Abstract
Free full text

Association of Reactive Oxygen Species Levels and Radioresistance in Cancer Stem Cells
Associated Data
Abstract
Metabolism of oxygen, while central to life, also produces reactive oxygen species (ROS) that have been implicated in processes as diverse as cancer, cardiovascular disease, and aging. It has recently been shown that central nervous system stem cells1, 2 and hematopoietic stem cells and early progenitors3-6 contain lower levels of ROS than their more mature progeny and that these differences appear to be critical for maintaining stem cell function. We hypothesized that epithelial tissue stem cells and their cancer stem cell (CSC) counterparts may also share this property. Here we show that normal mammary epithelial stem cells contain lower concentrations of ROS than their more mature progeny cells. Congruently, subsets of CSCs in some human and murine breast tumors contain lower ROS levels than corresponding non-tumorigenic cells (NTCs). Consistent with ROS being critical mediators of ionizing radiation-induced cell killing7, 8, CSCs in these tumors develop less DNA damage and are preferentially spared after irradiation compared to NTCs. Lower ROS levels in CSCs are associated with increased expression of free radical scavenging systems. Pharmacologic depletion of ROS scavengers in CSCs significantly decreases their clonogenicity and results in radiosensitization. These results indicate that, similar to normal tissue stem cells, subsets of CSCs in some tumors contain lower ROS levels and enhanced ROS defenses compared to their non-tumorigenic progeny, which may contribute to tumor radioresistance.
We began by asking whether low ROS concentrations that appear to be critical to self renewal of hematopoietic stem cells (HSCs)3, 5 are also a property of mammary epithelial stem cells9, 10 by isolating CD24medCD49fhighLin- mammary cells, a population enriched for mammary repopulating units (MRUs), and CD24highCD49flowLin- progenitor cells by flow cytometry (Supplementary Fig. S1) and measuring intracellular concentrations of prooxidants using 2′-7′-dichlorofluorescein diacetate (DCF-DA) staining3. Cells in the MRU-enriched population contained significantly lower concentrations of ROS than the progenitor-enriched cells in two different strains of mice (Fig. 1a-c). Specifically, the MRU-enriched populations displayed low to intermediate ROS levels, while the progenitor-enriched populations contained more uniformly high levels of ROS. Similarly, analysis of the two populations with MitoSOX Red, a highly selective detection method for mitochondrial superoxide, revealed lower superoxide levels in the MRU-enriched population (Fig. 1d). In order to assess if mammary repopulating activity was related to intracellular concentrations of ROS, we transplanted CD24medCD49fhighLin- cells based on their levels of DCF-DA staining. Mammary stem cells with both low and intermediate ROS levels gave rise to epithelial outgrowths when transplanted into cleared fat pads (Supplementary Table 1). Similar heterogeneity of ROS concentrations was recently demonstrated in HSC-enriched populations5, 11, where it may have functional significance in modulating the HSC-niche interaction12.
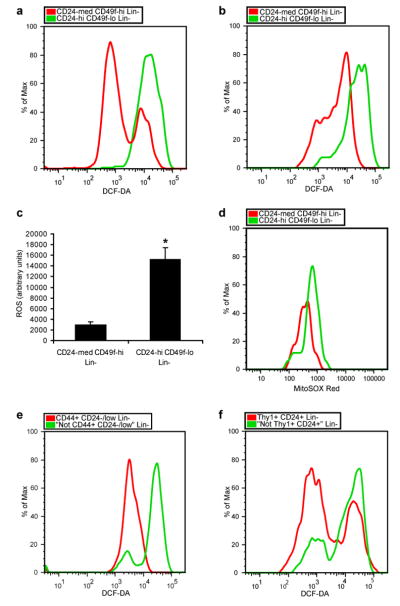
a, CD24medCD49fhighLin- mammary cells (mammary stem cell-containing population) and CD24highCD49flowLin- mammary cells (progenitor cell-containing population) were isolated from C57Bl/6J female mice using flow cytometry and intracellular ROS concentrations were measured by DCF-DA staining. b, as in a but using 29S1/SvImJ mice. c, Mean ± s.e.m. for replicates of a and b (n=6; p=0.001). d, as in b but using MitoSOX Red instead of DCF-DA. Data shown are representative of two independent experiments. e, CD44+CD24-/lowLin- breast cancer cells (CSC-containing population) and “Not CD44+CD24-/low” Lin- cells (non-tumorigenic population) were isolated from a primary human breast tumor by flow cytometry and ROS levels were analyzed using DCF-DA. f, as in e but using murine Thy1+CD24+Lin- breast cancer cells (CSC-containing population) and “Not Thy1+CD24+” Lin- cells (non-tumorigenic population) isolated from an MMTV-Wnt-1 breast tumor.
