Abstract
Background & aims
Genetic variations that affect innate immunity increase risk of ileal Crohn's disease (CD). However, the penetrance of susceptibility genes, including NOD2, is low, suggesting additional risk factors. Neutralizing autoantibodies (Ab) against granulocyte-macrophage colony-stimulating factor (GM-CSF Ab) reduce neutrophil antimicrobial function in patients with primary alveolar proteinosis (PAP). We investigated whether GM-CSF Ab regulates neutrophil function in CD.Methods
Serum samples from 354 adult and pediatric patients with inflammatory bowel disease (IBD) were analyzed for GM-CSF Ab and IBD markers. Levels of GM-CSF Ab were compared with patients' CD features and neutrophil function. Intestinal barrier function and nonsteroidal anti-inflammatory drug (NSAID)-induced injury were assessed in GM-CSF-null and NOD2-null mice.Results
Median GM-CSF Ab levels increased from 0.4 microg/mL in control serum to 2.4 microg/mL in pediatric CD and 11.7 microg/mL in adult CD serum and were associated with ileal involvement (P<.001). Ileal location, duration of disease, and increased GM-CSF Ab levels were associated with stricturing/penetrating behavior (odds ratio, 2.2; P=.018). The positive and negative predictive values of GM-CSF Ab for stricturing/penetrating behavior were comparable with that of other IBD serum markers. CD patients with increased GM-CSF Ab had reduced neutrophil phagocytic capacity and increased accumulation of pSTAT3+ neutrophils in the affected ileum. GM-CSF-null mice and NOD2-null mice in which GM-CSF was neutralized had defects in mucosal barrier function and developed a transmural ileitis following NSAID exposure.Conclusions
GM-CSF regulates ileal homeostasis in CD and in mouse models. CD patients with increases in serum GM-CSF Ab might benefit from GM-CSF administration.Free full text

Granulocyte-Macrophage Colony Stimulating Factor Auto-Antibodies in Murine Ileitis and Progressive Ileal Crohn’s Disease
Associated Data
Abstract
Background:
Genetic variations that affect innate immunity increase risk of ileal Crohn’s Disease (CD). However, the penetrance of susceptibility genes, including NOD2, is low, suggesting additional risk factors. Neutralizing auto-antibodies against granulocyte-macrophage colony stimulating factor (GM-CSF Ab) reduce neutrophil antimicrobial function in patients with primary alveolar proteinosis (PAP). We investigated whether GM-CSF Ab regulates neutrophil function in CD.
Methods:
Serum samples from 354 adult and pediatric patients with inflammatory bowel disease (IBD) were analyzed for GM-CSF Abs and IBD markers. Levels of GM-CSF Ab were compared with patients’ CD features and neutrophil function. Intestinal barrier function and severity of non-steroidal anti-inflammatory drug (NSAID)-induced injury were assessed in GM-CSF-null and Nod2-null mice.
Results:
Median GM-CSF Ab levels increased from 0.4 mcg/mL in control serum to 2.4 mcg/mL in pediatric CD and 11.7 mcg/mL in adult CD serum and were associated with ileal involvement (p<0.001). Ileal location, duration of disease and increased GM-CSF Ab levels were associated with stricturing/penetrating behavior (Odds Ratio: 2.2, p=0.018). The positive and negative predictive value of GM-CSF Abs for stricturing/penetrating behavior was comparable to that of other IBD serum markers. CD patients with increased GM-CSF Abs had reduced neutrophil phagocytic capacity and increased accumulation of pSTAT3+ neutrophils in the affected ileum. GM-CSF-null mice and Nod2-null mice in which GM-CSF was neutralized had defects in mucosal barrier function and developed a transmural ileitis following NSAID exposure.
Conclusions:
GM-CSF regulates ileal homeostasis in CD and in mouse models. CD patients with increases in serum GM-CSF Ab might benefit from therapeutic GM-CSF administration.
INTRODUCTION
The frequency of chronic inflammatory disorders including rheumatoid arthritis (RA), multiple sclerosis (MS), and the Inflammatory Bowel Diseases (IBDs), Crohn’s Disease (CD) and Ulcerative Colitis (UC), has increased dramatically over the past five decades 1. For each of these, polygenic variation in immunity is believed to interact with a variety of environmental factors to trigger inappropriate inflammatory respones to both endogenous and exogenous antigens. Under normal conditions, Granulocyte/Macrophage Colony-Stimulating Factor (GM-CSF) mediates priming of myeloid cell antimicrobial functions, and homeostatic responses to tissue injury 2–4. However, as GM-CSF is up-regulated in RA, MS, and IBD, it has been proposed that it may play a pro-inflammatory role in these settings, and therapeutic strategies to inhibit GM-CSF are being explored. We have found that endogenous GM-CSF autoantibodies (GM-CSF Ab) inhibit GM-CSF bioactivity and reduce neutrophil anti-microbial function in the rare lung disease, autoimmune Pulmonary Alveloar Proteinosis (PAP) 5. Surprisingly, we found that low levels of GM-CSF Ab exist ubiquitously in healthy individuals and modulate GM-CSF function (Uchida et al, submitted manuscript). We therefore asked whether GM-CSF Ab would influence neutrophil function and intestinal homeostasis in a chronic inflammatory condition, CD.
Recent genome wide association studies have shown that inherited defects in microbial pattern recognition and autophagy contribute to the pathogenesis of CD 6, 7. Loss of function mutations in NOD2 increase risk for CD 8, 9, and impair the ability of NOD2 to sense the bacterial product muramyl dipeptide (MDP) 10. However, the penetrance of susceptibility genes including NOD2 is low, suggesting the existence of additional risk factors 11. In this regard, nonsteroidal anti-inflammatory drugs (NSAID), which reduce intestinal epithelial cell survival and proliferation and increase permeability, are recognized as a common environmental trigger for relapses of disease 12. CD patients also exhibit primary defects in intestinal permeability and neutrophil antimicrobial functions not accounted for by CARD15 mutations or environmental exposures 13, 14. Studies demonstrating that GM-CSF corrects CD neutrophil function in vitro, and that GM-CSF administration can reduce disease activity in CD and experimental colitis, have pointed to a potential role for reductions in GM-CSF bioactivity 15–17. We therefore employed complementary patient-based and animal studies to determine whether GM-CSF Ab would suppress neutrophil function and disrupt the intestinal barrier, thereby promoting more aggressive transmural CD.
METHODS
Human Subjects.
Human studies were approved by the Cincinnati Children’s Hospital Medical Center (CCHMC), Medical College of Wisconsin (MCW), and University of North Carolina (UNC) Institutional Review Boards. IBD patients were diagnosed using standard clinical, endoscopic, and radiographic criteria, and CD location and behavior were as per the Montreal criteria 18. Stricturing disease was defined as constant luminal narrowing demonstrated by radiologic, endoscopic, or surgical examination combined with pre-stenotic dilatation and/or obstructive signs or symptoms. Internal penetrating disease was defined as evidence of entero-enteric or entero-vesicular fistulae, intra-abdominal abscesses or intestinal perforation demonstrated by radiologic or surgical examination. We utilized the first appearance of endoscopic or radiologic evidence of stricturing and/or internal penetrating behavior as the endpoint for “stricture/penetrating disease-free behavior.” In the vast majority of cases, this was based upon radiologic evidence as summarized above, in a patient with clinical signs and symptoms of stricturing and/or internal penetrating disease. A minority of patients also had endoscopy performed which confirmed stricturing disease. In most, a surgical procedure which confirmed the radiologic data followed shortly after, although this was not required to re-classify the disease behavior.
