Abstract
Purpose
This phase II trial was designed to define the role of O(6)-benzylguanine (O(6)-BG) in restoring temozolomide sensitivity in patients with recurrent or progressive, temozolomide-resistant malignant glioma and to evaluate the safety of administering O(6)-BG in combination with temozolomide.Patients and methods
Patients were accrued into two independent strata on the basis of histology: glioblastoma multiforme (GBM) and anaplastic glioma. Both temozolomide and O(6)-BG were administered on day 1 of a 28-day treatment cycle. Patients were administered a 1-hour O(6)-BG infusion at a dose of 120 mg/m(2) followed immediately by a 48-hour infusion at a dose of 30 mg/m(2)/d. Temozolomide was administered orally within 60 minutes of the end of the 1-hour O(6)-BG infusion at a dose of 472 mg/m(2). The primary end point was objective response rate. Secondary end points included progression-free survival, overall survival, and safety.Results
Sixty-six of 67 patients who enrolled were treated with temozolomide and O(6)-BG. One of 34 patients (3%) with GBM (95% CI, 0.1% to 15%) and five of 32 assessable patients (16%) with anaplastic glioma (95% CI, 5% to 33%) were responders. The most commonly reported adverse events were grade 4 hematologic events experienced in 48% of the patients.Conclusion
O(6)-BG when added to a 1-day dosing regimen of temozolomide was able to restore temozolomide sensitivity in patients with temozolomide-resistant anaplastic glioma, but there seemed to be no significant restoration of temozolomide sensitivity in patients with temozolomide-resistant GBM.Free full text

Phase II Trial of Temozolomide Plus O6-Benzylguanine in Adults With Recurrent, Temozolomide-Resistant Malignant Glioma
Abstract
Purpose
This phase II trial was designed to define the role of O6-benzylguanine (O6-BG) in restoring temozolomide sensitivity in patients with recurrent or progressive, temozolomide-resistant malignant glioma and to evaluate the safety of administering O6-BG in combination with temozolomide.
Patients and Methods
Patients were accrued into two independent strata on the basis of histology: glioblastoma multiforme (GBM) and anaplastic glioma. Both temozolomide and O6-BG were administered on day 1 of a 28-day treatment cycle. Patients were administered a 1-hour O6-BG infusion at a dose of 120 mg/m2 followed immediately by a 48-hour infusion at a dose of 30 mg/m2/d. Temozolomide was administered orally within 60 minutes of the end of the 1-hour O6-BG infusion at a dose of 472 mg/m2. The primary end point was objective response rate. Secondary end points included progression-free survival, overall survival, and safety.
Results
Sixty-six of 67 patients who enrolled were treated with temozolomide and O6-BG. One of 34 patients (3%) with GBM (95% CI, 0.1% to 15%) and five of 32 assessable patients (16%) with anaplastic glioma (95% CI, 5% to 33%) were responders. The most commonly reported adverse events were grade 4 hematologic events experienced in 48% of the patients.
Conclusion
O6-BG when added to a 1-day dosing regimen of temozolomide was able to restore temozolomide sensitivity in patients with temozolomide-resistant anaplastic glioma, but there seemed to be no significant restoration of temozolomide sensitivity in patients with temozolomide-resistant GBM.
INTRODUCTION
Despite recent advances in therapeutic regimens, the diagnosis of malignant glioma still carries a poor prognosis. Although alkylators such as temozolomide and polifeprosan 20 with carmustine implant (Gliadel, Guilford Pharmaceuticals-MGI Pharma, Bloomington, MN) are US Food and Drug Administration–approved for treatment of malignant glioma, their ability to prolong survival is short-lived. Thus, innovative therapeutic agents and strategies are imperative.
One novel approach to fighting this deadly disease is to target the mechanisms of resistance to chemotherapy. Because O6-alkylguanine-DNA alkyltransferase (AGT) has been shown to be a major factor in the resistance of tumor cells to alkylating agents such as carmustine and temozolomide,1–3 perhaps targeting this DNA repair protein can help restore chemotherapy activity.
