Abstract
Objective
Systemic juvenile idiopathic arthritis (JIA) is associated with macrophage activation syndrome. Macrophage activation syndrome bears a close resemblance to familial hemophagocytic lymphohistiocytosis (HLH). The development of familial HLH has been recently associated with mutations in MUNC13-4. The purpose of this study was to assess for possible sequence alterations in MUNC13-4 in patients with systemic JIA/macrophage activation syndrome.Methods
The MUNC13-4 sequence was analyzed in 18 unrelated patients with systemic JIA/macrophage activation syndrome, using 32 primer pair sets designed to amplify the 32 exons and at least 100 basepairs of the adjacent intronic regions. DNA samples obtained from 73 unrelated patients with systemic JIA and no history of macrophage activation syndrome and 229 unrelated healthy individuals were used as controls.Results
The biallelic sequence variants in MUNC13-4 reported in familial HLH were present in 2 of the 18 patients with JIA/macrophage activation syndrome. Further analysis of the MUNC13-4 sequences revealed an identical combination of 12 single-nucleotide polymorphisms (SNPs) in 9 of the remaining 16 patients with systemic JIA/macrophage activation syndrome (56%). Additional analysis suggested that these 12 SNPs (154[-19] g>a, 261[+26] c>g, 388[+81] g>a, 388[+122] c>t, 570[-60] t>g, 888 G>C, 1389[+36] g>a, 1992[+5] g>a, 2447[+144] c>t, 2599 A>G, 2830[+37] c>g, 3198 A>G) were inherited as an extended haplotype. In several patients, in addition to the described haplotype, there were other SNPs in the second allele of MUNC13-4. Moreover, 1 patient had a complex mutation with 2 changes, 2542 A>C and 2943 G>C, in a cis configuration. The haplotype was present in only 27 (12%) of 229 healthy control subjects (chi(2) = 23.5) and in 6 (8.2%) of 73 patients with systemic JIA and no history of macrophage activation syndrome.Conclusion
The data suggest an association between MUNC13-4 polymorphisms and macrophage activation syndrome in patients with systemic JIA.Free full text

Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis is Associated With MUNC13-4 Gene Polymorphisms
Abstract
Objective
Systemic Juvenile Idiopathic Arthritis (SJIA) is associated with macrophage activation syndrome (MAS). MAS bears close resemblance to familial hemophagocytic lymphohistiocytosis (FHLH). The development of FHLH has been recently associated with mutations in MUNC13-4 gene. The purpose of this study was to assess for possible sequence alterations in MUNC13-4 gene in SJIA/MAS.
Methods
MUNC13-4 sequence was analyzed in 18 unrelated patients with SJIA/MAS using 32 primer pair sets designed to amplify the 32 exons and at least 100 base pairs of the adjacent intronic regions. DNA samples from unrelated 73 SJIA patients without MAS history and 229 healthy unrelated individuals were used as controls.
Results
Bi-allelic sequence variants in MUNC13-4 gene reported in FHLH were present in two of 18 patients. Further analysis of the MUNC13-4 sequences revealed an identical combination of 12 single nucleotide polymorphisms (SNP) in 9 of remaining 16 SJIA/MAS patients (57%). Additional analysis suggested that these 12 SNPs [154(-19)g>a, 261(+26)c>g, 388(+81)g>a, 388(+122)c>t, 570(-60)t>g, 888G>C, 1389(+36)g>a, 1992(+5)g>a, 2447(+144)c>t, 2599A>G, 2830(+37)c>g, 3198A>G] were inherited as an extended haplotype. In several patients, in addition to the described haplotype, there were other SNPs in the second allele of the MUNC13-4 gene. Moreover, one patient had a complex mutation with two changes, 2542A>C and 2943G>C in a cis configuration. The haplotype was present only in 27 of 229 (12%) healthy controls (Chi Square =23.5) and in 6 of 73 (8.2 %) SJIA patients without MAS history.
Conclusions
The data suggest an association between MUNC13-4 gene polymorphisms and MAS in SJIA.
Macrophage Activation Syndrome (MAS) is a severe, potentially fatal condition associated with excessive activation of macrophages and T cells leading to an overwhelming inflammatory reaction. The main manifestations of MAS include fever, hepatosplenomegaly, lymphadenopathy, severe cytopenias, serious liver disease, and disseminated intravascular coagulation [1,2]. The pathognomonic feature of MAS is often found in bone marrow: numerous, well-differentiated macrophages phagocytosing hematopoietic elements. Although MAS has been reported in patients with different rheumatic diseases, it is most strongly associated with Systemic Juvenile Idiopathic Arthritis (SJIA). In fact, it accounts for much of the morbidity and mortality seen in this form of JIA. At least 10% of the patients with SJIA develop MAS [3]. The true incidence of MAS might be much higher since there are no validated diagnostic criteria and mild instances of MAS are not always recognized [4,5].