Given the conservation of low ROS levels in several types of normal tissue stem cells, we hypothesized that CSCs in some tumors may also contain lower concentrations of ROS than their non-tumorigenic progeny. In order to investigate ROS biology in human CSCs, we began by examining the expression of genes involved in ROS metabolism in primary human breast CSCs and NTCs. Using microarray data from human breast CSC-enriched populations and NTCs13 and a curated list of genes involved in ROS metabolism5 (see methods), Gene Set Enrichment Analysis (GSEA)14 revealed that the expression of ROS genes was highly overrepresented in the CD44+CD24-/lowLin- breast CSC-enriched population compared to NTCs (p<0.001; Supplementary Fig. S2). The ROS genes identified as the core enriched genes by GSEA included a number of important antioxidant genes (Supplementary Table 2). Thus, gene expression profiles of human breast CSC-containing populations suggest that they contain higher levels of antioxidant defense systems than NTCs.
Next, we directly assessed ROS levels in human tumor subpopulations. To do this the CD44+CD24-/lowLin- breast CSC-enriched population and the corresponding “Not CD44+CD24-/low” Lin- NTC population were purified from surgically resected breast tumors (Supplementary Fig. S3). DCF-DA staining revealed that the CSC-enriched population in the human breast tumors we examined contained significantly lower levels of prooxidants than the NTC population. In some breast tumors, the vast majority of cells in the CSC-containing fraction displayed a low ROS phenotype compared to NTCs (Fig. 1e) while in others it was restricted to a significant subset of CSCs (Supplementary Fig. S4). We found a similar enrichment of cells with low ROS concentrations in a head and neck tumor (Supplementary Fig. S4). Thus, CSC-enriched populations from some human tumors contain lower average intracellular ROS levels than corresponding NTC populations.
Recently, we have demonstrated that Thy1+CD24+Lin- cells in the majority of spontaneously developing breast tumors from MMTV-Wnt-1 mice are highly enriched for tumorigenic activity15 (Supplementary Fig. S5a). We therefore asked whether the CSC-enriched population in this model system also displays a low ROS phenotype. ROS analysis using DCF-DA revealed that in tumors in which the Thy1+CD24+Lin- population was enriched for CSCs, this population contained a significantly higher fraction of cells with low prooxidant levels than the “Not Thy1+CD24+” Lin- non-tumorigenic population (Fig. 1f). Thy1+CD24+Lin- cells contained two main sub-populations of cells based on ROS concentration, with the low ROS subpopulation being significantly overrepresented compared to NTCs (Supplementary Fig. S5b). In order to confirm the presence of CSCs within the low ROS subpopulation, we transplanted CSCs based on their DCF-DA staining and found that both the low and high ROS subsets of Thy1+CD24+Lin- cells gave rise to tumors in recipient animals (Supplementary Table 3). Thus, a subset of CSCs from these MMTV-Wnt-1 tumors displayed low baseline levels of ROS compared to NTCs.
It is well established that cell killing after exposure to ionizing radiation (IR) and a subset of cytotoxic chemotherapeutics is partially mediated by free radicals16. Given our observations of increased expression of ROS defense genes in CSCs, we were therefore interested in testing whether CSC-enriched populations develop less DNA damage after IR than NTCs. In order to examine DNA damage immediately after irradiation, we purified Thy1+CD24+Lin- cells and NTCs from MMTV-Wnt-1 tumors by flow cytometry and irradiated them on ice. Cells were then either left on ice or incubated at 37°C, before being analyzed using the alkaline comet assay17. While untreated cells did not display significantly different levels of DNA damage, there were fewer DNA-strand breaks in the Thy1+CD24+Lin- cells than NTCs immediately after exposure to IR (Fig. 2a-b). These findings are consistent with the hypothesis that enhanced expression of ROS defenses in CSCs contributes to reduced levels of DNA damage after irradiation.