One such agent that can irreversibly inactivate AGT is a low molecular weight substrate, O6-benzylguanine (O6-BG). In vitro1,4,5 and in vivo6,7 studies using tumor cell lines and subcutaneous brain tumor xenografts demonstrate that O6-BG increases the therapeutic effectiveness of temozolomide.
These studies suggest that combining O6-BG with temozolomide may circumvent resistance and restore chemotherapy sensitivity. However, before one can combine these two agents, one must answer the following two important questions: what dosing schedule of O6-BG is necessary to completely suppress AGT activity in brain tumors for at least 48 hours, and what is the maximum-tolerated dose (MTD) of temozolomide in a 1-day dosing regimen that can be combined with O6-BG?
Prior research endeavors have answered both of these questions. Weingart et al8 answered this first question in a phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-BG in adults with recurrent malignant glioma by establishing the dose of O6-BG to be 120 mg/m2 for 1 hour followed by a continuous infusion of 30 mg/m2/d for 2 days. Quinn et al9 answered the second question in a phase I trial of temozolomide plus O6-BG in adults with recurrent malignant glioma by establishing the MTD of temozolomide to be 472 mg/m2 when administered as a single dose in conjunction with the above dosing schedule of O6-BG.
The objectives of this phase II trial were twofold: to define the role of O6-BG in restoring temozolomide sensitivity in patients with recurrent or progressive, temozolomide-resistant malignant glioma and to evaluate the safety of administering O6-BG in combination with temozolomide.
PATIENTS AND METHODS
Patients
Eligible patients had a histologically confirmed diagnosis of progressive or recurrent glioblastoma multiforme (GBM) or anaplastic glioma (anaplastic astrocytoma [AA], anaplastic oligodendroglioma [AO], or anaplastic mixed AA and AO). These tumors must have shown resistance to temozolomide, defined as a ≥ 25% increase in tumor growth on contrast-enhanced magnetic resonance imaging (MRI) or computed tomography (CT) within 8 weeks of the last dose of temozolomide. Furthermore, all patients who experienced treatment failure with temozolomide as their most recent treatment were immediately enrolled onto the trial using temozolomide plus O6-BG. Patients were ≥ 18 years old and required to have a Karnofsky performance score of ≥ 60%. An interval of at least 2 weeks since prior surgical resection (if conducted) or 4 weeks since prior chemotherapy (6 weeks for a nitrosourea-based regimen) had to have elapsed for the patient to be enrolled onto the clinical trial. All patients had evidence of residual tumor on postoperative MRI before starting temozolomide plus O6-BG. Additional enrollment criteria included adequate pretreatment bone marrow function (hematocrit > 29%, total granulocyte count > 1,500 cells/μL, platelets > 100,000 cells/μL), renal function (serum creatinine < 1.5 mg/dL, serum urea nitrogen < 25 mg/dL), and hepatic function (serum AST and bilirubin < 1.5× upper limit of normal). For patients receiving corticosteroids, a stable dose for 1 week before entry onto the study was required, if clinically possible, with no escalation of dose above entry dose level. Patients of reproductive potential were required to take effective contraceptive measures for the duration of the study. The following patients were excluded from the study: pregnant women, potentially fertile women or men who were not using effective contraception method, and patients taking immunosuppressive agents other than corticosteroids. The protocol was reviewed and approved by the Duke University Health System institutional review board. Each patient signed an informed consent form.
Study Design and Treatment
This was a phase II, open-label, single-center trial with accrual goals defined within two separate strata: GBM and anaplastic glioma (AA or AO). Both temozolomide and O6-BG were administered on day 1 of a 28-day treatment cycle. First, patients were treated with a 1-hour bolus infusion of O6-BG at a dose of 120 mg/m2 followed immediately by a 48-hour continuous infusion of O6-BG at a dose of 30 mg/m2/d. Next, temozolomide was administered orally, in a fasting state, at a dose of 472 mg/m2 within 60 minutes of the end of the 1-hour administration of O6-BG infusion.