It is now recognized that MAS bears close resemblance to a group of histiocytic disorders collectively known as hemophagocytic lymphohistiocytosis (HLH) [2,6]. HLH is a term that describes a spectrum of disease processes characterized by accumulations of well-differentiated mononuclear cells with a macrophage phenotype [7]. In the contemporary classification of histiocytic disorders, HLH is further subdivided into primary, or familial HLH, and secondary, or reactive HLH (ReHLH) [7]. Clinically, however, they may be difficult to distinguish from each other [9]. Familial hemophagocytic lymphohistiocytosis (FHLH) is a constellation of rare autosomal recessive immune disorders. The clinical symptoms of FHLH usually become evident within the first 2 months of life although initial presentation as late as 22 years of age has been reported [8]. Secondary HLH tends to occur in older children and more often is associated with an identifiable infectious episode, most notably Epstein-Barr virus (EBV) or cytomegalovirus (CMV) infection. The exact pathophysiological relationship between MAS and HLH is not understood. Some pediatric rheumatologists view MAS as ReHLH occurring in a setting of a rheumatologic disease [6].
The pathological mechanisms of HLH/MAS are not fully understood. In HLH, there is uncontrolled proliferation of T cells and macrophages that has been linked to decreased NK-cell and cytotoxic T-cell function [10] often due to mutations in the gene encoding perforin [11]. Perforin is a protein which cytolytic cells utilize to induce apoptosis of target cells such as tumor cells or cells infected by viruses. It has been hypothesized by some authors that abnormal cytotoxic cells may fail to provide appropriate apoptotic signals for removal of activated macrophages and T cells during the contraction stage of certain immune responses [12]. Our recent observations suggest that as in HLH, MAS patients have profoundly depressed NK-cell function, often associated with abnormal perforin expression [13].
More recently, mutations in another gene, MUNC13-4, have been implicated in the development of hemophagocytic lymphohistiocytosis in about 10-30% of patients with inherited HLH [14]. The protein encoded by the MUNC13-4 gene is an essential effector of the cytolytic secretory pathway. MUNC13-4 protein is involved in vesicle priming function which follows granule docking and precedes plasma granule membrane fusion [14]. Therefore, it is an important player in the intracellular transport of perforin. Although the cytolytic cells of the patients with FHLH caused by MUNC13-4 mutations produce sufficient amounts of perforin, the poor ability to deliver perforin to the surface of the cells leads to profoundly decreased cytolytic activity against target cells. Here we present new data that suggests an association between MAS in SJIA and specific mutations and/or haplotypes in MUNC13-4 gene.
MATERIALS AND METHODS
Patients
Ninety one unrelated patients included in the study met the ELAR diagnostic criteria for Systemic Juvenile Idiopathic Arthritis [15]. 18 of 91 SJIA patients had developed MAS at some point during the course of the disease. The retrospective chart review of the remaining 73 patients did not reveal findings suggestive of MAS. The diagnosis of MAS in 17 patients was established by managing clinicians based on the combination of cytopenias, coagulopathy and liver dysfunction. One patient was included in this group based mainly on the presence of hemophagocytic macrophages in the bone marrow aspirate. Overall, diagnostic bone marrow aspiration was performed in 10 of 18 patients, and hemophagocytosis was demonstrated in all 10 samples. Ten of the 18 patients were seen in the Cincinnati Children’s Hospital Medical Center (CCHMC) Rheumatology clinic. The remaining eight patients were from other pediatric institutions; their DNA samples were submitted to the laboratory of Human Genetics at CCHMC to rule out FHLH. All 18 patients have been analyzed for PRF1 gene mutations in Exons 2 and 3 (coding regions) as previously described [13], and no mutations were identified. The patients were of diverse ethnic origins, including 14 Caucasians, 2 African Americans, 2 Latin American, one Native American, and one Asian American individuals from different regions of the United States and Canada. The subjects enrolled in this study at CCHMC provided written informed consent approved by the Institutional Review Board. A total of 229 healthy individuals from Southern Ohio population were included in the study as controls. The control group included 34 individuals of the African American descent.
