Abstract
Free full text

Evaluation of the ERETIC Method as an Improved Quantitative Reference for 1H HR-MAS Spectroscopy of Prostate Tissue
Abstract
The Electronic REference To access In vivo Concentrations (ERETIC) method was applied to 1H HR-MAS spectroscopy. The accuracy, precision, and stability of ERETIC as a quantitative reference were evaluated in solution and human prostate tissue samples. For comparison, the reliability of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid (TSP) as a quantitation reference was also evaluated. The ERETIC and TSP peak areas were found to be stable in solution over the short-term and long-term, with long-term relative standard deviations (RSDs) of 4.10% and 2.60%, respectively. Quantification of TSP in solution using the ERETIC peak as a reference and a calibrated, rotor-dependent conversion factor yielded results with a precision ≤2.9% and an accuracy error ≤4.2% when compared with the expected values. The ERETIC peak area reproducibility was superior to TSP’s reproducibility, corrected for mass, in both prostate surgical and biopsy samples (4.53% vs. 21.2% and 3.34% vs. 31.8%, respectively). Furthermore, the tissue TSP peaks exhibited only 27.5% of the expected area, which would cause an overestimation of metabolite concentrations if used as a reference. The improved quantification accuracy and precision provided by ERETIC may enable the detection of smaller metabolic differences that may exist between individual tissue samples and disease states.
High resolution magic angle spinning (HR-MAS) spectroscopy is a rapidly developing technique that allows solution-like NMR spectra to be obtained from intact tissue specimens while preserving the tissue for further pathologic and genetic study (1-8). The measurement of absolute metabolite concentrations in tissue would provide important biochemical information and allow changes in individual metabolites to be compared directly between samples. Current methods of quantification often rely on relative ratios of metabolites, which are not ideal because they do not permit detection of individual metabolite changes that may occur with a particular disease state. Furthermore, metabolite ratios can yield ambiguously high or low values when one metabolite peak area approaches zero, and thus skew the average among many samples. The measurement of absolute concentrations requires the presence of an endogenous reference, such as tissue water (6), or the addition of an external or internal reference standard to which other metabolites can be compared. Unsuppressed water is not ideal for quantitation because the water signal intensity is typically 1,000–10,000 times larger than the most concentrated metabolite in tissue, and it assumes that the density of water does not change between pathologies. External silicone rubber beads produce an NMR signal close to 0 ppm (4,9), but the peak area varies with spin rate and therefore must be calibrated (10). Additionally, the bead must be embedded in an insert, which isolates it from the tissue sample inside the HR-MAS rotor. Although these inserts reliably secure the silicone bead in the rotor and improve the B0 homogeneity, tissue and associated fluids can be trapped under the insert, leading to cross contamination between samples.
Numerous internal chemical standards [e.g., formate, 3-(trimethylsilyl)propionic-2-2-3-3-d4 acid (TSP)] have also been described for quantitative analysis in conventional solution NMR and HR-MAS spectroscopy (11). An ideal internal standard in tissue should be highly reproducible from sample to sample, nontoxic to the tissue or cells in the sample, chemically inert (metabolically inactive), nonvolatile, and nonhygroscopic. It should also resonate at a frequency that does not overlap with other peaks, possess a sufficiently long T2 to allow good signal detection, and possess a short enough T1 to prevent signal saturation when using a repetition time (TR) appropriate for the tissue. Unfortunately, most internal standards evaluated to date do not meet all of these criteria. Formate is metabolically inactive and reproducible; however, the T1 and T2 relaxation times of formate are much longer than those of endogenous metabolites and must be corrected for when analyzing the data (12). TSP is metabolically inactive, is nontoxic, and has desirable T1 and T2 relaxation times. Although it has been used as a quantification reference in HR-MAS spectroscopy of tissue (5), there are concerns about its reproducibility due to protein binding (12) or other interactions with the tissue. Additionally, because internal chemical standards are typically aqueous solutions that are often premixed with deuterium oxide (D2O) to provide a lock signal, they may be displaced into small spaces inside the spinning rotor that are outside of the RF coil’s detectable volume.