Temozolomide was commercially available from Schering-Plough Research Institute (Kenilworth, NJ). O6-BG was supplied by AOI Pharmaceuticals Inc (New York, NY).
Prophylactic antiemetics were permitted as needed. Neurologic stability was provided with the lowest corticosteroid dose when required. Colony-stimulating factors were permitted only for rescue from grade 4 neutropenia.
Surveillance and Follow-Up
The baseline examination included central review of tumor tissue, MRI (or CT if MRI was medically contraindicated), CBCs and blood chemistry tests, and a physical examination including a comprehensive neurologic examination. During therapy, weekly CBCs were obtained. Before subsequent cycles of chemotherapy, patients were required to repeat CBCs, blood chemistry tests, and a physical examination. In addition, after every two cycles of chemotherapy, patients were required to obtain repeat neuroimaging.
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria version 2.0. Repeat cycles of chemotherapy were administered on schedule only if the patient met the following re-treatment criteria: total granulocyte count > 1,500 cells/μL, platelets > 100,000/μL, hematocrit > 29 g/dL, AST ≤ 2.5× upper limit of normal, creatinine < 1.5× upper limit of normal, and total bilirubin within normal limits; all other toxicities must have resolved to baseline or grade 1. Temozolomide dose was reduced by 25% in any patient with grade ≥ 3 nonhematologic or grade 4 hematologic toxicity or if re-treatment was delayed by longer than 2 weeks because of any grade toxicity. Patients who were delayed 3 weeks from treatment were removed from the trial unless there was evidence of tumor response.
Objective assessments of overall response were based on tumor assessment from MRI scans (CT if MRI was medically contraindicated) interpreted in the light of corticosteroid use, as suggested by Macdonald et al,10 with appropriate support from the neurologic examination. This drug combination was administered for a maximum of 16 months or until unacceptable toxicity or tumor progression occurred.
Statistical Analysis
The primary end point was objective response rate (ORR) defined as the percentage of patients with complete response (CR) or partial response (PR) according to the modified Macdonald criteria. The secondary end points were safety, 6-month, and median progression-free survival (PFS), and 6-month and median overall survival (OS).
On the basis of a two-stage Simon minimax design11 with an α level of 10% and 90% power, 32 patients (18 patients in stage 1 of the study and an additional 14 patients in stage 2) within each stratum were required to test the null hypothesis that the true ORR was ≤ 5% versus the alternative hypothesis that the true ORR was ≥ 20%. At least one objective response within each stratum was needed in stage 1 to allow expansion of the trial to stage 2. At the end of the study, at least four objective responses within each stratum were needed to reject the null hypothesis.
Efficacy and safety analyses included all patients who received at least one dose of temozolomide and O6-BG. The number and proportion of patients who achieved an objective response (CR or PR) was summarized, along with the corresponding exact two-sided 95% CI, calculated by a method derived from the binomial distribution. PFS, defined as the time between initiation of treatment and the first occurrence of disease progression, disease, or death, and OS, defined as the time between initiation of treatment and death, were summarized by using the Kaplan-Meier method.12 Estimates for the median event time and 6-month rates were generated within the Kaplan-Meier framework.