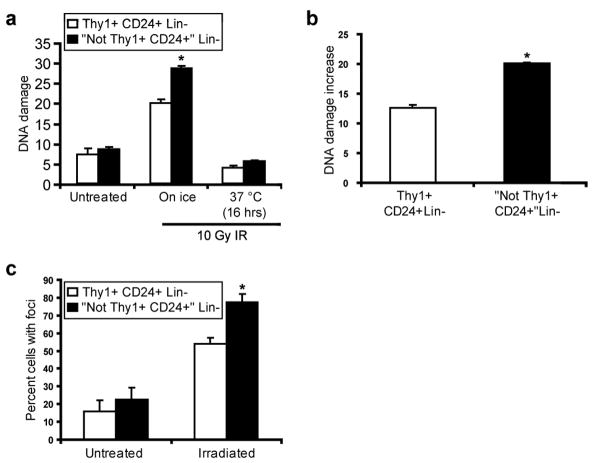
a, Thy1+CD24+Lin- cells (CSC-enriched population) and “Not Thy1+CD24+” Lin- non-tumorigenic cells were isolated from MMTV-Wnt-1 breast tumors by flow cytometry and irradiated with 10 Gy of IR. DNA damage was measured before irradiation, immediately after irradiation, and 16 hrs later using the alkaline comet assay. Mean of median tail moments ± s.e.m. (n=3; p=0.05). b, Using the data from a, the difference in median tail moments between the untreated and “on ice” time points was calculated. Mean ± s.e.m. (n=3; p=0.004). c, Thy1+CD24+Lin- cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells from MMTV-Wnt-1 tumors that were processed and collected 15 minutes after being irradiated in vivo with 1 Gy of IR were immunostained for γ-H2AX, a marker of DNA double strand breaks. Mean ± s.e.m. (n=2; p=0.04).
Since the alkaline comet assay mainly measures single strand breaks and since double strand breaks are important for IR-induced lethality18, we also analyzed levels of double strand breaks as reflected by phosphorylated histone 2AX (H2AX) nuclear foci after in vitro irradiation. As with the comet assay, we again observed significantly lower levels of DNA damage in Thy1+CD24+Lin- CSC-enriched cells than in NTCs (Supplementary Fig. S6). We also measured phosphorylated H2AX foci after in vivo irradiation of MMTV-Wnt-1 tumors, and again found that Thy1+CD24+Lin- cells contained fewer foci than NTCs (Fig. 2c). Thus, consistent with their lower baseline levels of ROS, Thy1+CD24+Lin- CSC-enriched cells isolated from these tumors developed less DNA strand breaks than NTCs after exposure to IR.
Given these findings, CSCs would be expected to preferentially survive exposure to IR in intact tumors. Mice bearing MMTV-Wnt-1 tumors were therefore treated with short, fractionated courses of IR and the percentage of the Thy1+CD24+Lin- CSC-enriched population before and after irradiation was analyzed using flow cytometry. On average, we found an approximately 2 fold increase in the percentage of the Thy1+CD24+Lin- CSC-enriched population compared with “Not Thy1+CD24+” Lin- NTCs in the irradiated tumors, suggesting that CSCs are relatively radioresistant compared with NTCs (Fig. 3a-b). We found a similar increase in the fraction of CD44+Lin- CSC-enriched population when we irradiated human head and neck cancer xenografts grown in immunodeficient mice (Fig. 3c). Other investigators have documented similar radioresistance of CSCs in brain tumors19 and a breast cancer cell line20. Thus, CSCs in some murine and human tumors are relatively radioresistant compared to their NTC counterparts.
