Abstract
Free full text

Digenic inheritance of non-syndromic deafness caused by mutations at the gap junction proteins Cx26 and Cx31
Abstract
Mutations in the genes coding for connexin 26 (Cx26) and connexin 31 (Cx31) cause non-syndromic deafness. Here, we provide evidence that mutations at these two connexin genes can interact to cause hearing loss in digenic heterozygotes in humans. We have screened 108 GJB2 heterozygous Chinese patients for mutations in GJB3 by sequencing. We have excluded the possibility that mutations in exon 1 of GJB2 and the deletion of GJB6 are the second mutant allele in these Chinese heterozygous probands. Two different GJB3 mutations (N166S and A194T) occurring in compound heterozygosity with the 235delC and 299delAT of GJB2 were identified in three unrelated families (235delC/N166S, 235delC/A194T and 299delAT/A194T). Neither of these mutations in Cx31 was detected in DNA from 200 unrelated Chinese controls. Direct physical interaction of Cx26 with Cx31 is supported by data showing that Cx26 and Cx31 have overlapping expression patterns in the cochlea. In addition, by coimmunoprecipitation of mouse cochlear membrane proteins, we identified the presence of heteromeric Cx26/Cx31 connexons. Furthermore, by cotransfection of mCherry-tagged Cx26 and GFP-tagged Cx31 in human embryonic kidney-293 cells, we demonstrated that the two connexins were able to co-assemble in vitro in the same junction plaque. Together, our data indicate that a genetic interaction between these two connexin genes can lead to hearing loss.
INTRODUCTION
Hearing loss is one of the most common inherited disorders and is a highly heterogeneous sensory disorder. Until now, over 100 loci and 46 different genes in which mutations cause monogenic nonsyndromic sensorineural hearing loss, have been reported (http:webhost.ua.ac.be/hhh/). Despite this heterogeneity, in many populations, up to 50% of autosomal recessive non-syndromic sensorineural hearing loss (AR-NSNHL) is associated with mutations in the locus DFNB1 (MIM 220290) on chromosome 13q12, which contains the two connexin (Cx) genes (GJB2 and GJB6). Cxs are membrane-spanning proteins that coassemble into intercellular gap junction channels. Gap junction channels mediate electrical and biochemical communication between adjacent cells and play vital roles as mediators of intercellular molecular signaling. Cx-linked deafness highlights the key role of gap junctions in the physiological processes of hearing. Colocalization of Cxs with the gap junction system in the inner ear suggests a role in cochlear electrolyte homeostasis. During auditory transduction, they are proposed to maintain membrane potentials by regulating the flow of potassium ions between the sensory epithelia of the inner ear (Simon and Goodenough 1998; White and Paul 1999). To date, mutations in the genes encoding three of these Cxs (GJB2 for Cx26 (MIM 121011), GJB6 for Cx30 (MIM 604418), and GJB3 for Cx31 (MIM 603324) are known to result in hearing impairment (Kelsell et al. 1997; Grifa et al. 1999; Xia et al. 1998). The defect in any two of the four alleles from GJB2 and GJB6 could result in hearing impairment. Thus, either monogenic or digenic inheritance can occur with these genes. Among individuals with DFNB1-associated AR-NSNHL, 98% are estimated to carry two identifiable mutations in GJB2, whereas 2% are reported to have mutations in both GJB2 and GJB6 (Genetests DFNB1, http://www.genetests.org/). Mutations in GJB3 have originally been shown to underlie an autosomal dominant form of non-syndromic deafness (DFNA2) in Chinese patients (Xia et al. 1998). We have also reported an autosomal recessive non-syndromic form of GJB3 mediated deafness in this population (Liu et al. 2000). In Spanish patients, several Cx31 variants have been associated with a syndromic form of neuropathy and hearing loss (Lopez-Bigas et al. 2000; 2001). Variations in the Cx31 gene have also been linked to nonsyndromic deafness in Brazilian patients (Alexandrino et al. 2004). Mutations in the GJB3 gene have also been reported to cause both autosomal dominant and recessive skin diseases (Plantard et al.. 2003; Richard et al. 1997; 1998; 2000).
Nevertheless, 10% to 50% of patients with prelingual nonsyndromic deafness carry a single heterozygous recessive mutation in the GJB2 gene. Although the finding that the del(GJB6-D13S1830) mutation provided an explanation for the deafness in as many as 30% to 70% of affected GJB2 heterozygotes in some populations, it has become clear that other mutations, both within DFNB1 and elsewhere involved in epistatic interactions with GJB2, contribute significantly to AR-NSNHL in most populations (del Castillo et al. 2003). Given the high prevalence of patients carrying only one mutant allele in GJB2 with apparent lack of the del(GJB6-D13S1830) and effect of pathogenic mutations of GJB2 and GJB3 in Chinese patients with autosomal recessive deafness, we initiated a study to determine whether there is functional interaction between the GJB2 and GJB3 genes. We provide evidence that mutations in the Cx26 and Cx31 genes can interact to cause hearing loss in digenic heterozygotes.
RESULTS
Mutations at the gap junction proteins Cx26 and Cx31 can interact to cause non-syndromic deafness
In total, 108 probands screened for mutations in the Cx26 gene were found to carry a single recessive mutant allele. In those samples, no mutation was detected on the second allele either in Cx26-exon-1/splice sites or in GJB6. To investigate the role of GJB3 variations along with GJB2 mutations for a possible combinatory allelic disease inheritance, we have screened patients with heterozygous GJB2 mutations for variants in Cx31 by sequencing. Analysis of the entire coding region of the Cx31 gene revealed the presence of two different missense mutations (N166S and A194T) occurring in compound heterozygosity along with the 235delC and 299delAT of GJB2 in 3 simplex families (235delC/N166S, 235delC/A194T and 299delAT/A194T).