RESULTS
Patient Characteristics
Sixty-seven patients were enrolled. Sixty-six patients received at least one dose of temozolomide and O6-BG. One patient with AA did not receive any protocol treatment because of withdrawal of consent and is excluded from all summaries. Patient demographics and baseline disease characteristics of the 66 patients who received protocol treatment are listed in Table 1. Central pathologic review confirmed that 34 patients had GBM, 28 patients had AA, and four patients had AO. The median age was 51 years, and the majority of patients were male. Fifty-five percent of patients had a Karnofsky performance score of ≤ 80. All patients had progressive or recurrent disease after prior therapy with temozolomide, and the majority of patients (59%) had at least two prior progressions. The median time from diagnosis to the start of therapy with temozolomide plus O6-BG was 60 weeks (range, 12 to 72 weeks).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | No. of Patients (N = 66) | % |
|---|---|---|
| Age, years | ||
    Median Median | 51 | |
    Range Range | 21-69 | |
| Sex | ||
    Male Male | 44 | 67 |
    Female Female | 22 | 33 |
| Karnofsky performance score, % | ||
    < 70 < 70 | 3 | 4 |
    70-80 70-80 | 33 | 51 |
    90-100 90-100 | 30 | 45 |
| No. of progressions | ||
    Median Median | 2 | |
    Range Range | 1-5 | |
| Time from diagnosis, weeks | ||
    Median Median | 60 | |
    Range Range | 12-702 | |
| Histological diagnosis | ||
    AA AA | 28 | 42 |
    AO AO | 4 | 6 |
    GBM GBM | 34 | 52 |
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; GBM, glioblastoma multiforme.
Response
The overall ORR for all histologies was 9% (95% CI, 3% to 19%). One (3%) of 34 patients with GBM (95% CI, 0.1% to 15%) and five (16%) of 32 patients with anaplastic glioma (95% CI, 5% to 33%) were responders to the combination of temozolomide and O6-BG. Table 2 details the response in each histology subgroup. One patient died as a result of a perforated cecum 2 days after treatment with this drug combination. Five patients with anaplastic glioma who had a response completed at least a full year of therapy. Of these five patients, disease progression did not occur in three patients for 131, 93, and 89 weeks, respectively, and the other two patients remain disease-free 185 and 164 weeks from enrollment, respectively. Of interest, one patient with an anaplastic glioma who completed a year of therapy remained progression free for 143 weeks but never realized a response by the modified Macdonald criteria. However, this patient's positron emission tomography scan was hypometabolic on completion of a full year of therapy.
Table 2.
Overall Survival, Progression-Free Survival, and Objective Response Rate According to Histology
| Variable | Overall | GBM | AA/AO | |||
|---|---|---|---|---|---|---|
| No. of Patients | 95% CI | No. of Patients | 95% CI | No. of Patients | 95% CI | |
| Overall survival | ||||||
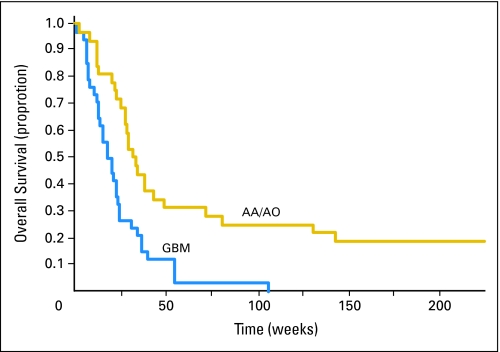
    Median, weeks* Median, weeks* | 24.7 | 20.9 to 31.0 | 19.4 | 13.7 to 24.3 | 33 | 28 to 49.6 |
    At 6 months, % At 6 months, % | 47 | 35 to 58 | 26 | 13 to 42 | 69 | 50 to 82 |
| Progression-free survival | ||||||
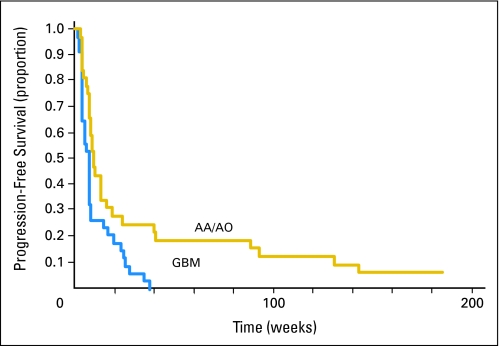
    Median, weeks* Median, weeks* | 7.9 | 7.6 to 9.6 | 7.5 | 4.2 to 7.9 | 9.6 | 7.9 to 16.1 |
    At 6 months, % At 6 months, % | 17 | 9 to 27 | 9 | 2 to 21 | 25 | 12 to 41 |
| Objective response rate,* % | 9 | 3 to 19 | 3 | 0.1 to 15 | 16 | 5 to 33 |
Abbreviations: AA, anaplastic astrocytoma; AO, anaplastic oligodendroglioma; GBM, glioblastoma multiforme.