The Electronic REference To access In-vivo Concentrations (ERETIC) method is a promising alternative to the use of chemical reference standards in HR-MAS spectroscopy of tissue and has several unique advantages. Because the ERETIC method employs a synthesized RF pulse during the acquisition period to produce its signal, no chemical substance needs to be added to the sample. Thus, there are no concerns about toxicity, chemical activity, binding, or visibility. The frequency, linewidth, and amplitude of the ERETIC signal may also be modified easily by the experimentalist and, the lineshape is independent of B0 homogeneity. Furthermore, there are no relaxation time considerations when using ERETIC because the linewidth can be chosen freely. Also, if the spectrometer is configured correctly, the RF coil loading caused by the sample reduces the ERETIC signal by the same amount as the metabolite signals from the sample, as expected for a quantitation reference (13). The ERETIC method has previously been shown to provide reproducible and robust concentration measurements in solution and solid state NMR (13,14); however, very little has been reported on the use of ERETIC with HR-MAS spectroscopy of tissue. The aim of this study was to evaluate the accuracy, precision, and reproducibility of ERETIC compared with TSP in HR-MAS spectroscopy of solutions and prostate tissues to establish its reliability as a quantitative reference.
MATERIALS AND METHODS
Hardware and ERETIC parameters
Spectral data were acquired at 11.7 T (500 MHz for 1H), 1°C, and a 2,250 Hz spin rate using a Varian INOVA spectrometer equipped with a 4-mm gHX nanoprobe. All samples were analyzed inside custom designed 20 or 35 μL leak-proof, wide-mouth zirconia rotors (Fig. 1), which can be purchased from Varian (Palo Alto, CA).
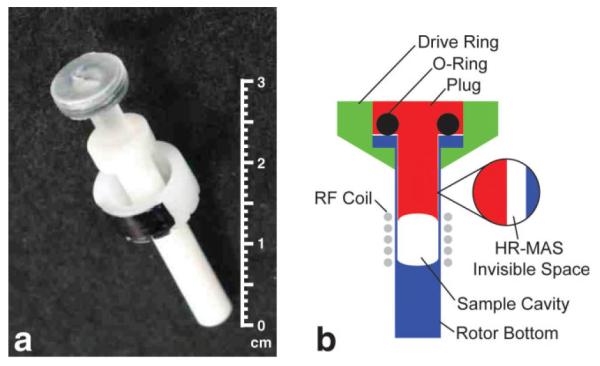
(a) A photograph and (b) a schematic of a wide-mouth zirconia HR-MAS rotor (not drawn to scale). The wide-mouth zirconia rotors were designed to keep the sample within a homogenous region of the RF coil. Although this was true for tissue samples, a small volume, referred to as the invisible volume (VI), of an aqueous sample was forced into the narrow gap between the walls of the rotor bottom and shaft of the plug when the rotor was spinning. Since this volume was outside of the RF coil, it was not detected by the HR-MAS experiment. Using Eq. [3], it was possible to correct for this invisible volume when calibrating the ERETIC signal with a solution sample.
Generation of the ERETIC signal required the development of a custom pulse sequence and modification of the system configuration as illustrated in Fig. 2. The output of the 1H synthesizer was shared between channels 1 and 3 within the system cabinet by connecting the synthesizer ports (SYNTH) for the waveform generators on the these two channels to the same synthesizer output. This parallel configuration ensured that the 1H transmitter and receiver were phase locked with the ERETIC transmitter. Channel 3 was set up to produce a programmable, low power output capable of generating an electronic signal with an amplitude comparable to the NMR signal produced by the sample. The low power signal was created by bypassing the amplifier on channel 3 and transmitting the signal from output #4 on the attenuator module. The channel 3 output and the filtered output from channel 2 were combined with a directional coupler (Varian, Palo Alto, CA), allowing the signals from both channels to be transmitted through the X channel of the nanoprobe as shown in Fig. 2 (13,15).