In family A, a profoundly hearing impaired proband was found to be heterozygous for a novel A to G transition at nucleotide position 497 of GJB3, resulting in an asparagine into serine substitution in codon 166 (N166S) and for the 235delC of GJB2 (Fig. 1b, d). Genotyping analysis revealed that the GJB2/235delC was inherited from the unaffected father and the N166S of GJB3 was inherited from the normal hearing mother (Fig. 1a). In families F and K, a heterozygous missense mutation of a G-to-A transition at nucleotide 580 of GJB3 that causes A194T, was found in profoundly deaf probands, who were also heterozygous for GJB2/235delC (Fig. 1g, i) and GJB2/299-300delAT (Fig. 1l, n), respectively. In Family F, the GJB2/235delC was inherited from the unaffected father and the A194T of GJB3 was likely inherited from the normal hearing deceased mother (Fig. 1f). In Family K, genotyping analysis revealed that the father transmitted the A194T/GJB3, while the mother is heterozygous for the GJB2/299-300delAT (Fig. 1k). Carriers of a single affected allele in the two families had normal hearing. To exclude the possibility that the identified mutations were DNA polymorphisms in the studied population, samples from 200 unrelated control subjects were analyzed for both mutations by sequencing. Both mutations are believed to be pathological, first because of their location and conservation (see below) and, second, because neither change has been observed in 200 randomly selected, unrelated, normal hearing Chinese subjects. In addition, the pathogenicity of the N166S mutation is probable: because the asparagine is located in the second extracellular loop (E2), which is the major determinant for specificity of heterotypic interactions between hemichannels. It has also been proposed that the E2 region might be involved in targeting of the hemichannels to the plasma membrane as well as in proper cellular distribution (Verselis et al. 1994; Trexler et al. 2000). Furthermore, previous studies suggest that the specificity of heterotypic interactions between hemichannels composed of different connexins is largely dictated by the primary sequence of the second extracellular loop (White et al. 1994). Our finding is that the replacement of asparagine with serine, a smaller amino acid with a β-hydroxyl group in the N166S mutation, disrupts the primary sequence of EC2. This may induce a defect in the EC2 secondary structure that impairs Cx31 mediated coupling of cochlear cells. The multiple alignment of this domain with its homologues in rat and mouse, shows that the asparagine at position 166 is in fact a serine in these two species. Secondary structure prediction shows this asparagine to be in a short loop region in between two short beta strands. The asparagine at codon 166 is absent in the other human beta-connexins. The fact that the serine is found in rat and mouse and is not present among other human beta-connexins indicate that this mutation may be nonpathogenic. However, serines are able to be phosphorylated. We would speculate that the mutation may have caused the addition of a phosphorylation signal on the protein, and that this in turn might disrupt its usual function in humans. Altogether, these observations may give an explanation that the digenic inheritance of this Cx31 mutation with the Cx26 mutation can lead to hearing impairment due to impaired heterotypic interactions.

The deaf proband is indicated by an arrow. GJB2/GJB3 genotypes are given below the respective pedigrees symbol (a, f and k). Direct sequence analysis showing the 235delC mutation (b and g) and wild type (WT) allele (c and h) of GJB2. Direct sequence analysis showing the 299–300delAT mutation (l) and wild type (WT) allele (m) of GJB2. Direct sequence analysis showing the 497A>G (N166S) mutation (d) and WT allele (e) of GJB3. Direct sequence analysis showing the 580G>A (A194T) mutation (i and n) and WT allele (j and o) of GJB3.
The altered alanine residue in A194T identified in Families F and K lies within the fourth transmembrane domain (M4) of the GJB3 gene, which has been postulated to play a role in connexin trafficking (White et al. 1994). Sequence alignments of Cx31 from human, bovin, mouse, rat and chicken show that A194 is a highly conserved alanine residue. This single base-pair substitution changed a hydrophobic, non polar residue (alanine) to a neutral polar, much larger amino acid (threonine), altering the hydropathy index from 0.14 to 0.42. Because charged residues are important for proteins trafficking, the A194T may result in accumulation of the Cx31 protein in intracellular compartments such as the Golgi apparatus or in other sites such as the endoplasmic reticulum or lysosomes (Bonifacino et al. 1991; Machamer et al. 1993). The A194T substitution might cause conformational changes within the Cx31 molecule or affect the ability of Cx31 to form heteromeric hemichannels. The relationship between hemichannel assembly may be complex, considering the different paradigms for connexin oligomerization (Musil and Goodenough 1993; Kumar et al. 1995). Many of the Cx26 mutant residues lie within the EC2 and TM4 domains. Mutations affecting these regions have also been reported in Cx32 underlying X-linked-Charcot-Marie-Tooth disease. Moreover, mutations in residues close to N166 and A194 identified in the families reported here, namely, M163L, R165W, F191L, and A197S in Cx26 (Matos et al. 2003; Rickard et al. 2001; Feng et al. 2002; Hamelmann et al. 2001) as well as F193C, S198F and G199R in Cx32, have been reported previously in patients with hearing impairment (Janssen et al. 1997). Interestingly, mutations identified in patients with the skin disease erythrokeratoderma variabilis (EKV) were located within all the protein domains of the Cx31 gene except for the EC2 and TM4 domains, which are main domains for deafness mutations. This correlation between location of mutations and phenotypes, together with the identification of pathological mutations associated with hearing loss in the same region of the EC2 and TM4 domains in these three connexin genes (Cx26, Cx31, and Cx32) suggested that the EC2 and TM4 domains are important to the function of the Cx31 protein in the inner ear and plays a vital role in forming connexons in the cells of the inner ear.