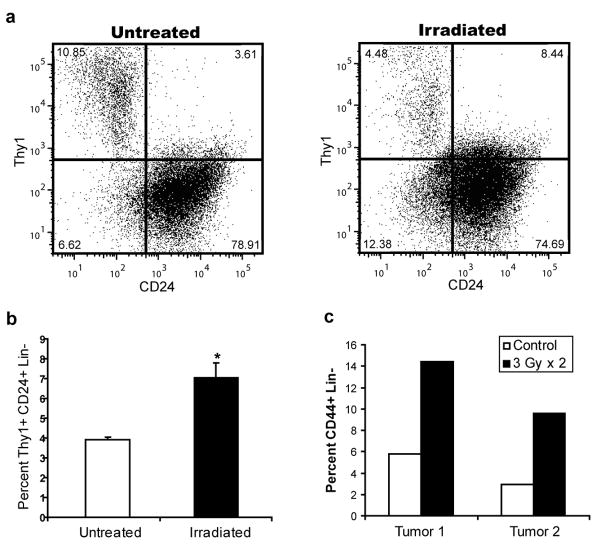
a, Breast tumors from MMTV-Wnt-1 mice were irradiated in vivo with 3 × 5 Gy or 5 × 2 Gy. Percentage of Thy1+CD24+Lin- cells in untreated and irradiated tumors were quantified by flow cytometry 72 hours after the last fraction was delivered. b, Mean ± s.e.m. for replicates of a (n=6; p=0.008). c, First generation xenografts established from two different primary human head and neck cancers were irradiated in vivo with 2 × 3 Gy. Percentage of CD44+Lin- cells was quantified as above.
Since a significant fraction of murine breast CSCs contained relatively low levels of ROS, we hypothesized that these cells may express enhanced levels of ROS defenses compared to their NTC counterparts. We were particularly interested in glutathione (GSH), a critical cellular reducing agent and antioxidant which has been implicated in chemotherapy and radiotherapy resistance of cancer cells21. Since our prior analyses revealed heterogeneity within CSC-enriched populations and since single cell gene expression studies have revealed significant variation in gene expression in other stem cell populations22, we investigated expression of critical GSH biosynthesis genes in MMTV-Wnt-1 CSCs and NTCs using single cell qRT-PCR. This analysis revealed significant overexpression of Gclm (p<0.001) and Gss (p<0.005) in a large fraction of cells within the CSC-enriched population, the former of which encodes the regulatory subunit of the enzyme (glutamate-cysteine ligase) that catalyzes the rate limiting step of GSH synthesis (Fig4a)23. Furthermore, Foxo1, a transcription factor implicated in the regulation of an anti-ROS gene expression program in HSCs5, was also overexpressed in CSCs compared to NTCs (p<0.001; Fig.4a). Other genes, including Hif1a, Epas1, and Foxo4 were not differentially expressed. Thus, genes controlling GSH biosynthesis were overexpressed by many cells within the CSC-enriched population isolated from this tumor.
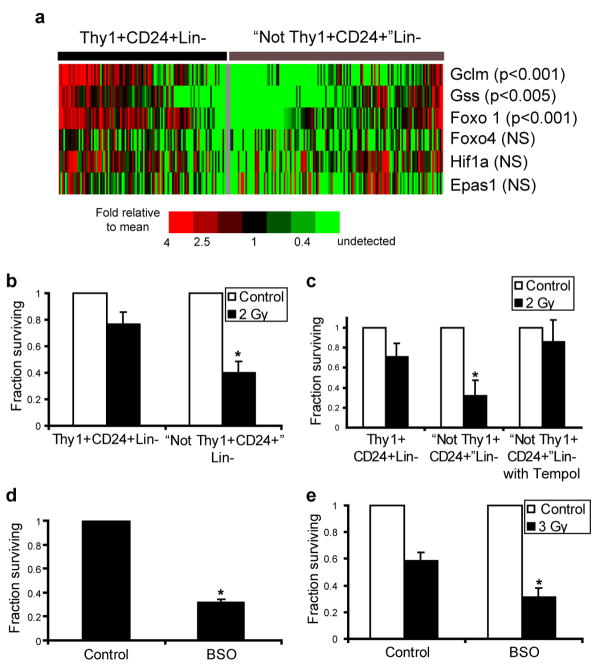
a, Single cell qRT-PCR analysis of gene expression in Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells. The heatmap displays mean centered CT values. b, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells before and after 2 Gy of ionizing radiation. Mean ± s.e.m. (n=3; p=0.001). c, Clonogenic survival of “Not Thy1+CD24+” Lin- non-tumorigenic cells in the presence or absence of the ROS scavenger tempol (10 mM). Mean ± s.e.m. (n=2; p=0.03). d, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells in the presence or absence of 24 hour pre-treatment with the glutathione synthesis inhibitor L-S,R-Buthionine Sulfoximine (BSO, 1 mM). Mean ± s.e.m. (n=3; p=0.002). e, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells after 3 Gy of ionizing radiation with or without BSO pretreatment. Mean ± s.e.m. (n=3; p=0.03).