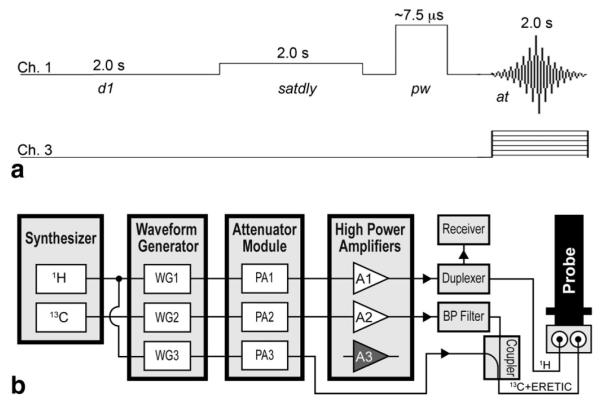
(a) Pulse sequence diagram of the ERETIC sequence and (b) system diagram of the Varian INOVA console high-lighting the modifications required for generating an ERETIC signal at the 1H frequency. The output of the 1H synthesizer was shared between the waveform generators (WG) for channels 1 and 3. The ERETIC signal was taken directly from the output of the Programmable Attenuator (PA) module, combined with the output from channel 2 using a directional coupler, and transmitted through the X channel of the nanoprobe. The transmission of the ERETIC waveform and the data acquisition were synchronized in the pulse sequence. (d1, relaxation delay; satdly, presaturation delay; pw, pulse width; at, acquisition time; BP, bandpass).
The ERETIC signal was created using Pandora’s Box (Pbox) software (Varian, Palo Alto, CA) to generate an exponentially decaying waveform with a user-defined decay constant and offset frequency and synthesized using the waveform generator on channel 3 as shown in Fig. 2. The desired power and phase for the ERETIC signal were applied to channel 3, allowing independent control of the ERETIC signal’s amplitude and phase relative to the rest of the spectrum. The 1H RF pulses were transmitted on channel 1, the ERETIC signal was transmitted on channel 3 during the data acquisition, and channel 2 was set and tuned to the 13C frequency.
Because the ERETIC signal was transmitted through the RF coil tuned to the 13C frequency, it was effectively transmitted through a detuned RF coil, and its transmission was unaffected by sample loading, as described by Ziarelli and Caldarelli (13). In short, the quality factor (Q) of the X channel RF coil at the ERETIC frequency was insensitive to the sample loading differences because the X coil was tuned to the 13C frequency, whereas the Q of the 1H RF coil at the ERETIC (1H) frequency was sensitive to sample loading differences. Thus, transmission of the ERETIC signal was constant for different loads. However, differences in sample loading or tuning of the 1H RF coil attenuated the reception of the ERETIC signal by the same amount as the NMR signal originating from the sample. As a result, the ERETIC signal was equivalent to an NMR signal from a fixed amount of compound added to the sample. The ERETIC signal was phase cycled in synchrony with the receiver to maintain phase coherence across the scans.
Sample Preparation and Acquisition
Deuterium oxide (D2O, 99.9% atom-D) and deuterium oxide containing 0.75 wt % 3-(trimethylsilyl)propionic-2-2-3-3-d4 acid (D2O + TSP) were purchased from a single batch manufactured by Sigma-Aldrich (St. Louis, MO). All solution samples were prepared by pipetting aliquots of D2O + TSP (weighed to ±0.01 mg) into an HR-MAS rotor shown in Fig. 1 and filling the remainder of the rotor with plain D2O. Samples containing 3.0 μL of D2O + TSP were prepared and analyzed for five consecutive days over three separate weeks and monthly for five months to test the short-and long-term stability of the ERETIC and TSP peak areas. Calibrations were performed separately on 20 and 35 μL rotors using four and five volumes, respectively, of TSP to determine rotor-specific conversion factors for ERETIC and the volume of solution invisible to the HR-MAS experiment, which were later used to calculate metabolite amounts. Then, the precision and accuracy of measurements made using ERETIC were evaluated in a 35 μL rotor by acquiring five spectra on samples containing 1, 15, and 30 μL of D2O + TSP. Spectra were also acquired at ERETIC transmitter power levels of −6, 0, 6, 12, and 18 dB to evaluate the linearity of the corresponding increase in ERETIC peak area.