PFS
The 6-month PFS was 17% overall (95% CI, 9% to 27%), 9% for GBM (95% CI, 2% to 21%), and 25% for anaplastic glioma (95% CI, 12% to 41%). The median PFS was 7.9 weeks overall (95% CI, 7.6 to 9.6 weeks), 7.5 weeks for GBM (95% CI, 4.2 to 7.9 weeks), and 9.6 weeks for anaplastic glioma (95% CI, 7.9 to 16.1 weeks). There was a significant statistical difference between the two histologies (P = .0049). The PFS data are illustrated by the Kaplan-Meier curve in Figure 1.
OS
The 6-month OS was 47% (95% CI, 35% to 58%), 26% for GBM (95% CI, 13% to 42%), and 69% for anaplastic glioma (95% CI, 50% to 82%). The median OS was 24.7 weeks (95% CI, 20.9 to 31.0 weeks), 19.4 weeks for GBM (95% CI, 13.7 to 24.3 weeks), and 33 weeks for anaplastic glioma (95% CI, 28.0 to 49.6 weeks). There was a significant statistical difference between the two histologies (P = .0004). The OS data are presented as a Kaplan-Meier curve in Figure 2.
Toxicity
Table 3 summarizes the adverse events (AEs). The most commonly reported AEs were grade 4 hematologic events experienced in 48% of the patients, for whom a 25% temozolomide dose reduction was required for subsequent dose administrations. The most frequent hematologic AEs were grade 4 neutropenia (47%), followed by grade 4 thrombocytopenia (12%). One patient died as a result of a perforated cecum 2 days after initiation of treatment with this drug combination. This event was unlikely to be drug related, given that before enrollment she complained of abdominal pain. One patient was not assessable for toxicity because the patient never received this drug combination.
Table 3.
Adverse Events
| Adverse Event | Grade 3 | Grade 4 | Grade 5 | |||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Hematologic | ||||||
    Anemia Anemia | 1 | 1 | ||||
    Lymphopenia Lymphopenia | 1 | 1 | ||||
    Thrombocytopenia Thrombocytopenia | 8 | 12 | ||||
    Neutropenia Neutropenia | 31 | 47 | ||||
    Neutropenia, febrile Neutropenia, febrile | 1 | 1 | ||||
| Nonhematologic | ||||||
    Infection without neutropenia Infection without neutropenia | 2 | 3 | ||||
    Seizure Seizure | 4 | 6 | ||||
    Thrombosis/embolism Thrombosis/embolism | 2 | 3 | ||||
    GI perforation GI perforation | 1 | 1 | ||||
DISCUSSION
Temozolomide and polifeprosan 20 with carmustine implant are both US Food and Drug Administration–approved drugs because of their ability to prolong survival in patients diagnosed with malignant glioma. Unfortunately, all patients with this disease will eventually experience treatment failure with these agents.
Resistance to both temozolomide and carmustine is mediated in part through the DNA repair protein AGT. Three clinical trials13–15 reported an inverse relationship between AGT levels and survival for patients with malignant glioma treated with carmustine. Esteller et al16 also confirmed this relationship, albeit using methylation of the AGT promoter gene in lieu of quantization of AGT levels. Likewise, this same inverse relationship between AGT levels and response was seen in patients with malignant glioma treated with preradiation temozolomide.17 Furthermore, Hegi et al18 have shown a relationship between inactivation of the AGT gene by promoter methylation and survival in patients with newly diagnosed GBM treated with surgery, radiotherapy, and temozolomide. These studies suggest that the efficacy of temozolomide and carmustine could be enhanced by depletion of tumor AGT.