Mouse model of ileitis.
Animal studies were approved by the CCHMC Institutional Animal Care and Use Committee. Card15 deficient (C15KO) mice were from Jackson Labs. Gm-csf deficient (GMKO) mice have been described 19. Mice were maintained in conventional housing. At four weeks of age mice received anti-GM-CSF antibody, 50 mcg, IP (Clone number 22E9, Endogen, Rockford IL). Controls received an isotype control antibody (Clone R35–95, BD Pharmigen). Two weeks later, WT, C15KO mice, and GMKO mice received a NSAID, piroxicam, at 200 ppm in the chow. Mice were sacrificed after one (WT and C15KO following GM-CSFAb administration) or two (WT and GMKO) weeks. Histopathology was graded in a blinded fashion using an established system for NSAID induced intestinal injury 20.
GM-CSF Auto-Antibody ELISA.
Anti-GM-CSF antibodies were quantified in human serum by enzyme-linked immunosorbent assay (ELISA) 5, 19. The sensitivity of this assay is 0.08 mcg/mL, with an inter-assay coefficient of variation of 15.6%. GM-CSF Ab were quantified in mouse serum by enzyme-linked immunosorbent assay (ELISA). Recombinant murine GM-CSF (R&D Systems) was used for the capture protein at a concentration of 0.1μg/ml in phosphate buffered saline.
IBD Serologies.
Serum concentration of ASCA, ANCA, CBir1, OmpC, and I2 were determined at the Cedar-Sinai site 21.
NOD2, ATG16L1, and IRGM Genotyping.
DNA was isolated from whole blood using the Puregene® kit (Gentra Systems, Minneapolis, MN). Genotyping for NOD2R702W (SNP8), G908R (SNP12), and 1007fs (SNP13), ATG16L1, and IRGM was performed in the CCHMC and MCW Genetics Core Laboratories 6, 7, 22.
Neutrophil CD11b Stimulation Index Assay.
Whole blood was incubated with rhGM-CSF at 37°C for 30 minutes followed by incubation with FITC-anti-human CD16, APC-anti-human CD14 and PE-anti-human CD11b antibodies (all from BD Biosciences). CD11b expression on neutrophils was evaluated using flow cytometry (FACS Calibur®). Neutrophils were gated according to their expression of CD16 and scatter properties.
Neutrophil Phagocytosis Assay.
Phagocytic capacity of neutrophils was evaluated as described 19. Neutrophil phagocytic capacity = (Geometric mean fluorescent intensity of neutrophil population)X(Percentage of beads positive neutrophils) X (absolute neutrophil count in the whole blood)/107.
Neutrophil Oxidative Burst Assay.
The production of hydrogen peroxide was measured in neutrophils in whole blood as described 23.
Neutrophil pSTAT3 Response to GM-CSF.
Peripheral blood leukocytes (PBL) were stimulated with PBS or GM-CSF, 10 ng/mL, and the frequency of neutrophils containing tyrosine phosphorylated STAT3 was determined 24.
Measurement of permeability and bacterial translocation in the animal model.
Ileal and colonic transcellular permeability to the fluorescent tracer fluorescein isothiocyanate-dextran with a molecular mass of 4,000 Da (FD-4) was determined using an everted gut sac method 25. Bacterial translocation to MLN was determined using standard methods with MLN homogenates plated onto brain-heart infusion and MacConkey agar (Becton Dickinson, Franklin Lakes, NJ).
Immunofluorescence (IF) for lamina propria cell populations.
Frozen tissue sections from mouse and human were prefixed in cold acetone and then air dried. The sections were fixed with 4% paraformaldehyde and then washed three times with cold PBS. Tissue sections were incubated with primary antibodies as follows: F4/80 (eBiosciences). CD3 (Santa Cruz), CD11c (BD Pharmigen), Neutrophil Elastase (NE, Santa Cruz), and pSTAT3 (Cell Signaling Technology, Danvers, MA) and images were captured using a fluorescence microscope (Ziess, Germany). The frequency of CD3+ cells and the total area for F4/80+ cells per hpf was determined using the NIH Image/J image processing and analysis program.
Data analysis.
Statistical analyses were performed using SAS® Version 9.1 (SAS Institute, Inc.) and GraphPad PRISM© Version 4.01. Univariate analyses were conducted to investigate any potential outliers as well as the normality of the data. Bivariate analyses were conducted to investigate for differential distribution of demographic and other variables between the CD patient and control groups. Continuous variables were analyzed using two sample t test and one-way ANOVA with Newman-Keuls test for multiple comparisons, or their non-parametric alternatives, the Mann-Whitney test or Kruskal-Wallis with Dunns test for multiple comparisons, when the normality of the data was suspected. Discrete variables were analyzed using Fisher’s exact test or Cochran-Mantel-Haenszel (CMH) test. Odds ratio estimates were computed with the 95% confidence intervals. Multivariate logistic regression analyses were conducted to observe conditional relationships between serum GM-CSF Autoantibody concentration and the outcomes of disease location, behavior, and surgery, while controlling for age, gender, duration of disease, and CARD15 SNP carriage.
RESULTS
Patient characteristics.
The clinical and demographic characteristics of the IBD patients are summarized in Supplemental Table I. These included 272 pediatric onset IBD patients, 82 adult onset IBD patients, 20 healthy pediatric controls, and 88 healthy adult controls. Neutrophil function was examined in a subset of pediatric onset CD patients whose clinical and demographic characteristics were similar to the overall pediatric group.
GM-CSF Ab and CD Location and Behavior.
We found that pediatric (A1) and adult (A2) onset CD patients with ileal or ileo-colonic involvement (L1 or L3) have increased circulating GM-CSF Ab which bind glycosylated GM-CSF, compared to disease controls with colon-only CD (L2) or UC, and healthy age-matched controls (Fig. 1A). Similar results were obtained with the assay detecting GM-CSF Ab binding to non-glycosylated GM-CSF (Suppl. Fig. 1). The median(IQ range) serum GM-CSF Ab concentration was equal to 0.4(0.2,0.6) mcg/ml in healthy pediatric controls, compared to 2.4(0.6,9) mcg/mL in ileal/ileo-colonic pediatric CD (p<0.001). Similarly, the median(IQ range) serum GM-CSF Ab concentration was equal to 0.4(0.2,1.2) mcg/mL in healthy adult controls, compared to 11.7(2,18) mcg/mL in ileal/ileo-colonic adult CD (p<0.001).