One-dimensional spectra were acquired on all solution samples with a calibrated 90° pulse, 40,000 complex points, 20,000 Hz spectral window, 2 s delay (d1), 2 s water presaturation period (satdly), 2 s acquisition time (6 s repetition time, fully relaxed), 4 steady state pulses, and 64 transients as depicted in Fig. 2. The phase and amplitude of the ERETIC peak were selected to match other peaks in the spectrum, and the signal was transmitted during acquisition using 0 dB of power, a full width at half height of 3.5 Hz, and an offset frequency equivalent to −0.5 ppm.
This study was approved by the Institutional Review Board at our institution and informed consent was obtained from all patients. Tissue samples were obtained from patients undergoing transrectal ultrasound guided prostate biopsies (n = 48) and radical prostatectomy surgeries (n = 12), frozen on dry ice, and stored at −80°C until the time of the HR-MAS experiment. The HR-MAS experiments were designed based on the methods described by Swanson et al. (5). In brief, to provide a lock signal and frequency reference, 3.0 μL of D2O+TSP was pipetted into the bottom of a tared rotor and weighed to ±0.01 mg, after which a biopsy (5.90 ± 1.15 mg) or surgical (18.70 ± 3.44 mg) sample was weighed and added to a 20 or 35 μL rotor, respectively. When necessary, the magnetic field homogeneity was optimized using an automated routine. A presat sequence analogous to the one described for the solution samples was used for tissue samples, but with 128 transients to obtain a signal to noise sufficient for quantification. In addition, the TR was reduced to 4 s by setting the d1 = 0 s because the metabolite T1 relaxation times in tissue were shorter. As in previously published HR-MAS spectra of prostate tissue (5), a spin rate of 2.25 kHz was selected in order to minimize the overlap of the spinning sidebands with the metabolites of interest, while maintaining the pathologic integrity of the tissue. Moreover, as illustrated in Fig. 3, with adequate water suppression, spinning sidebands due to residual water were usually negligible. Thus, the tissue HR-MAS experimental design minimized the impact of T1 saturation and spinning sidebands on the results.
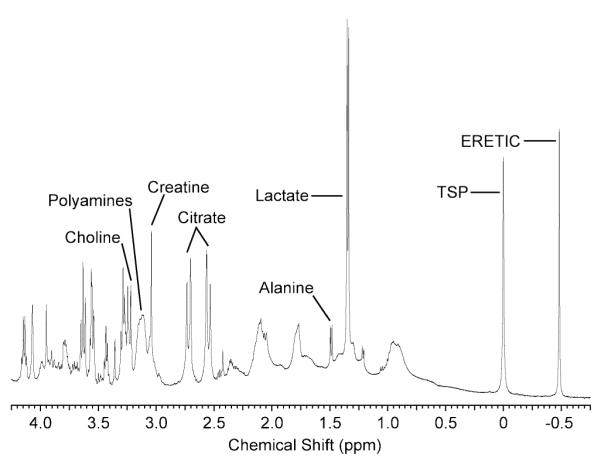
Representative HR-MAS spectrum of prostate surgical tissue with the ERETIC signal generated at a frequency of −0.5 ppm and with a transmitter power of 0 dB. The phase of the ERETIC signal was matched to the metabolites before the acquisition and produced a peak that had a phase and amplitude comparable to those of the tissue metabolites. The frequency of the ERETIC signal was well separated from the other peaks in the spectrum and allowed for accurate quantification of its peak.