In the present study, we have shown that the missense N166S and A194T mutations in GJB3 acts in a recessive manner in three unrelated Chinese patients. We have previously reported a recessive inheritance of an in-frame deletion and a missense mutation in Cx31 in a screening of 25 Chinese families affected with non-syndromic deafness (Liu et al. 2000). A missense mutation and a nonsense mutation of GJB3 have originally been shown to be associated with an autosomal dominant form of non-syndromic hearing loss in two Chinese DFNA2 families (Xia et al. 1998), indicating that Cx31 alterations are a cause of deafness in non-syndromic hearing loss patients in this population. In contrast, the studies described so far have shown that variations in Cx31 have no or a low genetic relevance in European populations and Caucasian in general. DFNA2 was first mapped in two large families with autosomal dominant non-syndromic deafness originating from Indonesia and the United States (Coucke et al. 1994). Three additional large DFNA2 families from Belgium and the Netherlands were subsequently reported (Van Camp et al. 1997). Extensive sequence analysis of the coding region and the 5′ UTR of GJB3 in all 5 original DFNA2 families (Coucke et al. 1994) revealed no mutations (Van Hauwe et al. 1999). Several different Cx31 alterations were found at similar frequencies in patients with deafness, patients with peripheral neuropathies and control subjects in the Spanish population (Lopez et al. 2000). Nonetheless, the dominant D66del mutation in Cx31 linked to neuropathy and deafness, was identified in one small family within 260 unrelated deaf patients in the same population with unilateral or bilateral high frequency NSHL (Lopez-Bigas et al. 2001). The failure in finding deafness causing mutations in Cx31 in screening of sporadic cases with NSHL performed in California and in Austria further confirms population relevance of Cx31 mutations in Chinese population (Mhatre et al. 2003; Frei et al. 2004).
Direct physical interaction between Cx26 and Cx31 in the mouse cochlea
Co-immunolabeling of cochlear sections with antibodies against Cx26 and Cx31 shows that Cx26 was expressed in fibrocytes of the lateral wall and spiral limbus (Fig. 2a). Cx26 immunoreactivity was also found in the supporting cells and inner sulcus cells where immunolabeling of Cx31 was also positive (Fig. 2b). Superimposed image (Fig. 2d) shows that the expression of Cx26 and Cx31 overlapped in the cochlea. To examine whether the two Cxs directly interact with each other by co-assembling into the same gap junction, we performed co-immunoprecipitation experiments of gap junctions from mouse cochlear tissues. Western blots confirmed the presence of Cx31 protein in the cochlea (Fig. 3a; left panel). The predominantly single band supported that the antibody specifically recognized the Cx31 protein and there was no cross reactions with Cx26. A faint band at the location of Cx30 indicated a weak cross reaction of Cx31 antibody with the Cx30 protein. Protein complexes immunoprecipitated by Cx31 were detected by Western blots using antibodies against both Cx26 and Cx31, and both bands were detected (Fig. 3a; right panel) indicating that the two molecules physically interact with each other.

Cochlear cryosections were cut at a thickness of 8 μm and labeled with an antibody against Cx26 (a) and Cx31 (b). DAPI staining gives the outline of cochlear structure (c). The superimposed image is given in (d). Abbreviations: SV: scala vestibule; SM: scala media; ST: scala tympani; SL: spiral limbus; SG: spiral ganglia.
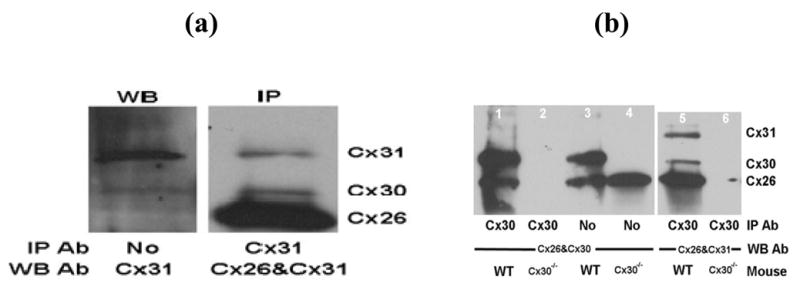
Western blot data confirmed the presence of Cx31 protein in the cochlea and the specificity of the antibody used. No cross-reactions were observed between the Cx26 and Cx31 antibodies (a; left panel). Detection of the Cx26 and Cx31 proteins by immunoblotting of the Cx31 immunoprecipited protein complex using antibodies against both Cx26 and Cx31 supported the physical interaction between the two connexins (a; right panel). The specificity of the interaction between Cx26 and Cx31 as well as that of the antibodies used is further confirmed by co-immunoprecipitation using both wild type (WT) and Cx30−/− mice, showing only the respective protein bands on the immunoblot in WT mice (b, lane 3). But a single band corresponding to Cx26 remained when Cx30−/− mice were used (b, lane 4).
Cx26 and Cx30 are the two major Cx isoforms found in the cochlea that are known to co-assemble in cochlear gap junctions (Ahmad et al. 2003). To further examine the specificity of the interaction between Cx26 and Cx31, we performed co-immunoprecipitation using both wild type (WT) and Cx30−/− mice. Protein complexes with or without immunoprecipitation by an antibody against Cx30 were examined by Western blotting analysis. Bands corresponding to Cx26 and Cx30 (Fig. 3b, lane 1) or Cx26 and Cx31 (lane 5) were detected if WT mice were used. In contrast, if cochlear tissue from Cx30−/− mice were used for immunoprecipitation by Cx30 antibody, no bands were detected (lanes 2 and 6). As controls testing for specificities of Cx26 and Cx30 antibodies, our Western blots showed no extra bands other than the two corresponding to Cx26 and Cx30 (Fig. 3b, lane 3) when WT mice were used. Only a single band corresponding to Cx26 remained when Cx30−/− mice were used (Fig. 3b, lane 4). Thus, our results support the findings of Abrams et al (2006) that Cx26, Cx30 and Cx31 can form heterotypic channels with each other. Co-assembly of Cx26 and Cx31 in the same gap junctions were further tested by reconstituting heteromeric gap junction in vitro. After cotransfection of HEK-293 cells using mCherry-tagged Cx26 and GFP-tagged Cx31 DNA constructs, we observed that all Cx26 gap junction plaques (labeled by mCherry in red, Fig. 4a) overlapped with GFP-tagged Cx31 gap junction plaques (Fig. 4b) (n>20). The in vitro data further supported that Cx26 and Cx31 are co-assembled in the same gap junctions.