Mutational Analysis by PCR and direct sequencing
Genomic DNA was isolated from peripheral blood or buccal swabs using standard techniques. To assess for possible sequence alterations in the MUNC13-4 gene, 32 primer pair sets were designed based on the genomic sequence of the MUNC13-4 gene to amplify the 32 exons (Figure 1) and included at least 100 base pairs of the adjacent intronic regions [14]. Amplified exons from patients and control subjects were purified by the EXOSapit (USB, Cleveland, Ohio) and sequenced by cycle sequencing using BigDye Terminator Sequencing Kit. Labeled products were separated by ABI 3730 automated DNA sequencer (Applied Biosystem, Forester city, California). Raw sequence data were then analyzed by Sequencher® 4.7 according to the consensus sequence retrieved from NCBI.
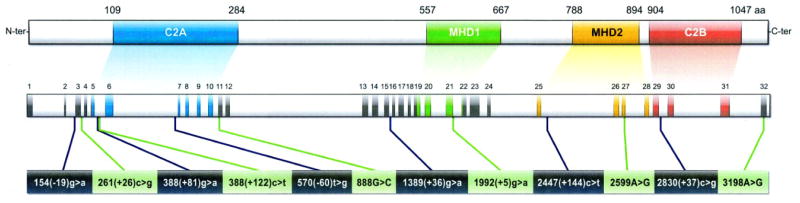
The upper panel represents the structure of the MUNC13-4 protein (adapted from [14]). The middle panel shows the corresponding genomic organization (the shaded areas correspond to the 32 exons). The lower panel shows the sites of the 12 single nucleotide polymorphisms (SNP) present in the MAS-associated haplotype described in the text. Note that only one of the 12 SNPs (i.e. 2595A>G) is non synonymous.
RESULTS
DNA samples from eighteen patients with SJIA and a history of MAS were sequenced as described in Methods. Bi-allelic sequence variants in MUNC13-4 gene reported in FHLH were present in two of these 18 patients. Both patients were of African American descent and shared one allele with the mutation that affects +3 position at a splicing donor site in intron 9 of the MUNC13-4 gene. Mutations at this position could result in abnormal alternative splicing of exon 9 during mRNA processing. Exon 9 is located in the C2A domain, which contain a conserved Ca2+ binding motif. Mutations in C2A domain modulate the binding affinity to Ca2+ and/or protein co-factors interacting with that region. Both of these patients also carried a second missense mutation (3145C>G and 1579 C>T, respectively). Interestingly, the latter patient had hemophagocytosis in the bone marrow, but did not meet the clinical criteria for the diagnosis of FHLH. This patient had been described in detail elsewhere as a case report (Hazen et al, A&R, in press). None of the MUNC13-4 mutations were found in normal controls.
Sequence information for the 16 remaining patients is summarized in Table 1 and reveals a common pattern of sequence variant for 9 of these 16 patients. This common pattern is highlighted in yellow in Table1 and includes an identical combination of 12 single nucleotide polymorphisms (SNP) in the MUNC13-4 gene. The specific pattern of interest is defined as 154 (-19) g>a, 261 (+26) c>g, 388 (+81) g>a, 388 (+122) c>t, 570 (-60) t>g, 888 G>C, 1389 (+36) g>a, 1992 (+5) g>a, 2447 (+144) c>t, 2599 A>G, 2830 (+37) c>g, 3198 A>G (See Figure 1).
Table 1
MUNC13-4 SNPs observed in SJIA patients with MAS.