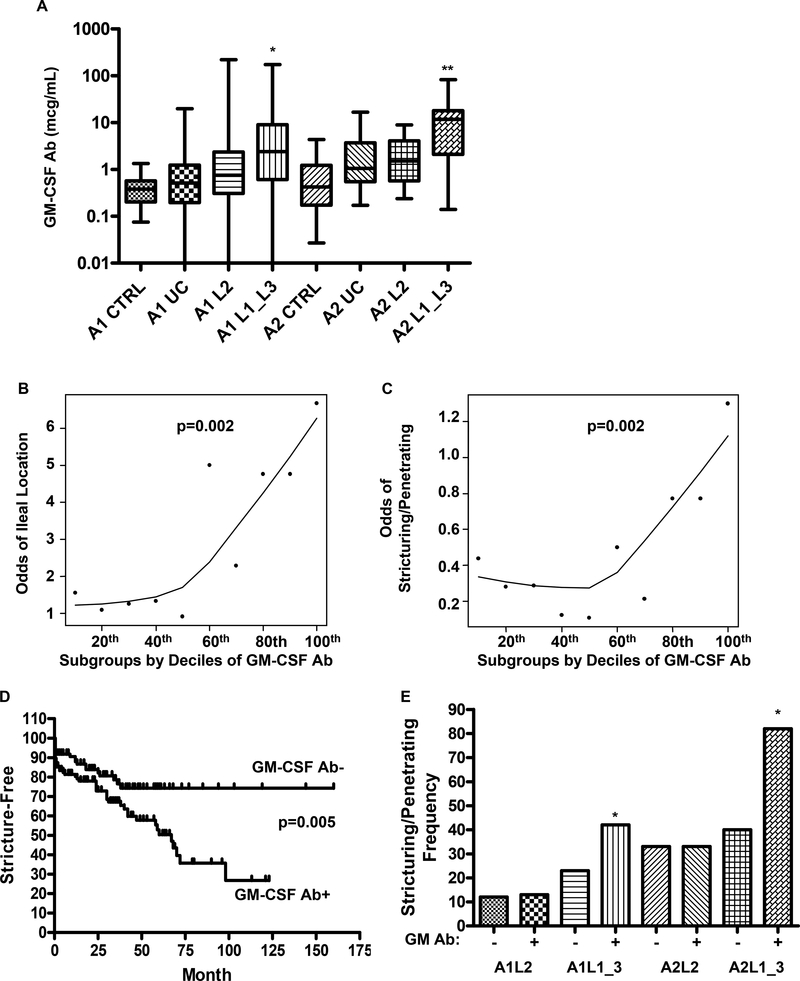
A) Serum GM-CSF autoantibody (GM-CSF Ab) concentration was determined by ELISA recognizing glycosylated GM-CSF in the pediatric (A1) and adult (A2) onset CD patients with colon-only (L2) or ileal (L1)/ileo-colonic (L3) location, UC patients and healthy controls (CTRL) summarized in Table I (n = 354 IBD and 108 controls). *p<0.001 vs. A1 CTRL, A1UC, and A1L2; **p<0.001 vs. A2 CTRL, A2UC. B & C) The relationship between the odds for B) ileal location and C) stricturing/penetrating behavior, and serum GM-CSF Ab concentration in CD is shown. Serum GM-CSF Ab was divided into subgroups by decile, and a smooth line was generated by robust locally weighted regression (LOWESS). D) Kaplan-Meier survival curve analysis was performed to determine the frequency of CD patients free from stricturing/penetrating disease behavior as a function of serum GM-CSF Ab level and duration of disease. E) The frequency of stricturing/penetrating disease behavior was determined in the groups shown. *p<0.01 vs. age & location matched GM-CSFAb-. In D & E: GM-CSF Ab+: serum concentration ≥ 1.6 mcg/ml. Data are shown as the median(range) or frequency.
Serum GM-CSF Ab did not vary by NOD2 or ATG16L1 susceptibility single nucleotide polymorphism (SNP) carriage (Suppl. Fig. 2A). The median(IQ range) serum GM-CSF Ab concentration was increased in CD patients carrying the IRGM susceptibility SNP, from 1.3(0.5,5) mcg/mL to 6.6(0.5,9.8) mcg/mL (p=0.04). However, only 22% of the overall cohort carried this SNP (Suppl. Fig. 2A). The median(IQ range) serum GM-CSF Ab concentration was higher in adult-onset CD patients compared to pediatric-onset CD patients. However, this did not vary with duration of disease (Suppl. Figs. 2B and 2C). The median (IQ range) serum GM-CSF Ab concentration was lower in patients who had received mesalamine (1(0.4,4.8) mcg/mL vs. 2(0.6,9) mcg/mL, p=0.03), and higher in patients who had received 6-mercaptopurine (1.8(0.5,7) mcg/mL vs. 0.9(0.2,2.6) mcg/mL, p=0.05). It did not vary based upon corticosteroid or infliximab exposure. The frequency of corticosteroid, 6-mercaptopurine, or infliximab exposure did not differ between patients with colon-only location (L2) and ileal (L1) or ileocolonic (L3) location. A higher frequency of patients with colon-only location were exposed to mesalamine. After controlling for concurrent immune suppressant use, serum GM-CSF Ab did not differ between subjects with inactive versus active disease.
The odds for ileal disease location (p=0.002 by Cochran-Armitage test for trend), and more aggressive stricturing/penetrating disease behavior (p=0.002) requiring surgery (p=0.006) each increased as the serum GM-CSF Ab concentration increased (Figs. 1B & 1C and data not shown). The risk increased appreciably in patients with serum GM-CSF Ab concentrations ≥ the median value in pediatric CD, 1.6 mcg/ml. We therefore defined elevated GM-CSF Ab as a serum concentration ≥ 1.6 mcg/ml. The median (IQ range) time for the development of stricturing/penetrating behavior was equal to 21(3,63) months at the CCHMC site, and 24(11,37) months at the MCW site. These data were not available for the UNC site. Kaplan-Meier survival curve analysis showed that increased serum GM-CSF Ab was associated with a more rapid rate of progression to stricturing/penetrating disease behavior (p=0.005, Fig. 1D).
After controlling for age and ileal disease location, elevated GM-CSF Ab was associated with a two-fold higher rate of stricturing/penetrating disease behavior (see Fig. 1E). This was limited to patients with ileal or ileo-colonic involvement. While 33% of the colon-only subjects (28/85) had elevated GM-CSF Ab, the frequency of stricturing/penetrating behavior was not increased in this group, after controlling for location and age of onset (see Fig. 1E).
Multivariate logistic regression analyses were conducted to observe conditional relationships between serum GM-CSF Ab concentration and disease location, behavior, and surgery, while controlling for age, gender, disease location, duration of disease, and CARD15 SNP carriage, within the pediatric onset cohort. Interactions found significant at the level of 0.1 in the univariate analysis were included. Data confirmed the previously reported associations between: 1) CARD15 SNP carriage, ileal location, and early surgery (disease duration < 36 months), 2) longer disease duration (> 36 months), stricturing behavior, and surgery, 3) ileal location, stricturing behavior, and surgery, and 4) female gender and surgery 22, 26. After controlling for these clinical, demographic, and genetic factors, elevated GM-CSF Ab remained highly associated with both ileal location, and stricturing/penetrating behavior and surgery, after accounting for ileal location (See Table I).
Table I.