In order to pharmacologically manipulate ROS levels separately in CSCs and NTCs, we employed in vitro culture conditions that allowed both cell populations to produce colonies upon co-culture with irradiated feeder cells. We found that Thy1+CD24+Lin- CSC-enriched cells were relatively radioresistant compared with NTCs (Fig. 4b and Supplementary Fig. S7). When exposed to 2 Gy, a dose commonly administered clinically during daily treatments of breast cancer patients, 2.0-fold +/- 0.2 more CSC-enriched colonies survived than NTC colonies. Next, we attempted to radioprotect NTCs by exposing them to the nitroxide antioxidant tempol24. Pretreatment with tempol radioprotected NTCs, and resulted in survival levels similar to those seen in the CSC-enriched population (Fig. 4c).
Given the overexpression of genes involved in GSH synthesis by CSCs, we wished to assess the sensitivity of these cells to ROS elevation via pharmacologic depletion of GSH. Exposure of Thy1+CD24+Lin- CSC-enriched cells to Buthionine Sulfoximine (BSO), which inhibits glutamate-cysteine ligase25, decreased their colony forming ability by approximately three fold (Fig. 4d). Finally, we asked if GSH depletion would radiosensitize CSCs. As shown in Fig. 4e, BSO pretreatment of Thy1+CD24+Lin- CSC-enriched cells led to significant radiosensitization. These data demonstrate the importance of low ROS levels and antioxidant defenses to CSC survival and radiosensitivity in these tumors.
Our data indicate that normal breast stem cells and a subset of CSCs in some tumors arising in both mice and humans contain lower levels of ROS than their cellular descendants. Taken together with previous reports of low ROS concentrations in other normal tissue stem cells, these findings suggest that stem cells in diverse systems have conserved this attribute, which likely helps to protect their genomes from endogenous and exogenous ROS-mediated damage. The mechanism leading to low ROS levels in some CSCs appears to be at least partially due to the increased production of free radical scavengers. Notably, there appears to be significant heterogeneity of ROS levels in both normal stem cell and CSC-enriched populations, which could reflect that the enriched populations contain both stem and non-stem cells and/or that ROS levels within stem cells can differ based on environmental factors that alter the balance of endogenous production and the expression of scavenging pathways. The low ROS subset found in the various stem cell populations may also represent a quiescent subpopulation. Heterogeneity of ROS levels may influence the extent to which CSC-enriched populations are resistant to therapies such as ionizing radiation.
In light of recent findings that CSCs in glioblastoma multiforme display enhanced DNA repair capabilities19, it appears that CSCs may resist standard cytotoxic therapies through a combination of mechanisms, and that these may be unique to a given tumor. In the case of human CSCs, the frequency with which these cells display low ROS levels or enhanced DNA repair remains to be determined, particularly since the normal transformation precursor may be either stem or progenitor cells26, 27 and since ROS concentration (Fig. 1 and 5, 28) and increased DNA repair capabilities appear to partially reflect differentiation state. Clinical therapies could likely be optimized by patient- and tumor-specific identification of CSC-resistance mechanisms and overcoming low ROS levels within CSCs may be a useful method for improving local and systemic oncologic therapies.
Methods Summary
Cells were analyzed, collected by FACS, and injected into recipient mice as described with minor modifications from mouse mammary glands10, human breast29 cancers, human head and neck30 cancers, and MMTV-Wnt-1 mouse tumors15. For human samples, informed consent was obtained after approval of protocols by the Stanford University and City of Hope Institutional Review Boards. For intracellular ROS analysis, cells were loaded with 10 μM DCF-DA (Invitrogen), incubated at 37°C for 30 min, and immediately analyzed by flow cytometry. Cells were re-sorted based on their level of DCF-DA staining for transplant experiments. For MitoSOX Red experiments, cells were loaded with 5 uM MitoSOX Red at 37°C for 20 min. DNA damage was evaluated using the single-cell gel electrophoresis assay under alkaline conditions17. For γ-H2AX immunostaining, purified cells were cytospun onto poly-L-lysine coated slides, fixed, permeabilized, and stained with a phospho-specific (Ser 139) histone H2AX antibody (Cell Signaling Technology) followed by a secondary Alexa Fluor 488-conjugated antibody (Invitrogen). For single cell gene expression analysis, cells were sorted into 96 well plates containing CellsDirect qRT-PCR mix (Invitrogen). After reverse transcription, genes were pre-amplified (22 cycles) using the same Taqman primers (Applied Biosystems) used for quantification. Products were analyzed using qPCR DynamicArray microfluidic chips (Fluidigm). For in vitro colony assays, cells were cultured in Epicult B medium (StemCell Technologies) with 5% serum in the presence of ~13,000 cm-2 irradiated NIH-3T3 cells. After 24-48 hrs, the media was replaced with serum-free Epicult B, and colonies counted ~7 days later. GSEA14 was performed using previously published microarray data13 of CSCs and NTCs from primary breast tumor samples and a curated list of ROS genes (Supplementary Table 4). Levels of significance were determined by Student's t-tests using α=0.05.