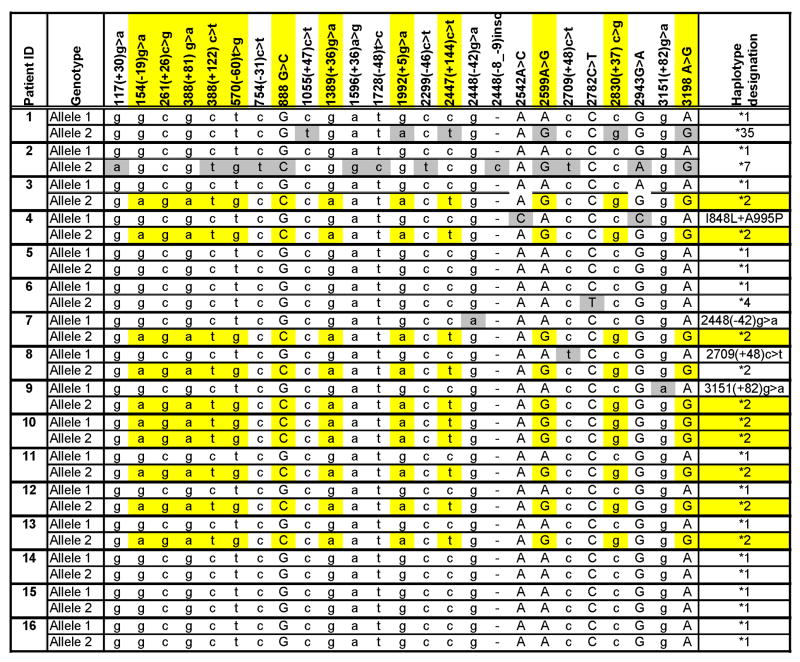 |
Capital letters indicate nucleotide substitutions in the coding regions, small case letters indicate changes in the introns or non-coding regions
The common pattern (highlighted in yellow) includes an identical combination of 12 SNPs in the MUNC13-4 gene inherited as an extended haplotype (designated “*2” with “*1” being the major allele). Note that patient #10 is homozygous for all 12 SNPs. In several patients, in addition to the presence of the described haplotype, there are other SNPs in the second allele of the MUNC13-4 gene (highlighted in grey). Patient #4 has a complex mutation with two changes, 2542 A>C (I848L) and 2943 G>C (A995P) in a cis configuration.
One of the SJIA/MAS patients (#10) was homozygous for all 12 SNPs suggesting that these SNPs might be inherited as an extended haplotype (two alleles highlighted by yellow shading in Table 1). To confirm this, the genomic sequence of the MUNC13-4 gene was then analyzed in the parents of one of the SJIA/MAS patients who was heterozygous for the 12 SNPs. This additional analysis revealed the presence of the same series of SNPs in one of the parents, suggesting that these SNPs are indeed likely to be inherited as an extended haplotype (designated “*2” in Table 1 with “*1” being the major allele). In several patients, in addition to the presence of the described haplotype, there were other SNPs in the second allele of the MUNC13-4 gene. Moreover, Patient #4 (Table 1) had a complex mutation with two changes, 2542 A>C (I848L) and 2943 G>C (A995P) in a cis configuration. This complex mutation has been seen in patients with FHLH [Zhang et al, unpublished observations].
Next, the minor allele frequency for the 12 SNPs was determined in a set of 229 local control DNAs. Haplotype determination in these samples was performed using direct sequencing. The minor allele frequencies and the total alleles assayed are shown in the last row of Table 1. Overall, the described haplotype (designated “*2” in Table 1) was present in 9 of the 16 MAS patients (57%) and only in 27 of 229 (12%) controls (Chi Square =23.5). In the control group, the proportion of Caucasian individuals carrying the haplotype (23 /195 or 12%) was similar to that in African American controls (4/34 or 12%).
To assess whether the described haplotype was associated with not only MAS, but also with SJIA in general, the study was expanded to include patients with SJIA without MAS history. The frequency of the haplotype in this group of patients was 6/73 (8.2%) compared to 27/229 (12%) in healthy controls (Chi Square=1.4), suggesting the association between the presence of the haplotype and MAS, but not SJIA in general.
DISCUSSION
The examination of the sequence of the MUNC13-4 gene in patients with Systemic Juvenile Idiopathic Arthritis revealed bi-allelic mutations previously reported in FHLH in two of 18 patients with MAS presenting as a complication of SJIA. The presence of bi-allelic mutations in the MUNC13-4 gene in these two patients is sufficient to establish the definite diagnosis of Familial HLH [9]. In addition these two patients also satisfied the criteria for systemic JIA [15] suggesting that there may be an overlap between the two diseases.
The analysis of the sequence of the MUNC13-4 gene in the remaining 16 MAS patients revealed a common pattern of sequence variants for 9 of these 16 patients. Increased frequency of the SNPs within the MUNC13-4 gene in MAS patients inherited as a unique haplotype is highly intriguing. It provides further support to the concept that there are pathophisiologic pathways common to both Familial Hemophagocytic Lymphohistiocytosis and SJIA. Given striking similarities in the clinical presentation of FHLH and MAS, the described MUNC13-4 gene polymorphisms may be relevant to the development of the predisposition to MAS in systemic JIA. Since the frequency of the described haplotype in SJIA patients without MAS history is similar to controls, it’s presence may help identify SJIA patients at long term risk for MAS.