Odds Ratio Estimates from Logistic Regression Analysis.
| Odds Ratio | p-value | |||
|---|---|---|---|---|
| Ileal Location | ||||
| Boy vs. Girl | 1.04 (0.56,1.92) | 0.9126 | ||
| Young vs. Old* | 0.75 (0.40,1.41) | 0.3748 | ||
| Duration>36 vs. ≤36 | 1.10 (0.60,2.02) | 0.7658 | ||
| CARD15 + vs. − | 2.84 (1.42,5.66) | 0.0031 | ||
| GM-CSF Ab high vs. low | 3.34 (1.80,6.20) | 0.0001 | ||
| Stricturing/Penetrating Behavior | ||||
| Boy vs. Girl | 0.70 (0.37,1.30) | 0.2543 | ||
| Young vs. Old* | 0.76 (0.39,1.48) | 0.4949 | ||
| Duration>36 vs. ≤36 | with | CARD15 + | 0.54 (0.19,1.55) | 0.2492 |
| Duration>36 vs. ≤36 | with | CARD15 − | 2.64 (1.23,5.67) | 0.0143 |
| CARD15 + vs. − | with | Duration > 36 | 0.50 (0.18,1.36) | 0.0914 |
| CARD15 + vs. − | with | Duration ≤ 36 | 2.45 (1.08,5.56) | 0.0952 |
| L1L3 vs. L2 | 2.81 (1.27,6.21) | 0.0106 | ||
| GM-CSF Ab high vs. low | 2.18 (1.14,4.18) | 0.0180 | ||
| Surgery | ||||
| Boy vs. Girl | 0.45 (0.23,0.88) | 0.0187 | ||
| Young vs. Old* | 1.01 (0.50,2.04) | 0.8772 | ||
| Duration>36 vs. ≤36 | with | CARD15 + | 0.51 (0.17,1.56) | 0.2314 |
| Duration>36 vs. ≤36 | with | CARD15 − | 3.20 (1.40,7.32) | 0.0074 |
| CARD15 + vs. − | with | Duration > 36 | 0.47 (0.16,1.34) | 0.0859 |
| CARD15 + vs. − | with | Duration ≤ 36 | 2.93 (1.19,7.20) | 0.0555 |
| L1L3 vs. L2 | 2.76 (1.15,6.60) | 0.0225 | ||
| GM-CSF Ab high vs. low | 2.14 (1.06,4.31) | 0.0340 | ||
GM-CSF Ab and IBD Serologies.
The frequency of ASCA sero-positivity was increased approximately five-fold in CD patients with elevated serum GM-CSF Ab, independent of disease location (Figs. 2A & 2B). The frequency of ANCA sero-positivity was reduced approximately four-fold in CD patients with colon-only location and elevated serum GM-CSF Ab. Because patients were followed for varying periods of time, we used progression-free probabilities to calculate PPV and NPV for stricturing/penetrating behavior. This relationship is shown in Figure 2C. Each line going from left to right depicts the PPV and NPV for each biomarker as a function of time, with the earliest time point (stricturing by 12 months after diagnosis) represented by the start of the line at the left lower end, the first closed circle on the line representing 60 months after diagnosis, and the longest duration of disease (108 months) at the upper right end. As shown in Table II, the PPV for the GM-CSF Ab assay increased from 21 percent at 12 months after diagnosis, to 71 percent at 108 months, while the NPV decreased from 89 percent to 73 percent. Similar results (PPV:67%, NPV:86%) were obtained for the adult onset CD patients, with a median duration of disease of 144 months. Therefore, throughout the duration of follow-up (108 months), the PPV and NPV for the GM-CSF Ab was comparable to current serological markers.
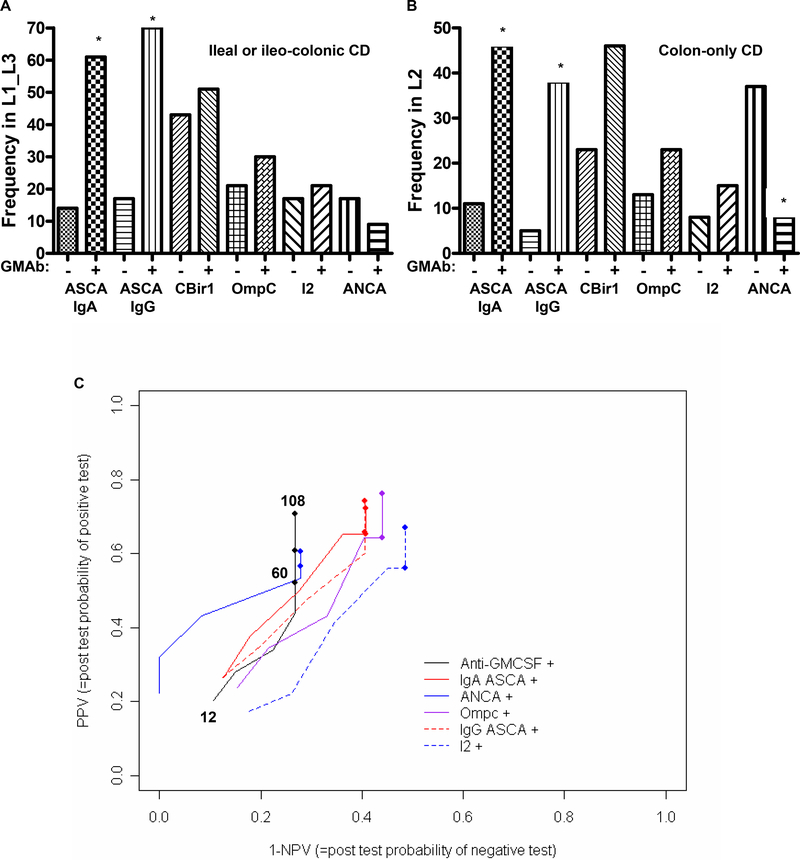
A & B) The frequency of sero-positivity for the IBD serological markers ASCA, CBir1, OmpC, I2, and ANCA was determined and is shown as a function of disease location and GM-CSF Ab level for a subset of the pediatric onset group (n=157) for which these values were obtained. *p<0.01 vs. location matched GM-CSFAb-. C) The positive and negative predictive value (PPV & NPV) of the GM-CSF Ab assay and the ASCA, ANCA, OmpC, and I2 markers for predicting progression to structuring/penetrating behavior was determined in the pediatric onset cohort. Because patients were followed for varying periods of time, we used progression-free probabilities to calculate PPV and NPV. This relationship was as shown. Each line going from left to right depicts the PPV and NPV for each biomarker as a function of time, with the earliest time point (stricturing by 12 months after diagnosis) represented by the start of the line at the left lower end, the first closed circle on the line representing 60 months after diagnosis, and the longest duration of disease (108 months) at the upper right end. GM-CSF Ab+: serum concentration ≥ 1.6 mcg/ml. Data are shown as the relative frequency.
Table II.
Positive and Negative Predictive Value of Serological Markers for Stricturing/Penetrating Behavior.
| Marker | 12* PPV | 12 NPV | 60 PPV | 60 NPV | 108 PPV | 108 NPV |
|---|---|---|---|---|---|---|
| GM-CSF Ab | 21 | 89 | 52 | 73 | 71 | 73 |
| ASCA IgA | 27 | 87 | 65 | 59 | 72 | 59 |
| ASCA IgG | 26 | 88 | 66 | 59 | 74 | 59 |
| ANCA | 22 | 100 | 57 | 72 | 61 | 72 |
| OmpC | 24 | 85 | 64 | 56 | 76 | 56 |
| I2 | 17 | 82 | 56 | 51 | 67 | 51 |
GM-CSF Ab and Neutrophil Function.