Cx26 and Cx31 colocalize in the same reconstituted gap junction –plaque by cotransfection and coimmunolabeling of mCherry -tagged Cx26 and GFP-tagged Cx31 in HEK-293 cells. Cx26-labeled gap junction plaques in red (a) overlapped with GFP-tagged Cx31 gap junction plaques (b) in cell pairs (n>20) we examined. Simultaneous presence of red (mCherry) and green (GFP) fluorescence in the same cell (c) was observed in the majority of cells from random views. Using the FuGene6 transfection reagent, about 40% cells was transfected with the Cx plasmids as determined by the expression of fluorescent protein.
DISCUSSION
Most cases of genetic deafness result from mutations at a single gene, but an increasing number of examples are being recognized in which recessive mutations at two loci are involved. In digenic inheritance, mutations in each of two unlinked genes are present in a single individual, and the combination of the two genetic hits causes a disease phenotype that is not apparent when an individual carries only one of these gene alterations. This mechanism complicates genetic evaluation and counseling, but provides an explanation for Connexin 26 heterozygotes who, for previously unknown reasons, are deaf. The del(GJB6-D13S1830) allele is most frequent in Spain, France, the United Kingdom, Israel, and Brazil, accounting for 5.0–9.7% of all the DFNB1 alleles (delCastillo et al. 2003; Marlin et al. 2005) and this finding has led to a substantial decrease of the percentages of monoallelic Cx26 mutation carriers in those countries. Interestingly, the del(GJB6-D13S1830) mutation is very rare in Southern Italy (one heterozygote/238 total screened cases) (delCastillo et al. 2003), but subsequent studies have shown that this large deletion is present in Northern Italy at frequencies similar to those of other European countries (Gualandi et al. 2004; del Castillo et al. 2005). However, the deletion has not been detected in Turkish, Italian, Austrian, Greek Cypriot and Chinese nonsyndromic hearing loss patients (del Castillo et al. 2003; Gunther et al. 2003; Uyguner et al. 2003; Neocleous et al. 2006; Liu et al. 2002). This may indicate that other mutations, both within DFNB1 and elsewhere may be involved in epistatic interactions with GJB2, in addition to the existence of mutations in the cis transcription regulatory elements that have not yet been considered, or it is not detected because of the technical limitations of the methods used for mutation analysis.
In the Cx31 gene, currently more than 10 different mutations have been found in patients with deafness from Chinese, Brazilian, and Spanish populations (Xia Liu et al. 1998; Liu et al. 2001; Lopez-Bigas et al. 2001; Alexandrino al. 2004). However, the pathogenicity of most of these sequence alterations still remains questionable. The so far reported mutation spectrum in Cx31 includes missense, deletion, and stop mutations are located in the first and second extracellular loop as well as in the third transmembrane domain of the connexin 31 protein. Of these Cx31 mutations, two have been described to act as recessive alleles and 2 mutant alleles are associated with dominant deafness in Chinese population (Xia et al. 1998; Liu et al. 2000). Mutations in GJB2 is one of the most common cause of non-syndromic deafness in the Chinese population (Liu et al. 2002; 2006; Liu et al. 2002; Dai et al. 2007). More than 25 different GJB2 deafness-causing mutations have been identified in Chinese patients with non-syndromic deafness including missense, stop mutations and small deletions or insertions across all Cx26 protein domains (Dai et al. 2007; Liu et al. 2006). The high prevalence of Cx26 mutations in the Chinese population is mainly due to the high frequency of the 235delC deletion, which has a common ancient founder in China and Far East, as has been shown by haplotype analysis (Yan et al. 2003), at a frequency of 16.3% in Chinese cohorts with NSHL (Dai et al. 2007) and accounts for up to 80% of pathogenic GJB2 alleles in DFNB1 patients in this population (Liu et al. 2002).
Our data have shown that Cx31 was expressed in the supporting cells and cells at the tip of the of spiral limbus that partially overlapped with the Cx26 cellular expression pattern. The overlapped localization suggested that it is feasible for the two Cxs to physically interact with each other. More evidence was provided by co-immunoprecipitation and co-localization after co-transfection. We have shown that in the cochlea Cx26 and Cx31 are able to form heterotypic channels like Cx26 and 30 (Cx26/Cx30) (Yum et al. 2007) and Cx31 and 32 (Cx31/Cx32) (Abrams et al. 2006), which provide a base of interaction of these connexins. Indeed, We have demonstrated that a digenic form of non-syndromic recessive deafness was caused by mutations in Cx26 and Cx31 and our data suggested that loss of any two of the four alleles from GJB2 and GJB3 can result in hearing impairment.
Several mutated connexins have been reported to mistarget gap junctions and/or fail to oligomerize correctly into hemichannels, or to alter specific permeability properties of gap junctions (Martin et al. 1999; Marziano et al. 2003; Bruzzone et al. 2003; Mese et al. 2004). In the present study, we show that double heterozygous patients for Cx26 and Cx31 mutations are deaf. The simultaneous loss of one GJB2 and one GJB6 allele may have reduced the dosage of both genes’ products. This finding suggests that the two connexins may not be functionally equivalent and cannot thus compensate each other. The inability of other connexins to functionally compensate for the absence of another is a common feature of connexin-based disorders (Simon and Goodenough 1998), and has been interpreted as an indication that a specific complement of connexin channels is required to meet the specific physiological needs of a particular tissue. It is well known now that all connexins are not made equal: in fact they are differences in size and ionic selectivity and have distinct gating mechanisms. It has also been shown that channels composed of different connexins have different conductances (Veenstra 1996), and permeabilities to ions (Beblo and Veenstra 1997) and fluorescent dyes (Elfgang et al. 1995; Cao et al. 1998). We demonstrate that Cx26/Cx31, like the Cx26/Cx30 proteins, are co-expressed and can physically interact in the mouse cochlea. The cochlear cells would then be expected to exhibit a variety of single-channel events consisting of homomeric/homotypic Cx26 or Cx31 channels or homomeric/heterotypic Cx26/Cx31 channels, depending on combinations of connexins forming connexons, hemichannels, and full channels. However, it is not clear exactly what proportion of cochlear gap junctions exist in homomeric and heteromeric forms, which makes analysis of different Cx26/Cx31 molecular configurations difficult. We have examinated the functional status of homomeric gap junction channels formed by the N166S and A194T mutant proteins by monitoring the calcium diffusion and by using dye transfer studies in transfected gap junctions-deficient HEK293 cells. Compared with WT-Cx31, neither of the two Cx31 variants showed obvious changes in ionic permeability (Data not shown). However, we cannot exclude the possibility that the pathogenesis of hearing loss caused by the N166S and A194T mutations in GJB3 is due to their deleterious effect on biochemical coupling. Thus, the patho-genetic nature of the N166S and A194T remains to be elucidated. Nevertheless, an important implication of our results is that it would be useful to screen patients from other populations to establish the likely frequency of GJB2/GJB3 mutations among those in whom deafness could be attributed to GJB2 mutation in a heterozygous condition.