Data Analysis
Spectra were processed and analyzed using Advanced Chemistry Development’s 1D NMR Processor, Version 9.15. (ACD/Labs, Toronto). Each FID was zero-filled to 262,144 complex points and Fourier transformed (FT). All tissue FIDs were multiplied by a 0.5 Hz exponential filter prior to the FT. The spectra were then phased manually, after which the baseline was corrected by fitting a 6th order polynomial curve to the spectrum and subtracting it from the spectrum. The TSP and ERETIC signals were quantified using peak areas, making the analysis less sensitive to sample-to-sample variations in B0 homogeneity and T2 that would affect the TSP lineshape. In the solution spectra, peak areas were measured by integrating a region spanning 100 Hz on each side of the peak’s center, which was greater than 50 times the full width at half maximum of the peaks of interest (16). Whereas, peak areas in tissue spectra were measured using Lorentzian–Gaussian peak fitting as previously described (5) because the baseline near TSP was not flat enough to accurately measure its peak area with integration.
A conversion factor (ME) equal to the number of protons equivalent to the ERETIC peak area was empirically derived in order to assess the quantification accuracy of ERETIC. ME is related to the number of millimoles of TSP protons visible to the HR-MAS experiment, MTSP, as shown in Eq. [1]:
where ATSP is the area of the TSP peak, and AE is the area of the ERETIC peak. If a portion of the volume of TSP is invisible to the HR-MAS experiment (VI) then Eq. [1] can be modified to include the total volume of TSP (VT) the concentration of TSP protons, [TSPH], and VI as follows:
Equation [2] is only valid for VT > VI. In this study, ME and VI were determined separately for each rotor by plotting the ratio of (ATSP/AE) versus VT, which was calculated from the weights of the TSP solutions and then performing a linear regression on the data. ME and VI were calculated from the slope and y-intercept, respectively, obtained from the linear regression. The plot used for the linear regression can be modeled by rewriting Eq. [2] as shown in Eq. [3]:
The precision and accuracy of the measured amount of TSP protons were assessed using a separate set of spectra recorded from solutions with varying TSP concentrations. The moles of TSP protons were calculated for these experiments using ME, VI, the measured peak areas from the spectra, and Eq. [4]:
The precision of the measured amount of TSP protons was evaluated by computing a relative standard deviation (RSD) for the measurements. The accuracy of the measured amount of TSP protons was then determined using Eq. [5]:
where
The linearity of the increase in the ERETIC peak area with the increase in the ERETIC power as well as the slope of the increase was assessed by analyzing the data acquired with five different ERETIC power levels spanning −6 to 18 dB. The peak area versus transmit power data was fit to a linear model using the curve fitting toolbox in Matlab. The conversion of the power values from the dB scale to the linear scale was assumed to be perfect because it was computed by the spectrometer software and was applied to the data before fitting. Prior to fitting the data, the measured peak areas were normalized by the slope determined using the first data point and the origin. The normalization removed the scaling factors introduced by the preamplifier, the analog-to-digital converter, and the other electronics in the transmit and receive chains of the spectrometer. After normalizing the data, the slope of the fit would be equal to one if the ERETIC transmitter was a linear, one-to-one system.
Finally, the variability of the TSP and ERETIC peak areas in the prostate tissue spectra were evaluated by computing the RSD for spectra recorded from the biopsy and surgical samples, separately. In addition, the error of the TSP peak areas was calculated by comparing the measured amount of TSP protons determined from the spectra to the amount expected based on the mass of the TSP added to the rotor. The measured amount of TSP protons was calculated using the TSP and ERETIC peak areas, ME, and Eq. [4]. For the surgical samples, MTSP was also corrected for invisible volume of TSP by using the VI determined from the calibration data. It was not possible to make this correction for the biopsy samples because VI>VT for those samples. The findings from the error analysis were validated by comparing the TSP and ERETIC signals in a representative prostate surgical spectrum to those in a solution spectrum acquired using the same HR-MAS protocol.
RESULTS
The weekly RSD of the ERETIC peak area in solution ranged from 2.09% to 4.25% over three weeks and was 4.10% over the five-month period (Fig. 4). The RSD of the TSP peak area corrected for mass was slightly higher than that of the ERETIC peak over three weeks and slightly lower over five months, but the difference was not significant in either case (combined P > 0.05). ERETIC peak areas in individual spectra deviated from the long term peak area mean by a minimum of 0.98% and a maximum of 8.35% and were within 5% of the mean peak area in 80% of the spectra.