Supplementary Material
Supp Figures
Supp Methods
Acknowledgments
We thank D. Spitz for helpful discussions and D. Menke, D. Rossi, and J. Seita for technical assistance. This work was supported by grants from the National Institutes of Health (M.F.C. and I.L.W.), the Virginia and D.K. Ludwig Foundation (M.F.C. and I.L.W.), the Breast Cancer Research Foundation (M.F.C.), the Machiah foundation (T.K.), the American Society for Therapeutic Radiology and Oncology (M.D.), and the Radiological Society of North America (M.D.). M.D. is a recipient of the Leonard B. Holman Research Pathway fellowship.
Footnotes
Author Contributions M.D. and R.W.C. contributed equally to this work. M.D, R.W.C., N.L., T.K., M.J.D., A.K., D.Q., J.S.L., L.A., and M.W. performed the experiments. B.J., M.J.K, I.W., F.W., G.S., C.G., B.P., J.S., and S.K.L. aided in human tumor tissue acquisition. G.S. designed a pre-operative protocol allowing for tissue acquisition. M.D., R.W.C., and M.F.C. designed the experiments and wrote the manuscript. S.R.Q., J.M.B., and I.L.W. provided intellectual input and aided in experimental design.
Author Information Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nature07733
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2778612?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101953362
Article citations
Enrichment of cancer stem cell subpopulation alters the glycogene expression profile of colorectal cancer cells.
Discov Oncol, 15(1):647, 12 Nov 2024
Cited by: 0 articles | PMID: 39532788 | PMCID: PMC11557779
RhoA-ROCK2 signaling possesses complex pathophysiological functions in cancer progression and shows promising therapeutic potential.
Cancer Cell Int, 24(1):339, 14 Oct 2024
Cited by: 0 articles | PMID: 39402585 | PMCID: PMC11475559
Review Free full text in Europe PMC
Reactive oxygen species from non-thermal gas plasma (CAP): implication for targeting cancer stem cells.
Cancer Cell Int, 24(1):344, 22 Oct 2024
Cited by: 0 articles | PMID: 39438918 | PMCID: PMC11515683
Review Free full text in Europe PMC
Cancer stem cells in meningiomas: novel insights and therapeutic implications.
Clin Transl Oncol, 24 Sep 2024
Cited by: 0 articles | PMID: 39316249
Review
Full-course NIR-II imaging-navigated fractionated photodynamic therapy of bladder tumours with X-ray-activated nanotransducers.
Nat Commun, 15(1):8240, 19 Sep 2024
Cited by: 0 articles | PMID: 39300124 | PMCID: PMC11413067
Go to all (1,437) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A radical bailout strategy for cancer stem cells.
Cell Stem Cell, 4(3):196-197, 01 Mar 2009
Cited by: 12 articles | PMID: 19265655
Low production of reactive oxygen species and high DNA repair: mechanism of radioresistance of prostate cancer stem cells.
Anticancer Res, 33(10):4469-4474, 01 Oct 2013
Cited by: 34 articles | PMID: 24123017
Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling.
Semin Cancer Biol, 35:39-44, 25 Sep 2015
Cited by: 74 articles | PMID: 26392376
Review
Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.
Mol Cancer, 16(1):10, 30 Jan 2017
Cited by: 264 articles | PMID: 28137309 | PMCID: PMC5282724
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: U54 CA126524
Grant ID: R01 CA100225
Grant ID: U54 CA126524-04
Grant ID: R01 CA100225-05




