-4, and Dks-8 cells were plated at 50,000 cells per well, transfected with pGL3-181 bp or pGL3-181 bp MT promoter plasmids, then harvested 24 hours later for luciferase assay.
In contrast to the lack of effect on KLF6 promoter activity by Ras signaling, the p21 promoter luciferase construct is responsive to changes in KLF6 splicing21. GC boxes within this p21 promoter (pGL3 -181 bp) are required for KLF6-mediated transcriptional regulation, and levels of KLF6 SV1 are inversely correlated with activity of this promoter construct. The GC box-specific p21 promoter construct (pGL3 -181 bp MT) has a mutated CCAAT box, which eliminates responsiveness to most transcription factors except KLF6 and other, related GC box-binding proteins. Either the GC-box p21 promoter plasmid21, or a normal p21 promoter plasmid, was co-transfected as described above. When oncogenic K-Ras and a p21 promoter-luciferase construct were co-expressed in 293T cells, the Ras-induced increase in KLF6 SV1 also resulted in decreased GC box-specific p21 promoter activity, but no change in total p21 promoter activity (Fig 5D). Conversely, somatic cell genetic deletion of an oncogenic K-Ras allele, which decreased KLF6 SV1 mRNA, increased GC box-specific p21 promoter activity, with no change in total p21 promoter activity (Fig 5E). The lack of change in the total p21 promoter-luciferase activity was verified by qRT-PCR of p21 mRNA (data not shown). Although many other transcription factors can also bind GC boxes, this data when combined with previous results, suggests that changes in KLF6 alternative splicing occur independently of its own transcriptional regulation but can affect KLF6 transcriptional activity on other promoters such as p21.
Discussion
We propose a novel mechanism of tumor suppressor inactivation in liver cancer via Ras-mediated enhanced alternative splicing of KLF6 into growth-promoting isoforms. KLF6 SV1 expression is increased in an oncogenic Ras/PI3-K/Akt-dependent manner, thereby altering the relative ratio of KLF6Full to SV1, which leads to enhanced cellular proliferation. Ras transduces its signal through the splice regulatory protein ASF/SF2, which, when abrogated, leads to significantly increased KLF6 SV1 mRNA, indicating a requirement for ASF/SF2 to allow KLF6Full mRNA expression. Moreover, our data also demonstrate that ASF/SF2-mediated changes in KLF6 alternative splicing are not coupled to changes in KLF6 transcription. A model incorporating these observations is depicted in Figure 6, in which EGF receptor tyrosine kinase activity recruits Ras to the inner cell membrane, transducing signals through PI3-K, Akt, and possibly mTOR, and finally to ASF/SF2. When activated by Akt, this splice regulatory protein enhances KLF6 alternative splicing, increasing SV1 mRNA expression, and altering the KLF6Full/SV1 ratio.
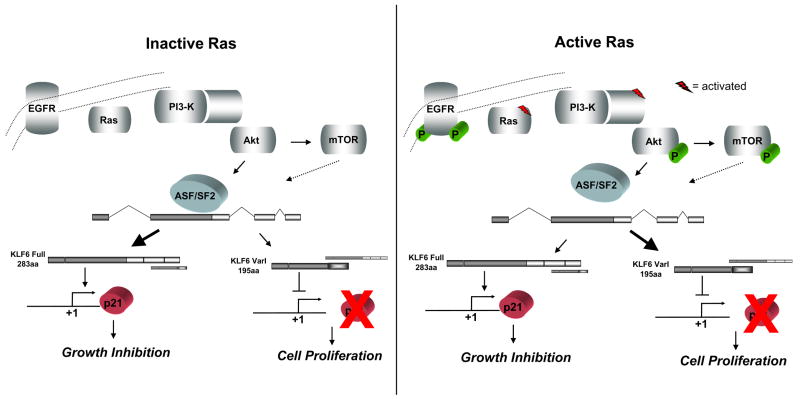
A model linking Ras signaling to KLF6 alternative splicing. When Ras signaling is not activated, ASF/SF2 generates primarily KLF6Full, which leads to p21-mediated growth inhibition. When Ras is activated, signals are transduced through PI3-K/Akt to ASF/SF2, up-regulating KLF6 SV1 expression and leading to cellular proliferation. EGFR can potentially activate Ras signaling. Although not tested here, mTOR may also play a role in ASF/SF2-dependent or -independent KLF6 alternative splicing.
The primary function of ASF/SF2 and other SR protein family members is splice-site selection35, a role that can potentially affect KLF6 splicing selection. SR proteins interact with pre-mRNAs through their arginine and serine-rich domains, which also promote protein-protein interactions with core splicing machinery proteins36. ASF/SF2 shuttles between the nucleus and cytoplasm, and in addition to its splicing regulation, may also affect cytoplasmic mRNA stability37. It is likely that ASF/SF2 directs spliceosome assembly to promote production of KLF6Full. Conversely, activation of Ras signaling can lead to phosphorylation of ASF/SF2, which leads to enhanced generation of KLF6 SV1.
Recently, ASF/SF2 has been identified as a proto-oncogene that is amplified in human tumors and can transform immortalized mouse fibroblasts38. ASF/SF2 can alter splicing of the tumor suppressor BIN1 (exon inclusion results in abolished inhibition of c-myc), MNK2 (increased expression of catalytically active splice isoform leading to phosphorylation of eIF4E), and the translation regulatory kinase S6K1 (increased expression of oncogenic isoform), resulting in altered functions of these proteins, all of which contribute to ASF/SF2’s oncogenic potential38. Since ASF/SF2 can regulate alternative splicing in a concentration-dependent manner, this protein might affect splicing of different sets of target pre-mRNAs, including KLF6. These data highlight ASF/SF2’s complex network of targets, which when alternatively spliced, can lead to context-dependent and possibly divergent biological outcomes.