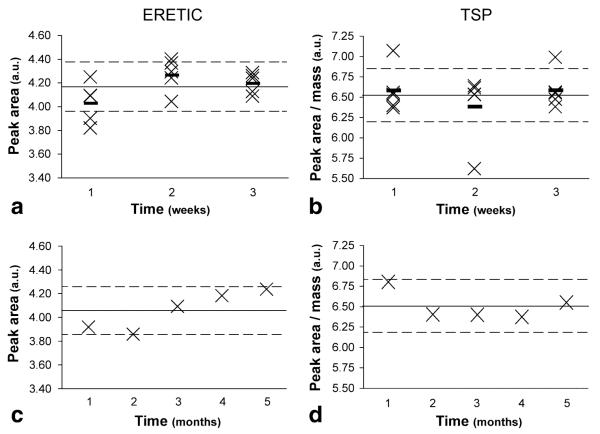
ERETIC peak area and TSP peak area (corrected for mass) from identically prepared solution samples acquired (a and b) onfive consecutive days over three separate weeks and (c and d) over five months. The solid line represents the mean peak area over the relevant time period, the dotted lines represent 5% deviations from the mean, and the small dashes in plots a and b represent the weekly means. Samples were prepared by adding 3 μL of D2O + TSP (weighed to 0.01 mg) and filling the remainder of the rotor volume with plain D2O. The average weekly RSD of the ERETIC peak area was 4.25%, 3.29%, and 2.09% during each week, respectively, whereas the average relative standard deviation (RSD) of the TSP peak area was 4.37%, 6.76%, and 3.57% during the same periods. The average monthly RSD was 4.10% and 2.60% for ERETIC and TSP, respectively.
The ratio of TSP and ERETIC peak areas was plotted versus the total volume of the TSP solution for each of the rotor sizes in Fig. 5. A linear relationship was established with an R2 of 0.999 for both the 20 and 35 μL rotors using least squares regression analysis. Next, ME and VI were derived using Eq. [3]. At an ERETIC transmit power of 0 db, ME equaled 0.235 and 0.253 μmol of protons in the 20 and 35 μL rotors, respectively. Whereas, VI was 4.31 and 1.56 μL for the 20 and 35 μL rotors, respectively.
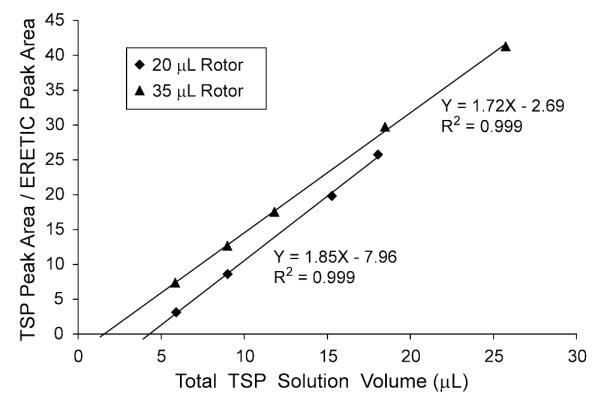
Plot of the TSP peak area (ATSP)/ERETIC peak area (AE) versus the total volume of the TSP solution determined by weight in the 20 and 35 μL rotors. A linear regression was performed on the data to calculate the rotor-specific conversion factors (ME) and the HR-MAS invisible volumes (VI) contained in Eq. [3]. ME represents the equivalent amount of protons for the ERETIC peak and can be used for quantification of metabolites; VI is the volume of the solution not detected by the HR-MAS experiment. The VI for the 20 μL rotor was 176% greater than the VI for the 35 μL rotor because of small differences in the rotor geometries.
Serial studies were then conducted to validate the accuracy and precision of these conversion factors. The number of μmoles of TSP protons was calculated using the mass of D2O + TSP and compared to the number of protons calculated using ERETIC, as summarized in Table 1. For each amount of TSP, the ERETIC-derived number of protons was accurate to within 4.1% and precise to within 2.9% of the weighed measurement.