Depletion of tumor AGT activity by a selective inhibitor, O6-BG, enhances the cytotoxicity of chloroethylators and methylators including temozolomide and carmustine.1,4,7,17,19–21 However, the main limitation in systemic administration of alkylating agents in combination with O6-BG is their potential for dose-related toxicity to the hematopoietic system. This was seen in animal studies22–24 and confirmed in a phase I clinical trial,25 where systemic administration of O6-BG and carmustine markedly enhanced myelosuppression and reduced the carmustine MTD from 200 to 40 mg/m2, representing an 80% dose reduction. This profound reduction in the carmustine dose was most likely the underlying factor in the failure of this drug combination to cause frank tumor regression in the phase II trial.26
Given that temozolomide is inherently less toxic than carmustine, particularly to hematopoietic cells, it was hypothesized that perhaps temozolomide in combination with O6-BG would produce less myelosuppression than carmustine in combination with O6-BG. Thus, a phase I clinical trial was performed in patients with malignant glioma to determine the MTD of single-dose temozolomide in combination with O6-BG.9 When combined with O6-BG, the MTD of temozolomide was found to be 472 mg/m2. The requirement for an 80% dose reduction seen with carmustine when combined with O6-BG was seen to a lesser extent (50%) with temozolomide when combined with O6-BG.
Thus, one of the aims of this study was to evaluate the safety of administering O6-BG in combination with temozolomide, especially to the hematopoietic system. As expected, myelosuppression was the most commonly reported AE. Grade 4 hematologic events were experienced in 48% of the patients for whom a 25% temozolomide dose reduction was required for subsequent dose administrations. The most frequent hematologic AEs were grade 4 neutropenia (47%) followed by grade 4 thrombocytopenia (12%).
Another aim of this study was to identify the role of O6-BG in restoring temozolomide sensitivity in patients with recurrent or progressive temozolomide-resistant malignant glioma. Unfortunately, the lack of a clinical rationale to justify a biopsy at recurrence precluded quantitation of tumor AGT levels, which could have provided insights if other non-AGT mechanisms of resistance were operational. We determined that O6-BG when added to a 1-day dosing regimen of temozolomide was able to restore temozolomide sensitivity in patients with temozolomide-resistant anaplastic glioma, but there seemed to be no significant restoration of temozolomide sensitivity in patients with temozolomide-resistant GBM.
Despite the encouraging response to temozolomide and O6-BG in patients with anaplastic glioma, it is somewhat disappointing that a greater response and improvement in PFS and OS were not realized in GBM, especially if AGT was indeed the primary mechanism responsible for tumor resistance to temozolomide. Although O6-BG in combination with temozolomide was able to reverse the resistance in a minority of patients with recurrent or progressive, temozolomide-resistant malignant glioma, it was unable to reverse this resistance in the majority of these patients. The explanation for the inability of this combination to reverse resistance may lie in the two following reasons.