Another important question is whether the observed polymorphism in the MUNC 13-4 is associated with abnormal function of the MUNC13-4 protein and, thus, directly contributes to the development of MAS. Another possibility is that the described haplotype may extend either upstream or downstream of the MUNC13-4 gene and involve additional polymorphisms in other immunologically relevant genes. Therefore, further studies are necessary to establish the extent of the MAS-associated haplotype. Another important question to address in the future studies is whether the frequency of the described haplotype is also increased in Reactive HLH.
Acknowledgments
Supported, in part, by the NIH grant AR047784 and by a Translational Research Initiative Grant from Children’s Hospital Research Foundation of Cincinnati.
Contributor Information
Kejian Zhang, Division of Human Genetics, Children’s Hospital Medical Center, Cincinnati, OH 45229.
Jennifer Biroschak, Division of Human Genetics, Children’s Hospital Medical Center, Cincinnati, OH 45229.
David N. Glass, William S. Rowe Division of Rheumatology, Children’s Hospital Medical Center, Cincinnati, OH 45229.
Susan Thompson, William S. Rowe Division of Rheumatology, Children’s Hospital Medical Center, Cincinnati, OH 45229.
Terri Finkel, Children’s Hospital of Philadelphia, PA 19104, USA.
Murray H. Passo, William S. Rowe Division of Rheumatology, Children’s Hospital Medical Center, Cincinnati, OH 45229.
Bryce A. Binstadt, University of Minnesota, Minneapolis, MN 55455.
Alexandra Filipovich, Division of Hematology/Oncology, Children’s Hospital Medical Center, Cincinnati, OH 45229.
Alexei A. Grom, William S. Rowe Division of Rheumatology, Children’s Hospital Medical Center, Cincinnati, OH 45229.
References
Full text links
Read article at publisher's site: https://doi.org/10.1002/art.23734
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2779064?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Functional role of UNC13D in immune diseases and its therapeutic applications.
Front Immunol, 15:1460882, 14 Oct 2024
Cited by: 0 articles | PMID: 39469717 | PMCID: PMC11513310
Review Free full text in Europe PMC
Clinical and genetic analysis of macrophage activation syndrome complicating juvenile idiopathic inflammatory myopathies.
Pediatr Res, 24 Aug 2024
Cited by: 0 articles | PMID: 39181985
Single center clinical analysis of macrophage activation syndrome complicating juvenile rheumatic diseases.
Pediatr Rheumatol Online J, 22(1):58, 23 May 2024
Cited by: 0 articles | PMID: 38783316 | PMCID: PMC11112803
New discoveries in the genetics and genomics of systemic juvenile idiopathic arthritis.
Expert Rev Clin Immunol, 20(9):1053-1064, 02 May 2024
Cited by: 0 articles | PMID: 38641907
Review
Advancements and progress in juvenile idiopathic arthritis: A Review of pathophysiology and treatment.
Medicine (Baltimore), 103(13):e37567, 01 Mar 2024
Cited by: 0 articles | PMID: 38552102 | PMCID: PMC10977530
Review Free full text in Europe PMC
Go to all (107) article citations
Other citations
Wikipedia
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis.
Arthritis Rheumatol, 66(12):3486-3495, 01 Dec 2014
Cited by: 101 articles | PMID: 25047945 | PMCID: PMC4321811
Brief Report: Novel UNC13D Intronic Variant Disrupting an NF-κB Enhancer in a Patient With Recurrent Macrophage Activation Syndrome and Systemic Juvenile Idiopathic Arthritis.
Arthritis Rheumatol, 70(6):963-970, 02 May 2018
Cited by: 22 articles | PMID: 29409136 | PMCID: PMC5984660
Genetic loci contributing to hemophagocytic lymphohistiocytosis do not confer susceptibility to systemic-onset juvenile idiopathic arthritis.
Arthritis Rheum, 58(3):869-874, 01 Mar 2008
Cited by: 19 articles | PMID: 18311812 | PMCID: PMC2675009
Associations between interleukin-10 polymorphisms and susceptibility to juvenile idiopathic arthritis: a systematic review and meta-analysis.
Eur Cytokine Netw, 29(1):16-26, 01 Mar 2018
Cited by: 2 articles | PMID: 29748155
Review
Funding
Funders who supported this work.
NIAMS NIH HHS (6)
Grant ID: K08 AR054317-01
Grant ID: AR-47363
Grant ID: K08 AR054317
Grant ID: P60 AR047784
Grant ID: P60 AR047784-05
Grant ID: AR-047784