Table 1
Precision and Accuracy of the Number of TSP Protons (μmol) Measured Using the ERETIC Method From Solution Samples of Equal Volume
| TSPW | TSPE | Precision (%) | Accuracy (%) |
|---|---|---|---|
| 0.386 | 0.402 ± 0.004 | 0.99 | 4.13 |
| 6.07 | 5.84 ± 0.17 | 2.91 | −3.65 |
| 12.11 | 12.04 ± 0.29 | 2.37 | −0.58 |
The left column shows the number of HR-MAS visible TSP protons calculated using the mass of TSP in the rotor (weighed to 0.01 mg) and the HR-MAS invisible volume. The second column shows the mean and standard deviations of the same protons calculated using ME, VI, and the ERETIC and TSP peak areas from five consecutive acquisitions. The third column shows the precision of the measurement expressed as the RSD. The fourth column shows the accuracy obtained using Eq. [5].
The ERETIC peak area increased linearly with the ERETIC transmitter power as the power was increased from −6 to 18 dB (Fig. 6). However, the rate of the increase in the ERETIC peak areas was not one, as would be expected from an ideal spectrometer. The slope of the Matlab fit to peak area data was 1.187 after the data were normalized to remove arbitrary scaling factors introduced by the spectrometer’s electronics (R2 = 0.999). Because the slope was 18.7% greater than the theoretically expected value of 1, the increase in the ERETIC peak area is 18.7% greater than theoretically predicted for a 24 dB boost in power.
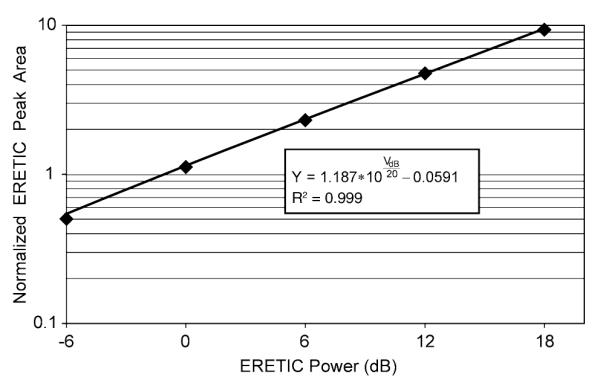
A plot of the linearity of ERETIC signal area with ERETIC power. The diamonds represent the average of three HR-MAS measurements taken on the same solution sample after they were normalized to remove arbitrary scaling factors introduced by the spectrometer’s electronics. The black line is a plot of the fit obtained on the data from Matlab. Since the leading coefficient of the fitted model is 1.187, the model indicates that the ERETIC signal area does not exhibit the one-to-one correlation with the ERETIC power that would be expected from an ideal spectrometer.
Figure 3 demonstrates the addition of the ERETIC signal to a representative prostate surgical tissue spectrum at a frequency of −0.5 ppm, where it does not overlap with other metabolite peaks. The peak had a Lorentzian line-shape, a full width at half height of 3.5 ± 0.01 Hz, and a phase and amplitude comparable with the peaks originating from the tissue metabolites. In prostate biopsy and surgical tissues, the RSD of the ERETIC peak area was significantly lower than that of the TSP peak area corrected for TSP mass (4.53% compared with 21.2% in surgical tissue, P < 0.001; 3.33% compared with 31.7% in biopsy tissue, P < 0.001), whereas no significant difference was found between ERETIC and TSP peak area RSDs in solution (Table 2). A plot of ERETIC peak area versus TSP peak area corrected for the HR-MAS visible volume (plot not shown) showed little correlation (R2 = 0.24), indicating that variation in TSP was not caused by differences in coil loading or coil tuning between samples.