We have utilized GM-CSF dependent up regulation of cell surface CD11b on neutrophils as a sensitive assay for the neutralizing effect of GM-CSF Ab in whole blood 19. GM-CSF dependent CD11b activation on neutrophils was substantially reduced in CD patients with elevated serum GM-CSF Ab, compared to CD patients with low serum GM-CSF Ab, and healthy controls (Fig. 3A). Neutrophil phagocytic capacity was also reduced in CD patients with elevated serum GM-CSF Ab, to a level comparable to that observed in PAP (Fig. 3B). Moreover, neutrophil phagocytic capacity was inversely related to serum GM-CSF Ab concentration, in both CD patients and healthy controls (Fig. 3C). By comparision, mean(SEM) neutrophil oxidative burst did not differ between healthy controls (95±5) and CD patients with high (99±15) or low (125±21) serum GM-CSF Ab. At the time of myeloid cell evaluation, 14% of subjects were receiving corticosteroids, 76% 6-MP or methotrexate, and 44% infliximab. Neutrophil function did not vary based upon these concomitant medications, or by disease location or activity.
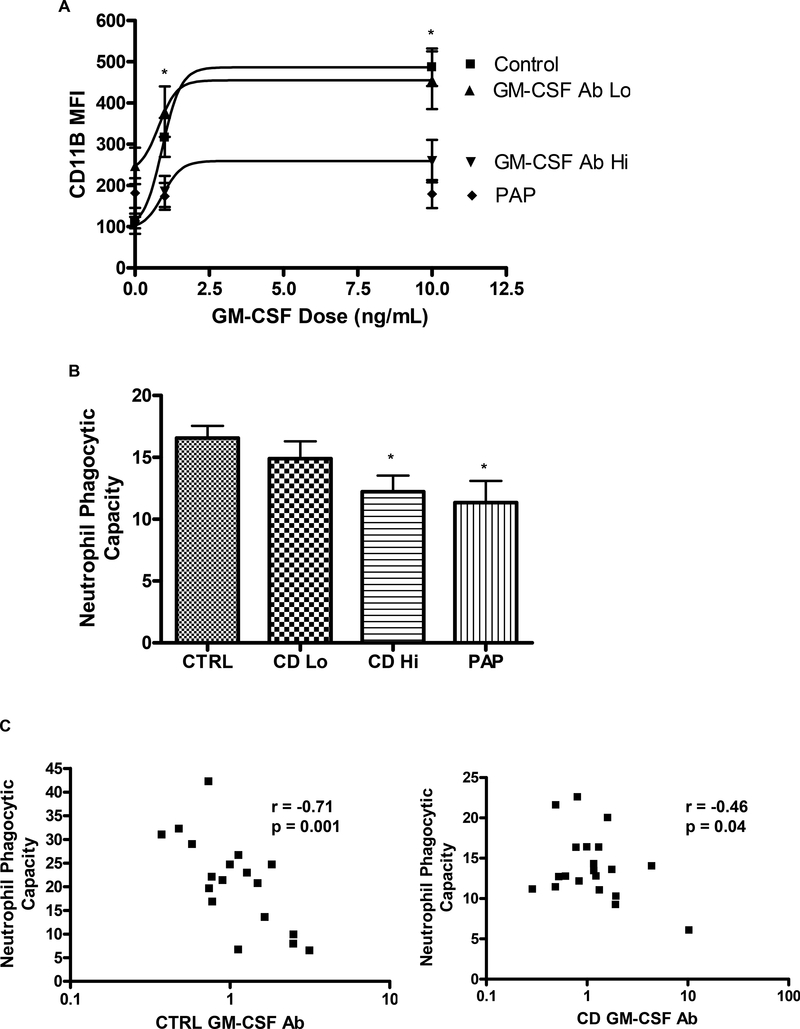
A) Neutrophils were stimulated with GM-CSF (0, 1, or 10 ng/ml) in whole blood samples obtained from healthy controls, CD patients with low (GM-CSF Ab Lo) or high (GM-CSF Ab Hi) serum GM-CSF Ab, and disease controls with Primary Alveolar Proteinosis (PAP), and the mean fluorescent intensity (MFI) for cell surface CD11b was determined by flow cytometry (n = 6 per group). Data are shown as the mean (SEM), *:p<0.05 versus GM-CSF Ab Hi group at same GM-CSF dose. B) Neutrophil phagocytic capacity was determined in whole blood samples obtained from healthy controls (CTRL), CD with low (CD Lo) or high (CD Hi) serum GM-CSF Ab, and disease controls with PAP by flow cytometry (n = 6–14 per group). *:p<0.05 vs CTRL. C) The Pearson correlation between serum GM-CSF Ab and neutrophil phagocytic capacity was determined in healthy controls (CTRL) and CD patients.
Neutrophil STAT3 activation.
Dysregulated STAT3 activation may exert a pro-inflammatory effect by promoting leukocyte survival in CD. As shown in Figs. 4A & 4B, the frequency of pSTAT3+ circulating neutrophils was increased in CD patients with clinically inactive disease and elevated serum GM-CSF Ab, compared to those with clinically inactive disease and low serum GM-CSF Ab. A similar trend was observed for CD patients with clinically active disease. Consistent with this, GM-CSF stimulation reduced STAT3 activation in isolated CD neutrophils (see Fig. 4C). As shown in Fig. 4D, the overall frequency of neutrophils, as measured by neutrophil elastase staining, and the frequency of neutrophils containing tyrosine phosphorylated STAT3 (pSTAT3), was increased in ileal sections from CD patients with elevated GM-CSF Ab.