MATERIAL AND METHODS
Subjects and DNA samples
Probands/families were ascertained through the nationwide epidemiologilcal survey in Beijing, China (Dai et al. 2007). Informed consent was obtained from all of the participants. The study protocol was approved by the Ethics Committee of the Chinese PLA General Hospital and from institutional review board (IRB) at University of Miami. In total, 108 Chinese families affected with non-syndromic deafness without obvious dominant inheritance patterns were included in this study. All the probands showed a congenital, bilateral, severe to profound, sensorineural hearing impairment with normal hearing parents. The clinical history was obtained and an examination was conducted on each individual by one of the investigators, with special emphasis on identifying potential environmental causes of hearing loss such as infections, trauma, and information on exposure to known or possible ototoxic drugs or for evidence of syndromic forms of deafness. The hearing of all affected and unaffected individuals in the present series was examined using pure tone audiometry. Air conduction thresholds were measured at 250 Hz, 500 Hz, 1 kHz, 2 kHz, 4 kHz, 6 kHz and 8 kHz. Bone conduction thresholds were determined to identify the type of hearing loss. Oto-immittance measurements were undertaken on all individuals and all were otoscopically examined. In addition, 200 Chinese control individuals with normal hearing were also analyzed. DNA was extracted from peripheral blood leukocytes using a commercially available kit.
Mutational analysis
After exclusion of GJB2 including exon 1, GJB6, SLC26A4, and the A1555G mutation in the 12SrRNA gene (MTRNR1) as potential causes of hearing loss in the 108 probands with only one GJB2 mutant allele, the full GJB3 coding region was analyzed. The coding exon (exon 2) and flanking intronic regions of GJB2 gene were PCR amplified with forward primer 5′-TTGGTGTTTGCTCAGGAAGA-3′ and reverse primer 5′GGCCTACAGGGGTTTCAAAT 3′. PCR amplification of Cx31 was performed using the forward 5′-TACGATGGTTTTTCCTCTAATTCT-3′ and reverse 5′-TTGCATAACTTAGTGAACTCAGAG-3′ primer sets based on the Cx31 sequence (BC012918). The PCR products were purified with the QIAquick spin column purification kit (Qiagen, Valencia, CA). Purified amplicons were cycle sequenced by using the Big Dye Terminator Cycle Sequencing Kit (PE Biosystems) and run on an ABI 3100 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA,) according to manufacturer recommendations (PE Biosystems). DNA sequence variations were identified by alignment of the subject’s DNA sequence to the wild type sequences AY280971 (GJB2) and BC012918 (GJB3) at the NCBI interface (http://www.ncbi.nlm.nih.gov/blast/bl2seq/bl2.html). Numbering of the genes begins with the nucleotide A of the ATG start codon in exon 2 as cDNA position number 1. The sequences were analyzed using Genetool Lite software and the genes Genebank sequences.
Immunolabeling of Cx31 in cochlear cryosections
Cochleas were obtained from adult mice (strain CD-1) and cochlear cryosections were prepared as previously described (Sun et al., 2005). Cochlear sections were blocked first with bovine serum albumin (2%) plus the serum (20%) of the host animal to generate the secondary antibodies prior to incubating with primary antibodies (1:200 dilution in PBS) overnight at 4 °C. Anti-Cx31 (Rabbit IgG, Zymed Laboratories, Southern San Francisco, CA) was used to label the cryosections. After washing in PBS three times the sections were incubated with secondary antibodies (1:500 dilution in PBS) for about 2 h at room temperature. Labeled sections were mounted in an anti-fade medium (Molecular Probes, Eugene, OR) and examined with a confocal microscope (Zeiss LSM, Carl Zeiss USA, Shrewsbury, PA).
Preparations and immunoprecipitation of gap junctions
The purified gap junctions from mouse cochlear tissues were prepared as previously described. Primary antibody against Cxs26, 30 or 31 (Zymed Laboratories, Southern San Francisco, CA) was added to the supernatant at 1:250 dilution and incubated overnight. Antibodies bound to connexons were precipitated by protein A-linked Sepharose beads using protocol as described earlier (Diez et al. 1999). Individual connexin protein or immunoprecipited protein complexes were separated by SDS PAGE and detected by immunoblotting using specific antibodies (1:1000 dilution in blocking solution) at 4 °C for 12–15 h using protocol previously described (Diez et al. 1999). Immunolabeled proteins were visualized by using enhanced chemiluminescence (Super-Signal, Pierce, Rockford, IL) exposed to X-ray films (Hyper Film, Amersham Biosciences, Piscataway, NJ).
Construction of the pCx31-eGFP-N1 clone
The human Cx31 cDNA was epitope tagged at the carboxyl terminus by subcloning into the green fluorescent protein vector, pEGFP-N1 (Clontech Laboratories, Inc., S. San Francisco, CA). The primers (5′-GTCAGATCCGCTAGCATGGACTGGAAGACA -3′ and 5′-AAGCTTGAGCTCGAGGATGGGGGTCAGGTT -3′) were designed such that the PCR product contained NheI and XhoI restriction enzyme sites for subsequent subcloning into the peGFP-N1 vector.