First, the dose reduction in temozolomide necessary to avoid hematologic toxicity when combined with O6-BG may be an underlying factor in the failure of this drug combination to restore sensitivity. As a 1-day dosing regimen, temozolomide required a 50% dose reduction when delivered with O6-BG. Alternative routes of administration of either O6-BG or alkylators have been investigated in hopes of reducing systemic toxicity while maximizing efficacy. Administration of O6-BG locally by intracerebral infusion through an Ommaya reservoir with concomitant oral administration of temozolomide was attempted in one patient.27 This therapy was well tolerated and caused tumor stabilization for four months, but its success at depleting AGT and thus prolonging survival above and beyond the effect of temozolomide alone is unknown at this time. The administration of carmustine locally in the form of polifeprosan 20 with carmustine implant in combination with systemic O6-BG was performed in a phase I8 and a phase II clinical trial.28 Both of these trials support the idea that local administration of carmustine in the form of polifeprosan 20 with carmustine implant in combination with systemic O6-BG may mitigate the systemic toxicities of alkylators with improved efficacy.28
Second, combining O6-BG with a 5-day dosing regimen of temozolomide may be more effective than a 1-day dosing regimen, especially if the total dose of temozolomide can be increased. To optimize a 5-day dosing regimen of temozolomide in combination with O6-BG, one could hypothesize that suppressing AGT beyond 48 hours for at least 5 days while administering temozolomide may improve efficacy. Because we know that the above O6-BG regimen completely suppresses O6-BG for at least 48 hours, perhaps repeating this exact regimen every 48 hours for a period of 5 to 7 days may optimize AGT suppression while administering a 5-day dosing regimen of temozolomide. Although other O6-BG regimens have been used in combination with temozolomide29 and polifeprosan 20 with carmustine implant,8 there is no evidence to suggest that these dosing regimens are adequate to suppress AGT levels in brain tumors.
Although the current trial of O6-BG when added to a 1-day dosing regimen of temozolomide was able to restore temozolomide sensitivity in patients with temozolomide-resistant anaplastic glioma, no significant restoration of temozolomide sensitivity was seen in patients with temozolomide-resistant GBM. The limited response seen in GBM propels the research in a direction that explores alternative dosing regimens of temozolomide and O6-BG and alternative administration routes for chemotherapy. Thus, additional investigation in combining temozolomide and O6-BG in a 5-day dosing schedule needs to be undertaken in a phase I trial. Also, additional exploration of the safety and efficacy of combining polifeprosan 20 with carmustine implant with O6-BG should be pursued. In addition, the role of drug delivery needs to be addressed, given that it is possible that potentially higher interstitial pressure seen in GBMs compared with anaplastic gliomas may be limiting drug delivery. This question might be addressed in future studies by incorporation of dynamic contrast-enhanced MRIs to evaluate tumor blood flow and potentially drug delivery.
Footnotes
Supported by National Institute of Neurological Disorders and Stroke Grant No. 5P50 NS20023-25, National Institutes of Health (NIH) Specialized Program of Research Excellence Grant No. 5P50 CA 108786-4, NIH Merit Award R37 CA 011898-38, and NIH Grant No. 1 R21 CA 101298-01; S.X.J. was supported by NIH Grant No. TL1 RR024126.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00613093.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Henry S. Friedman, Keryx Biopharmaceuticals (C) Stock Ownership: None Honoraria: David A. Reardon, Schering-Plough Research Funding: Henry S. Friedman, Schering-Plough Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer A. Quinn, David A. Reardon, James J. Vredenburgh, Darell D. Bigner, James E. Herndon, Henry S. Friedman
Financial support: Darell D. Bigner, Henry S. Friedman
Administrative support: Darell D. Bigner, Henry S. Friedman
Provision of study materials or patients: Jennifer A. Quinn, David A. Reardon, Annick Desjardins, James J. Vredenburgh, Jeremy N. Rich, Allan H. Friedman, John H. Sampson, Henry S. Friedman
Collection and assembly of data: Jennifer A. Quinn, Sara Xiaoyin Jiang, David A. Reardon, James J. Vredenburgh, Jeremy N. Rich, Sridharan Gururangan, Allan H. Friedman, John H. Sampson, Roger E. McLendon, Amy Walker
Data analysis and interpretation: Jennifer A. Quinn, Sara Xiaoyin Jiang, David A. Reardon, James J. Vredenburgh, Sridharan Gururangan, Darell D. Bigner, Roger E. McLendon, James E. Herndon
Manuscript writing: Jennifer A. Quinn, Sara Xiaoyin Jiang, James E. Herndon, Henry S. Friedman
Final approval of manuscript: Jennifer A. Quinn, Sara Xiaoyin Jiang, David A. Reardon, Annick Desjardins, James J. Vredenburgh, Jeremy N. Rich, Sridharan Gururangan, Allan H. Friedman, Darell D. Bigner, John H. Sampson, Roger E. McLendon, James E. Herndon, Henry S. Friedman
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2008.18.8417
Read article for free, from open access legal sources, via Unpaywall:
https://ascopubs.org/doi/pdfdirect/10.1200/JCO.2008.18.8417
Subscription required at intl.jco.org
http://intl.jco.org/cgi/content/full/27/8/1262
Subscription required at intl.jco.org
http://intl.jco.org/cgi/reprint/27/8/1262.pdf
Free to read at intl.jco.org
http://intl.jco.org/cgi/content/abstract/27/8/1262
Citations & impact
Impact metrics
Article citations
Enhancing glioblastoma cytotoxicity through encapsulating O6-benzylguanine and temozolomide in PEGylated liposomal nanocarrier: an in vitro study.