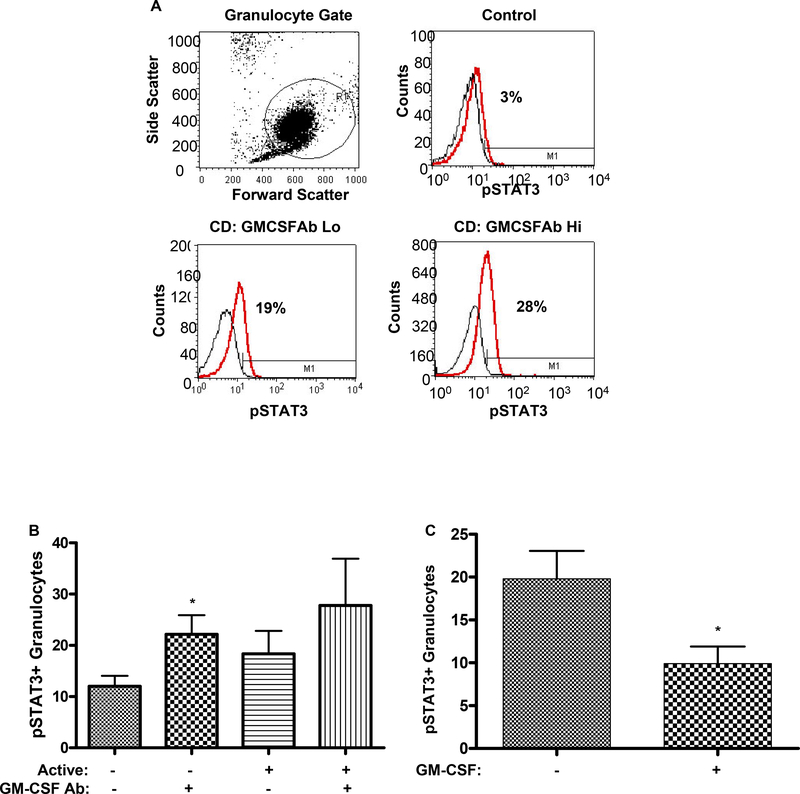
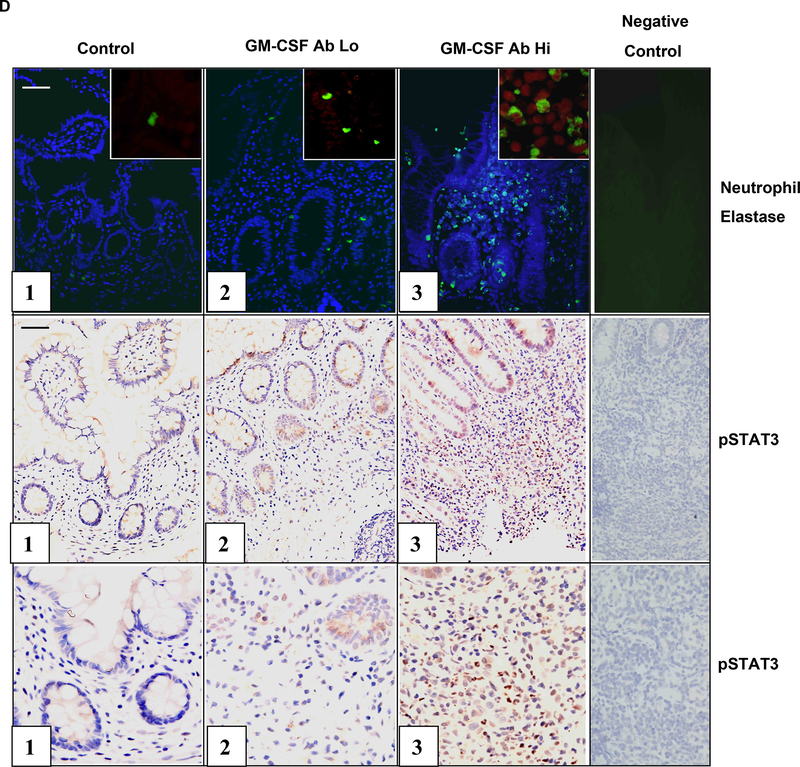
A) The frequency of circulating neutrophils in healthy controls and CD patients with low (GM-CSFAb Lo) or high (GMCSFAb Hi) serum GM-CSF Ab containing tyrosine phosphorylated STAT3 (pSTAT3) was determined by intra-cellular staining for pSTAT3 and flow cytometry as shown. B) The frequency of circulating neutrophils containing pSTAT3 was determined in CD patients in remission (active-), or with clinically active disease (active+), and low or high serum GM-CSF Ab (n=5–7 per group). Data are shown as the mean (SEM), *p<0.05 vs. GM-CSF Ab Lo with inactive disease. C) Circulating neutrophils obtained from CD patients were stimulated with GM-CSF (10 ng/mL) or PBS for 30 minutes. The frequency of neutrophils containing pSTAT3 was determined by flow cytometry (n=20). *p<0.05 vs. untreated sample. D) The frequency of neutrophil elastase (NE) staining or pSTAT3+ cells was determined by immunofluoresence (IF) or immunohistochemistry in ileal sections obtained from healthy controls, or CD patients with low (GM-CSF Ab Lo) or high (GM-CSF Ab Hi) serum GM-CSF Ab. For the IF images, the NE stain (green) is shown with co-labeling for cell nuclei with DAPI (blue), or in the inset, pSTAT3 (red). Images representative of 5–7 per group are shown.
GM-CSF Ab and murine neutrophil and barrier function.
In order to directly test the effect of GM-CSF Ab upon susceptibility to ileal injury, we administered a neutralizing GM-CSF Ab or isotype control to WT and card15 deficient (C15KO) mice and two weeks later examined barrier function and the response to NSAID (piroxicam) exposure. GM-CSF Ab administration yielded a circulating median(IQ range) level of 19(15,42) mcg/mL, compared to 3.5(1.1,11) mcg/mL in isotype-treated controls (Fig. 5A, p=0.007). GM-CSF activation of cd11b on neutrophils was almost completely inhibited following GM-CSF Ab administration (Fig. 5B). Ileal para-cellular permeability was increased in gm-csf deficient mice (GMKO), card15 deficient mice (C15KO), and GM-CSF Ab pre-treated WT and C15KO, relative to WT controls (Fig. 5C). This was specific to this segment of the intestine, as colonic para-cellular permeability did not differ between the groups (data not shown). Bacterial translocation to mesenteric lymph nodes (MLN) was increased in GMKO mice, and in C15KO and GM-CSF Ab treated WT, compared to WT controls (Fig. 5D). Importantly, GM-CSF Ab treatment increased bacterial translocation in C15KO, compared to C15KO alone. These results suggested that loss of GM-CSF and nod2 function disrupts ileal barrier function with regard to bacterial translocation in an additive manner.
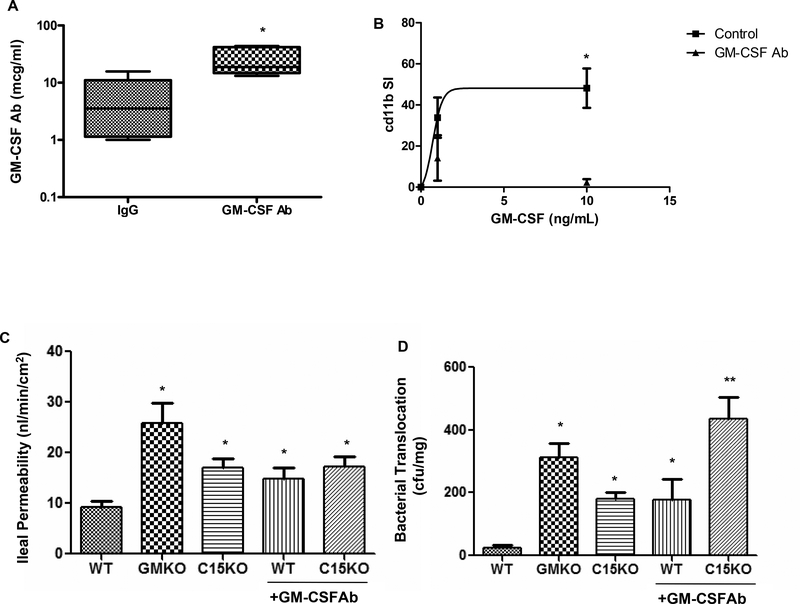
A) GM-CSF antibody (50 mcg IP) or isotype control were administered to wild type mice and serum was obtained two weeks later. The circulating level of GM-CSF Ab (mcg/mL) was determined by ELISA (n = 6 per group). *p<0.05 vs. IgG treated control. B) GM-CSF antibody (GM-CSF Ab, 50 mcg IP) or isotype control (IgG) were administered to wild type mice. Two weeks later, circulating leukocytes were obtained and stimulated with GM-CSF (0, 1, or 10 ng/mL) in whole blood samples and cell surface cd11b abundance was determined by flow cytometry. The cd11b stimulation index was calculated and is shown (n = 6 per group). *p<0.05 vs. GM-CSF Ab treated group at GM-CSF dose of 10 ng/mL. C) Ileal para-cellular permeability to FITC-dextran were determined using the everted gut sac method in mice fed regular chow, with and without GM-CSF Ab pre-treatment for two weeks as shown (n = 10–12 per group). *p<0.05 versus WT on regular chow. D) Bacterial translocation to draining mesenteric lymph nodes was determined in mice fed regular chow, with and without GM-CSF Ab pre-treatment for two weeks as shown (n = 10–12 per group). *p<0.05 versus WT on regular chow, **p<0.05 vs C15KO on regular chow. WT: wild type, GMKO: gm-csf deficient, C15KO: card15 deficient, GM-CSF Ab: received neutralizing GM-CSF antibody, 50 mcg IP. Data are shown as the A) median (range) or B-D) mean (SEM).