Previous work by our laboratory linked regulation of KLF6 alternative splicing to the splice factor SRp4025, a protein known to act downstream of Ras/Akt-signaling41. In this model, a single nucleotide polymorphism in KLF6 intron 1 abrogated ASF/SF2’s binding site and created a SRp40 motif, increasing KLF6 alternative splicing25. Although first discovered in prostate cancer samples, HCCs may also harbor this SNP, providing a second potential mechanism favoring enhanced KLF6 splicing to be tested in future studies.
Our experimental findings suggest an expanded role for Ras in human hepatocellular carcinoma. Not only does oncogenic Ras lead to significant changes in transcriptional and translational programs, but it also regulates alternative splicing programs for KLF6 and possibly other tumor suppressors.
These data also raise an interesting question of how extracellular signals converge to regulate alternative splicing events in promoting tumor progression. Targeted analysis of the molecular pathways that regulate KLF6 alternative splicing will provide further insight into the role of this gene in the progression of human cancer.
Acknowledgments
Grant Support: SY, Howard Hughes Medical Institute Medical Student Research Fellowship; SLF, NIH Grants DK37340 and DK56621, Dept of Defense DAMD17-03-1-0100; XZ, Howard Hughes Medical Institute Medical Student Research Fellowship; JAM, NCI-RO1-CA122332; JML, NIDDK 1R01DK076986-01, I+D Program Spain (SAF-2007-61898), Samuel Waxman Cancer Research Foundation; AV, Fundacion Pedro Barrie de la Maza and a recipient of a National Cancer Center Fellowship
We thank: A. Chan of Mount Sinai School of Medicine, New York, NY, for the DN PI3-K, H-RasR12, and K-RasV12 constructs; M. Schwartz (Mount Sinai), J. Bruix (BCLC group-Hospital Clinic) and V. Mazzaferro (National Cancer Institute, Milan) for providing HCC samples; A. Dhillon of Beatson Institute for Cancer Research, United Kingdom, for the HCT116 cell lines (Hkh-2 and Hke-3) and DLD-1 cell lines (Dko-4 and Dks-8); Y. Kloog of Tel-Aviv University and S. Reif of Souraski Medical Center, Israel, for the FTS.
Footnotes
The authors declare no conflict of interest.
Author contributions: SY, GN, SLF designed research; SY, GN, XZ, RG, STK, EH, AV, JL, MT performed research; KA, SS, TS, contributed new reagents/analytic tools; SY, GN, JAM, JML, SLF analyzed data; SY and SLF wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1053/j.gastro.2008.02.015
Read article for free, from open access legal sources, via Unpaywall:
http://www.gastrojournal.org/article/S0016508508002461/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1053/j.gastro.2008.02.015
Article citations
Decoding the role of aberrant RNA alternative splicing in hepatocellular carcinoma: a comprehensive review.
J Cancer Res Clin Oncol, 149(19):17691-17708, 29 Oct 2023
Cited by: 1 article | PMID: 37898981
Review
The role of alternative pre-mRNA splicing in cancer progression.
Cancer Cell Int, 23(1):249, 24 Oct 2023
Cited by: 1 article | PMID: 37875914 | PMCID: PMC10594706
Review Free full text in Europe PMC
Posttranslational splicing modifications as a key mechanism in cytarabine resistance in acute myeloid leukemia.
Leukemia, 37(8):1649-1659, 08 Jul 2023
Cited by: 2 articles | PMID: 37422594 | PMCID: PMC10400425
How Driver Oncogenes Shape and Are Shaped by Alternative Splicing Mechanisms in Tumors.
Cancers (Basel), 15(11):2918, 26 May 2023
Cited by: 0 articles | PMID: 37296881 | PMCID: PMC10251868
Review Free full text in Europe PMC
Pharmacological activities of Artemisia absinthium and control of hepatic cancer by expression regulation of TGFβ1 and MYC genes.
PLoS One, 18(4):e0284244, 13 Apr 2023
Cited by: 1 article | PMID: 37053209 | PMCID: PMC10101520
Go to all (69) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Hepatocyte growth factor enhances alternative splicing of the Kruppel-like factor 6 (KLF6) tumor suppressor to promote growth through SRSF1.
Mol Cancer Res, 10(9):1216-1227, 02 Aug 2012
Cited by: 48 articles | PMID: 22859706
Identification of miRNAs that specifically target tumor suppressive KLF6-FL rather than oncogenic KLF6-SV1 isoform.
RNA Biol, 11(7):845-854, 12 Jun 2014
Cited by: 30 articles | PMID: 24921656 | PMCID: PMC4179959
Enhanced hepatocarcinogenesis in mouse models and human hepatocellular carcinoma by coordinate KLF6 depletion and increased messenger RNA splicing.
Hepatology, 56(4):1361-1370, 27 Aug 2012
Cited by: 27 articles | PMID: 22535637 | PMCID: PMC3412196
The role of KLF6 and its splice variants in cancer therapy.
Drug Resist Updat, 12(1-2):1-7, 18 Dec 2008
Cited by: 85 articles | PMID: 19097929
Review
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: R01 CA122332
NIDDK NIH HHS (8)
Grant ID: DK37340
Grant ID: R01 DK076986
Grant ID: R01 DK037340-22
Grant ID: 1R01DK076986-01
Grant ID: R01 DK037340
Grant ID: R01 DK056621
Grant ID: R56 DK056621
Grant ID: DK56621