Expression analysis of wild-type and mutant human Cx31 in Human Embryonic Kidney (HEK) 293 cells
GJ-deficient HEK293 cells (American Type Culture Collection, Manassas, VA) grown to 80%. HEK 293 cells were grown at 37°C in an incubator (5% CO2, under a moist atmosphere), in MEM medium supplemented with penicillin and streptomycin (0.5% v/v final concentration) and foetal bovine serum (10% v/v final concentration; all reagents for cell culture were from CellGro, Mediatech, Herndon, VA, USA). One day prior to transfection, cells were dissociated by trypsin-EDTA for 2 min, seeded on glass coverslip in the culture medium and allow to grow up to 60–80% confluence. Transfection with pCx31-eGFP-N1 was performed using the FuGene6 transfection reagent (Roche Diagnostics Corp., Indianapolis, IN, USA) according to the protocol provided by the manufacturer. After 24h of incubation, culture medium was replaced. Functional assays were carried out at room temperature, 36 to 72h after transfection.
Acknowledgments
We thank the families for their kind participation in this study. This work was supported by NIH grants DCR01 05575 and NSFC 30528025 and 30728030 (China).
References
- Abrams CK, Freidin MM, Verselis VK, Bargiello TA, Kelsell DP, Richard G, Bennett MVL, Bukauskas FF. Properties of human connexin 31, which is implicated in hereditary dermatological disease and deafness. Proc Natl Acad Sci. 2006;103:5213–5218. [Europe PMC free article] [Abstract] [Google Scholar]
- Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. [Abstract] [Google Scholar]
- Alexandrino F, Oliveira CA, Reis FC, Maciel-Guerra AT, Sartorato EL. Screening for mutations in the GJB3 gene in Brazilian patients with nonsyndromic deafness. J Appl Genet. 2004;45:249–254. [Abstract] [Google Scholar]
- Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol. 1997;109:509–522. [Europe PMC free article] [Abstract] [Google Scholar]
- Bonifacino JS, Cosson P, Shah N, Klausner RD. Role of potentially charged transmembrane residues in targeting proteins for retention and degradation within the endoplasmic reticulum. EMBO J. 1991;10:2783–2793. [Europe PMC free article] [Abstract] [Google Scholar]
- Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D’Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett. 2003;533:79–88. [Abstract] [Google Scholar]
- Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, H-ulser DF, Willecke K, Nicholson BJ. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci. 1998;111:31–43. [Abstract] [Google Scholar]
- Coucke P, Van Camp G, Djoyodiharjo B, Smith SD, Frants RR, Padberg GW, Darby JK, Huizing EH, Cremers C, Kimberling WJ, Oostra BA, Van de Heyning PH, Willems PJ. Linkage of autosomal dominant hearing loss to the short arm of chromosome 1 in two families. N Engl J Med. 1994;331:425–431. [Abstract] [Google Scholar]
- Dai P, Yu F, Han B, Yuan Y, Li Q, Wang G, Liu X, He J, Huang D, Kang D, Zhang X, Yuan H, Schmitt E, Han D, Wong LJ. The prevalence of the 235delC GJB2 mutation in a Chinese deaf population. Genet Med. 2007;9:283–289. [Abstract] [Google Scholar]
- Del Castillo I, Moreno-Pelayo MA, Del Castillo FJ, Brownstein Z, Marlin S, Adina Q, Cockburn DJ, Pandya A, Siemering KR, Chamberlin GP, Ballana E, Wuyts W, Maciel-Guerra AT, Alvarez A, Villamar M, Shohat M, Abeliovich D, Dahl HH, Estivill X, Gasparini P, Hutchin T, Nance WE, Sartorato EL, Smith RJ, Van Camp G, Avraham KB, Petit C, Moreno F. Prevalence and Evolutionary Origins of the del(GJB6-D13S1830) Mutation in the DFNB1 Locus in Hearing-Impaired Subjects: a Multicenter Study. Am J Hum Genet. 2003;73:1452–1458. [Europe PMC free article] [Abstract] [Google Scholar]
- del Castillo FJ, Rodríguez-Ballesteros M, Alvarez A, Hutchin T, Leonardi E, de Oliveira CA, Azaiez H, Brownstein Z, Avenarius MR, Marlin S, Pandya A, Shahin H, Siemering KR, Weil D, Wuyts W, Aguirre LA, Martín Y, Moreno-Pelayo MA, Villamar M, Avraham KB, Dahl HH, Kanaan M, Nance WE, Petit C, Smith RJ, Van Camp G, Sartorato EL, Murgia A, Moreno F, del Castillo I. A novel deletion involving the connexin-30 gene, del(GJB6-d13s1854), found in trans with mutations in the GJB2 gene (connexin-26) in subjects with DFNB1 non-syndromic hearing impairment. J Med Genet. 2005;42:588–594. [Europe PMC free article] [Abstract] [Google Scholar]
- Deschenes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J Neurosci. 1997;17:9077–9084. [Europe PMC free article] [Abstract] [Google Scholar]
- Diez JA, Ahmad S, Evans WH. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur J Biochem. 1999;262:142–148. [Abstract] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, Hülser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. [Europe PMC free article] [Abstract] [Google Scholar]
- Feng Y, He C, Xiao J, Fang J, Zhao S, Tian X, Mei L, Xia K, Tang X. An analysis of a large hereditary postlingually deaf families and detecting mutation of the deafness genes. Chin J Otorhinolaryngol. 2002;37:348–351. [Abstract] [Google Scholar]
- Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. [Abstract] [Google Scholar]
- Frei K, Ramsebner R, Hamader G, Lucas T, Schoefer C, Baumgartner WD, Wachtler FJ, Kirschhofer K. Lack of association between Connexin 31 (GJB3) alterations and sensorineural deafness in Austria. Hear Res. 2004;194:81–86. [Abstract] [Google Scholar]