GM-CSF bioactivity and ileal susceptibility to NSAID injury.
There were no differences in intestinal epithelial architecture or lamina propria cellularity between GMKO, C15KO, and WT mice, with or without GM-CSF Ab administration, under basal conditions (Fig. 6 and data not shown). Following NSAID (piroxicam) exposure, WT and C15KO mice exhibited a modest degree of ileal inflammation (Fig. 6A). GMKO mice exhibited a greater degree of transmural ileal inflammation and more extensive ulceration. GM-CSF Ab pre-treatment increased ileal injury with piroxicam in C15KO, but not WT, mice. By comparison, a modest degree of colonic inflammation, which did not differ between the groups, was observed following NSAID exposure (Fig. 6B). The area per hpf for f4/80+ macrophages was increased in both WT and C15KO mice following GM-CSF Ab administration and piroxicam exposure (Suppl. Fig. 3A). The frequency of basal CD3+ lymphocytes increased in C15KO mice under these conditions (63±25 vs. 134±41, p=0.06), while the overall frequency of CD3+ lymphocytes decreased in WT mice (412±65 vs. 255±65, p=0.04, Suppl. Fig. 3B and data not shown). The frequency of neutrophil elastase (NE) staining and cd11c+ dendritic cells was not significantly different between the groups. The increased frequency of ileal laminia propria macrophages and T cells in C15KO mice was similar to that observed in newly diagnosed pediatric CD (Suppl. Fig. 3). Collectively, these data suggest that loss of GM-CSF bioactivity specifically increases susceptibility to ileal injury.
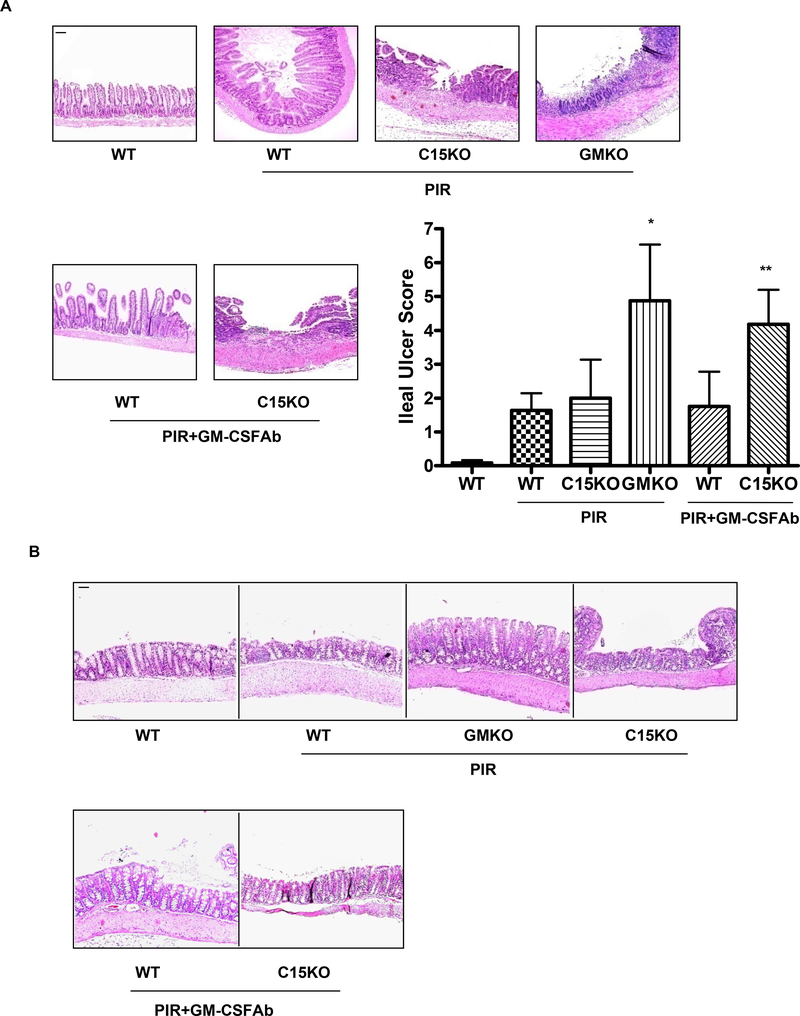
A & B) Gm-csf deficient (GMKO), card15 deficient (C15KO) or wild type (WT) control mice were treated with a neutralizing GM-CSF antibody (GM-CSF Ab, 50 mcg IP) or isotype control and were placed on regular chow, or chow containing the NSAID piroxicam (PIR, 200 ppm) two weeks later. The effect upon A) ileal and B) colonic histopathology was determined (n = 10–12 per group). Data are shown as the mean (SEM), *p<0.05 versus WT on piroxicam, **p<0.05 vs C15KO on piroxicam. Original magnification, × 100; bar = 50 μm.
DISCUSSION
Recent studies have suggested that defects in innate immune functions including pattern recognition (NOD2) and autophagy (ATG16L1 and IRGM) increase risk for the development of CD involving the ileum 6–9. However, the population-attributable risk is low, indicating that additional risk factors must exist 11. Watanabe et al have recently reported the detection of multiple anti-cytokine auto-antibodies in healthy individuals 34. We have identified GM-CSF Ab as a novel risk factor which reduce neutrophil antimicrobial functions and increase the likelihood of aggressive ileal CD requiring surgery. Serum GM-CSF Ab level was not influenced by CARD15 SNP carriage, and was independently associated with ileal location and stricturing/penetrating behavior. Our ongoing studies will determine whether serum GM-CSF Ab level is a heritable trait, influenced in part by CD susceptibility genes including IRGM, and whether other anti-cytokine auto-antibodies are increased under these conditions.