3 Biotech, 14(11):275, 23 Oct 2024
Cited by: 0 articles | PMID: 39450422 | PMCID: PMC11499494
DNA damage response in brain tumors: A Society for Neuro-Oncology consensus review on mechanisms and translational efforts in neuro-oncology.
Neuro Oncol, 26(8):1367-1387, 01 Aug 2024
Cited by: 1 article | PMID: 38770568
Review
MEN1 Deficiency-Driven Activation of the β-Catenin-MGMT Axis Promotes Pancreatic Neuroendocrine Tumor Growth and Confers Temozolomide Resistance.
Adv Sci (Weinh), 11(35):e2308417, 23 Jul 2024
Cited by: 1 article | PMID: 39041891 | PMCID: PMC11425246
Enhancing Temozolomide (TMZ) chemosensitivity using CRISPR-dCas9-mediated downregulation of O<sup>6</sup>-methylguanine DNA methyltransferase (MGMT).
J Neurooncol, 169(1):129-135, 19 May 2024
Cited by: 1 article | PMID: 38762829
The Versatile Attributes of MGMT: Its Repair Mechanism, Crosstalk with Other DNA Repair Pathways, and Its Role in Cancer.
Cancers (Basel), 16(2):331, 11 Jan 2024
Cited by: 7 articles | PMID: 38254819 | PMCID: PMC10814553
Review Free full text in Europe PMC
Go to all (171) article citations
Other citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT00613093
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phase I trial of temozolomide plus O6-benzylguanine 5-day regimen with recurrent malignant glioma.
Neuro Oncol, 11(5):556-561, 16 Mar 2009
Cited by: 24 articles | PMID: 19289491 | PMCID: PMC2765345
Phase 1 trial of temozolomide plus irinotecan plus O6-benzylguanine in adults with recurrent malignant glioma.
Cancer, 115(13):2964-2970, 01 Jul 2009
Cited by: 20 articles | PMID: 19402172 | PMCID: PMC2748258
Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma.
J Clin Oncol, 23(28):7178-7187, 01 Oct 2005
Cited by: 134 articles | PMID: 16192602
Procarbazine, lomustine and vincristine for recurrent high-grade glioma.
Cochrane Database Syst Rev, 7:CD011773, 26 Jul 2017
Cited by: 19 articles | PMID: 28744879 | PMCID: PMC6483418
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: P50 CA108786
Grant ID: 5P50 CA 108786-4
Grant ID: R37 CA 011898-38
Grant ID: R21 CA101298
Grant ID: R37 CA011898
Grant ID: 1 R21 CA 101298-01
NCRR NIH HHS (2)
Grant ID: TL1 RR024126
Grant ID: RR024126
NINDS NIH HHS (2)
Grant ID: P50 NS020023
Grant ID: 5P50 NS20023-25