- Grifa A, Wagner CA, D’Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nature Genet. 1999;23:16–18. [Abstract] [Google Scholar]
- Gualandi E, Ravani A, Berto A, et al. Mutation in Italian non-syndromic hearing loss patients carrying a single GJB2 mutated allele. Acta Otolaryngol Suppl. 2004;552:29–34. Occurrence of del(GIB6-D13S1830) [Abstract] [Google Scholar]
- Gunther B, Steiner A, Nekahm-Heis, Albegger K, Zorowka P, Utermann G, Janecke A. The 342-kb deletion in GJB6 is not present in patients with non-syndromic hearing loss from Austria. Hum Mutat. 2003;22:180–184. [Abstract] [Google Scholar]
- Hamelmann C, Amedofu GK, Albrecht K, Muntau B, Gelhaus A, Brobby GW, Horstmann RD. Pattern of connexin 26 (GJB2) mutations causing sensorineural hearing impairment in Ghana. Hum Mutat. 2001;18:84–85. [Abstract] [Google Scholar]
- Janssen EAM, Kemp S, Hensels GW, Sie OG, de Die-Smulders CEM, Hoogendijk JE, de Visser M, Bolhuis PA. Connexin32 gene mutations in X-linked dominant Charcot-Marie-Tooth disease. Hum Genet. 1997;99:501–505. [Abstract] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. [Abstract] [Google Scholar]
- Kumar NM, Friend DS, Gilula NB. Synthesis and assembly of human β1 gap junctions in BHK cells by DNA transfection with the human β1 cDNA. J Cell Sci. 1995;108:3725–3734. [Abstract] [Google Scholar]
- Lopez-Bigas N, Rabionet R, Martinez E, Banchs I, Volpini V, Vance JM, Arbones ML, Estivill X. Identification of seven novel SNPS (five nucleotide and two amino acid substitutions) in the Connexin 31 (GJB3) gene. Hum Mutat. 2000;15:481–482. [Abstract] [Google Scholar]
- Lopez-Bigas N, Olive M, Rabionet R, Ben-David O, Martinez-Matos JA, Bravo O, Banchs I, Volpini V, Gasparini P, Avraham KB, Ferrer I, Arbones ML, Estivill X. Connexin 31 (GJB3) is expressed in the peripheral and auditory nerves and causes neuropathy and hearing impairment. Hum Mol Genet. 2001;10:947–952. [Abstract] [Google Scholar]
- Liu XZ, Xia XJ, Xu LR, Pandya A, Liang CY, Blanton SH, Brown SD, Steel KP, Nance WE. Mutations in connexin31 underlie recessive as well as dominant nonsyndromic hearing loss. Hum Mol Genet. 2000;9:63–67. [Abstract] [Google Scholar]
- Liu Y, Ke X, Qi Y, Zhu P. Connexin 26 gene (GJB2): prevalence of mutations in Chinese population. J Hum Genet. 2002;47:688–690. [Abstract] [Google Scholar]
- Liu XZ, Xia XJ, Ke XM, Ouyang XM, Du LL, Liu YH, Angeli S, Telischi FF, Nance WE, Balkany T, Xu LR. The prevalence of connexin 26 (GJB2) mutations in the Chinese population. Hum Genet. 2002;111:394–397. [Abstract] [Google Scholar]
- Liu XZ, Ouyang XM, Yan D. The genetic deafness in Chinese population. Journal of Otology (in English) 2006;1:1–10. [Google Scholar]
- Machamer CE, Grim MG, Esquela A, Chung SW, Rolls M, Ryan K, Swift AM. Retention of a cis Golgi protein requires polar residues on one face of a predicted α-helix in the transmembrane domain. Mol Biol Cell. 1993;4:695–704. [Europe PMC free article] [Abstract] [Google Scholar]
- Martin PE, Coleman SL, Casalotti SO, Forge A, Evans WH. Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal heriditary deafness. Hum Mol Genet. 1999;8:2369–2376. [Abstract] [Google Scholar]
- Marziano NK, Casalotti SO, Portelli AE, Becker DL, Forge A. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet. 2003;12:805–812. [Abstract] [Google Scholar]
- Mhatre AN, Weld E, Lalwani AK. Mutation analysis of Connexin 31 (GJB3) in sporadic non-syndromic hearing impairment. Clin Genet. 2003;63:154–159. [Abstract] [Google Scholar]
- Marlin S, Feldmann D, Blons H, Loundon N, Rouillon I, Albert S, Chauvin P, Garabedian EN, Couderc R, Odent S, Joannard A, Schmerber S, Delobel B, Leman J, Journel H, Catros H, Lemarechal C, Dollfus H, Eliot MM, Delaunoy JL, David A, Calais C, Drouin-Garraud V, Obstoy MF, Goizet C, Duriez F, Fellmann F, Helias J, Vigneron J, Montaut B, Matin-Coignard D, Faivre L, Baumann C, Lewin P, Petit C, Denoyelle F. GJB2 and GJB6 mutations: genotypic and phenotypic correlations in a large cohort of hearing-impaired patients. Arch Otolaryngol Head Neck Surg. 2005;131:481–487. [Abstract] [Google Scholar]
- Matos T, Caria H, Galhardo I, Dias O, Andrea M, Correia C, Fialho G. A novel M163L mutation in GJB2 gene associated with autosomal dominant isolated hearing loss. European Human Genetics Conference European Society of Human Genetics 2003 [Google Scholar]
- Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet. 2004;115:191–199. [Abstract] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma-membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. [Abstract] [Google Scholar]
- Neocleous V, Aspris A, Shahpenterian V, Nicolaou V, Panagi C, Ioannis Ioannou I, Kyamides Y, Anastasiadou V, Leonidas AP. High Frequency of 35delG Mutation and Absence of del(GJB6-D13S1830) in Greek Cypriot Patients with Nonsyndromic Hearing Loss. Genetic Testing. 2006;10:285–289. [Abstract] [Google Scholar]
- Plantard L, Huber M, Macari F, Meda P, Hohl D. Molecular interaction of connexin 30.3 and connexin 31 suggests a dominant-negative mechanism associated with erythrokeratodermia variabilis. Hum Molec Gene. 2003;12:3287–3294. [Abstract] [Google Scholar]
- Richard G, Lin JP, Smith L, Whyte YM, Itin P, Wollina U, Epstein E, Jr, Hohl D, Giroux JM, Charnas L, Bale SJ, DiGiovanna JJ. Linkage studies in erythrokeratodermias: fine mapping, genetic heterogeneity and analysis of candidate genes. J Invest Derm. 1997;109:666–671. [Abstract] [Google Scholar]
- Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH, Jr, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nature Genet. 1998;20:366–369. [Abstract] [Google Scholar]
- Richard G, Brown N, Smith LE, Terrinoni A, Melino G, MacKie RM, Bale SJ, Uitto J. The spectrum of mutations in erythrokeratodermias--novel and de novo mutations in GJB3. Hum Genet. 2000;106:321–329. [Abstract] [Google Scholar]
- Rickard S, Kelsell DP, Sirimana T, Rajput K, MacArdle B, Bitner-Glindzicz M. Recurrent mutations in the deafness gene GJB2 (connexin 26) in British Asian families. J Med Genet. 2001;38:530–533. [Europe PMC free article] [Abstract] [Google Scholar]
- Simon AM, Goodenough DA. Diverse functions of vertebrate gap junctions. Trends Cell Biol. 1998;8:477–483. [Abstract] [Google Scholar]
- Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol. 2005;288:C613–C623. [Abstract] [Google Scholar]
- Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000;79:3036–3051. [Europe PMC free article] [Abstract] [Google Scholar]
- Uyguner O, Emiroglu M, Uzumcu A, Hafiz G, Ghanbari A, Baserer N, Yuksel-Apak M, Wollnik B. Frequencies of gap- and tight junction mutations in Turkish families with autosomal-recessive nonsyndromic hearing loss. Clin Genet. 2003;64:65–69. [Abstract] [Google Scholar]
- Van Camp G, Coucke PJ, Kunst H, Schatteman I, Van Velzen D, Marres H, van Ewijk M, Declau F, Van Hauwe P, Meyers J, Kenyon J, Smith SD, Smith RJ, Djelantik B, Cremers CW, Van de Heyning PH, Willems PJ. Linkage analysis of progressive hearing loss in five extended families maps the DFNA2 gene to a 1.25-Mb region on chromosome 1p. Genomics. 1997;41:70–74. [Abstract] [Google Scholar]
- Van Hauwe P, Coucke PJ, Declau F, Kunst H, Ensink RJ, Marres HA, Cremers CW, Djelantik B, Smith SD, Kelley P, Van de Heyning PH, Van Camp G. Deafness linked to DFNA2: one locus but how many genes? Nat Genet. 1999;213:263. [Abstract] [Google Scholar]
- Veenstra RD. Size and selectivity of gap junction channels formed from different connexins. J Bioenerg Biomemb. 1996;28:327–337. [Abstract] [Google Scholar]
- Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. [Abstract] [Google Scholar]
- White TW, Bruzzone R, Wolfram S, Paul DL, Goodenough DA. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994;125:879–892. [Europe PMC free article] [Abstract] [Google Scholar]
- White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283–310. [Abstract] [Google Scholar]
- Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet. 1998;20:370–373. [Abstract] [Google Scholar]
- Xia AP, Ikeda K, Katori Y, Oshima T, Kikuchi T, Takasaka T. Expression of connexin31 in the developing mouse cochlea. NeuroReport. 2000;11:2449–2453. [Abstract] [Google Scholar]
- Yan D, Park HJ, Ouyang XM, Pandya A, Doi K, Erdenetungalag R, Du LL, Matsushiro N, Nance WE, Griffith AJ, Liu XZ. Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in East Asians. Hum Genet. 2003;114:44–50. [Abstract] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–C1048. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1007/s00439-008-0602-9
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2737700?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Pathogenic variants in HGF give rise to childhood-to-late onset primary lymphoedema by loss of function.
Hum Mol Genet, 33(14):1250-1261, 01 Jul 2024
Cited by: 0 articles | PMID: 38676400
Genetic analysis of 106 sporadic cases with hearing loss in the UAE population.
Hum Genomics, 18(1):59, 07 Jun 2024
Cited by: 0 articles | PMID: 38844983
Engineering APOBEC3A deaminase for highly accurate and efficient base editing.
Nat Chem Biol, 20(9):1176-1187, 29 Mar 2024
Cited by: 3 articles | PMID: 38553609
Digenic inheritance involving a muscle-specific protein kinase and the giant titin protein causes a skeletal muscle myopathy.
Nat Genet, 56(3):395-407, 01 Mar 2024
Cited by: 3 articles | PMID: 38429495
Simulation-predicted and -explained inheritance model of pathogenicity confirmed by transgenic mice models.
Comput Struct Biotechnol J, 21:5698-5711, 18 Nov 2023
Cited by: 2 articles | PMID: 38074473 | PMCID: PMC10700547
Go to all (64) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Diseases (4)
- (1 citation) OMIM - 220290
- (1 citation) OMIM - 604418
- (1 citation) OMIM - 121011
- (1 citation) OMIM - 603324
Nucleotide Sequences (2)
- (2 citations) ENA - BC012918
- (1 citation) ENA - AY280971
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall.
Neurobiol Dis, 108:195-203, 17 Aug 2017
Cited by: 32 articles | PMID: 28823936 | PMCID: PMC5675824
[Sequence analysis of GJB3 in Chinese deafness population who carry one heterozygous GJB2 pathogenic mutation].
Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 45(4):287-290, 01 Apr 2010
Cited by: 2 articles | PMID: 20627047
[Study of the relation between Cx31 gene and hereditary hearing impairment].
Zhonghua Er Bi Yan Hou Ke Za Zhi, 39(6):344-348, 01 Jun 2004
Cited by: 2 articles | PMID: 15469079
Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins.
Hum Mutat, 16(3):190-202, 01 Sep 2000
Cited by: 123 articles | PMID: 10980526
Review
Funding
Funders who supported this work.
NIDCD NIH HHS (3)
Grant ID: R01 DC005575
Grant ID: R01 DC005575-08
Grant ID: R01 DC012115
PHS HHS (1)
Grant ID: DCR01 05575