Several groups have performed studies to determine whether IBD serologies can predict disease progression and the need for surgery 27. Consistently, high titer ASCA+ CD patients have been more likely to have fibrostenosing small bowel disease and require ileocecal resection 21, 28. We found that elevated serum GM-CSF Ab yielded a PPV and NPV with regard to stricturing/penetrating CD which was comparable to these serological markers. It should be noted that a potential weakness of this approach is the difficulty in defining precisely when a patient progresses to stricturing/penetrating behavior. Future studies will prospectively test this relationship, and determine whether these may be associated with shared genetic influences.
A variety of defects in neutrophil function have been described in CD, without a clear etiology 29. This has been shown to paradoxically reduce neutrophil accumulation at sites of acute ileal or rectal injury 14, 30. The relative defect in acute neutrophil chemotaxis in the CD patients was largely overcome by G-CSF administration, and in a small study, was not associated with NOD2 genotype 14. More recently, CD neutrophils have also been shown to exhibit dysregulated apoptosis; this was related to alterations in cellular BAX/BCL-2 31. Defective neutrophil function may in turn promote accumulation of bacterial products and stimulation of the mucosal adaptive immune system. GM-CSF is required for neutrophil priming, a process which enhances acute responses to bacteria including chemokine production, chemotaxis, adhesion, and phagocytosis 32. In vitro stimulation with GM-CSF restores the function of CD neutrophils, and GM-CSF administration reduces mucosal injury in murine colitis due to TNBS or DSS administration 16, 17. Our study has now identified a novel cause for neutrophil dysfunction in CD, elevated GM-CSF Ab.
Our results extend these recent studies regarding neutrophil function to show that GM-CSF is also required for intestinal barrier function and homeostatic responses to gut injury. Data in our novel animal model of ileal CD showed that, on a background of nod2 deficiency, GM-CSF Ab administration led to a transmural ileitis triggered by NSAIDs resembling CD in humans. These features of the animal model are quite consistent with the increased intestinal permeability, increased bacterial adherence and penetration, and defective counter-regulatory responses which characterize CD 33. Ongoing studies will aim to define the basis for the pathogenic features associated with GM-CSF Ab which do (increased bacterial translocation and NSAID-induced injury) and do not (reduced neutrophil antimicrobial function and increased ileal permeability) appear to interact with nod2 deficiency in the host. Taken together, our results suggest that therapeutic GM-CSF administration may be of particular benefit in CD patients with defective innate immunity due to elevated GM-CSF Ab.
ACKNOWLEDGEMENTS
This work was supported by the Crohn’s and Colitis Foundation of America (LD & XH), the Broad Medical Research Program (LD & SP), the Cincinnati Children’s Hospital Research Foundation Translational Research Institute (LD), the National Institutes of Health (NIH)-supported Cincinnati Children’s Hospital Research Foundation Digestive Health Center (1P30DK078392-01, BT), and NIH grant R01 DK058259 (LD). Tissue sections were prepared in the Integrative Morphology Core of the Digestive Health Center. We thank Dr. Claudio Fiocchi (Cleveland Clinic Foundation) for critical reading of the manuscript. Ramona Bezold and Kathleen Lake coordinated the patient-based studies, while Nicholas Peterson and Anne Ryan provided outstanding technical assistance.
Grant support: This work was supported by the Crohn’s and Colitis Foundation of America, the Broad Medical Research Program, the Bioinformatics, Flow Cytometry, and Microarray cores of the National Institutes of Health (NIH)-supported Cincinnati Children’s Hospital Research Foundation Digestive Health Center (1P30DK078392-01), and NIH grants R01 DK078683 and DK068164. Ramona Bezold and Kathleen Lake provided outstanding support with subject recruitment.
Abbreviations:
| CD | Crohn’s Disease |
| GM-CSF Ab | Granulocyte-Macrophage Colony Stimulating Factor auto-antibodies |
| IBD | Inflammatory Bowel Disease |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MDP | muramyl dipeptide |
| MS | multiple sclerosis |
| NPV | negative predictive value |
| NSAID | non-steroidal anti-inflammatory drug |
| PAP | Primary Alveolar Proteinosis |
| PBL | peripheral blood leukocytes |
| PGN | peptidoglycan |
| PPV | positive predictive value |
| RA | rheumatoid arthritis |
| SNP | single nucleotide polymorphism |
| STAT | signals transducers and activators of transcription |
| UC | Ulcerative Colitis |
Footnotes
Financial disclosures: Marla Dubinsky, MD is a consultant for Prometheus Labs. Otherwise, the authors have no financial arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product. Writing assistance: not applicable
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1053/j.gastro.2008.12.046
Read article for free, from open access legal sources, via Unpaywall:
http://www.gastrojournal.org/article/S0016508508023044/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1053/j.gastro.2008.12.046
Article citations
Pathogenesis-driven treatment of primary pulmonary alveolar proteinosis.
Eur Respir Rev, 33(173):240064, 01 Jul 2024
Cited by: 0 articles | PMID: 39142709 | PMCID: PMC11322829
Review Free full text in Europe PMC
Intestinal stroma guides monocyte differentiation to macrophages through GM-CSF.
Nat Commun, 15(1):1752, 26 Feb 2024
Cited by: 0 articles | PMID: 38409190 | PMCID: PMC10897309
Leaky gut, circulating immune complexes, arthralgia, and arthritis in IBD: coincidence or inevitability?
Front Immunol, 15:1347901, 20 Mar 2024
Cited by: 0 articles | PMID: 38571963 | PMCID: PMC10987687
Current Approach to Risk Factors and Biomarkers of Intestinal Fibrosis in Inflammatory Bowel Disease.
Medicina (Kaunas), 60(2):305, 10 Feb 2024
Cited by: 1 article | PMID: 38399592 | PMCID: PMC10889938
Review Free full text in Europe PMC
Immunophenotype associated with high sustained antibody titers against enzyme replacement therapy in infantile-onset Pompe disease.
Front Immunol, 14:1301912, 04 Jan 2024
Cited by: 3 articles | PMID: 38250073 | PMCID: PMC10798041
Go to all (77) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Granulocyte-macrophage colony stimulating factor blockade promotes ccr9(+) lymphocyte expansion in Nod2 deficient mice.
Inflamm Bowel Dis, 17(12):2443-2455, 04 Mar 2011
Cited by: 8 articles | PMID: 21381154 | PMCID: PMC3111853
Innate dysfunction promotes linear growth failure in pediatric Crohn's disease and growth hormone resistance in murine ileitis.
Inflamm Bowel Dis, 18(2):236-245, 18 Feb 2011
Cited by: 10 articles | PMID: 21337672 | PMCID: PMC3057426
Granulocyte-macrophage colony-stimulating factor autoantibodies: a marker of aggressive Crohn's disease.
Inflamm Bowel Dis, 19(8):1671-1680, 01 Jul 2013
Cited by: 55 articles | PMID: 23749272 | PMCID: PMC3707315
Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn's disease.
Am J Physiol Gastrointest Liver Physiol, 306(6):G455-65, 06 Feb 2014
Cited by: 35 articles | PMID: 24503766
Review
Funding
Funders who supported this work.
NIDDK NIH HHS (6)
Grant ID: R01 DK058259
Grant ID: 1P30DK078392-01
Grant ID: R01 DK078683
Grant ID: R01 DK068164
Grant ID: T32 DK007727
Grant ID: P30 DK078392