Table 2
Relative Standard Deviation (RSD) of the TSP Peak Area (Corrected for Mass) and ERETIC Peak Area in Solution, Biopsy, and Surgical Tissues
| Sample | TSP (%) | ERETIC (%) | P value |
|---|---|---|---|
| TSP solution (n = 19) | 4.39 | 4.06 | >0.05 |
| Prostate surgical (n = 12) | 21.2 | 4.53 | <0.001 |
| Prostate biopsy (n = 48) | 31.8 | 3.34 | <0.001 |
Additionally, the TSP peak areas in the tissue spectra were considerably smaller than what was expected based on the mass of the TSP added to the sample. For the prostate surgical spectra, the peak area was (46.7 ± 14)% less than the expected area after correcting for the invisible volume. The TSP peak area in the prostate biopsy spectra was (84.3 ± 8.1)% less than the expected area, without correcting for the invisible volume. These large differences were verified by comparing the ERETIC and TSP peaks from a surgical spectrum to those from a solution spectrum acquired using the same HR-MAS protocol and a comparable amount of TSP. The ERETIC peak from the solution spectrum was 22.1% larger than the peak in the surgical spectrum due to lower RF coil loading caused by the solution sample, further substantiated by a 14.3% shorter 90° pulse width for the solution sample. After amplifying the surgical spectrum by 22.1% to correct for the loading difference, the ERETIC peaks were nearly identical, whereas the TSP peak was substantially smaller for the surgical spectrum (Fig. 7). In order to match the linewidths of TSP peaks in the surgical and solution spectra, the TSP peak in the solution spectrum was apodized with a 2.9 Hz Lorentzian filter to facilitate the visual comparison presented in Fig. 7. The error in the TSP peak area for the surgical spectrum in Fig. 7 was 41.4% and its TSP peak area was 28.7% of the corresponding peak in the solution spectrum.
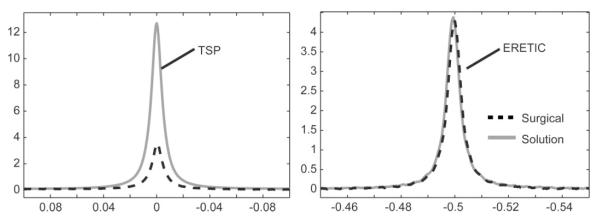
Comparison of the (a) TSP and (b) ERETIC peaks from a prostate surgical sample and solution sample with a comparable amount of TSP. The surgical sample was amplified by 22.1% to correct for loading differences between the two spectra, determined by the differences in the ERETIC peak area and the 90° pulse width. The TSP peak from the solution spectrum was apodized by 2.9 Hz to match the linewidths of the TSP peaks in the two spectra. Since the TSP peak area in the tissue spectrum was 28.7% of the peak area in the solution spectrum, a significant amount of the TSP was not detected in the HR-MAS experiment.
DISCUSSION
In this study, the ERETIC method was used to generate a stable and robust electronic quantitative reference signal for HR-MAS spectroscopy applications. The ERETIC signal was then added reproducibly to prostate tissue data acquisitions at a consistent frequency and in phase with other metabolites. Modifications for the addition of ERETIC were minimal and used only standard components found on many modern spectrometers and a commercially available directional coupler. Even though ERETIC has been used successfully in solution and solid state NMR applications (13,14), little work has been done showing its feasibility as a quantitative reference standard for HR-MAS spectroscopy of tissue.
The ERETIC peak area was found to be stable in solution over the short term (weekly intervals) and long term (5 months), with a RSD of 4.06% over all experiments. This level of stability was comparable to that observed with TSP using the same acquisition parameters. However, the stability of ERETIC signal was slightly lower than previously reported (15). The lower stability was not surprising because the amplitude of the ERETIC signal was chosen to be relatively low to mimic the intensity of the metabolite signals observed in tissue. The lower amplitude produced a signal-to-noise (SNR) that was more than sufficient for these studies, but lower than what was used in the previous reports. The lower SNR resulted in an higher RSD, as has been published (17). In addition, small differences in the baseline correction applied during postprocessing and variations in the probe tuning from sample to sample would also impact the ERETIC peak area stability. Lastly, a small coupling between the ERETIC transmitter and the digital receiver inside the system cabinet was observed on the INOVA spectrometer that contributed to the lower ERETIC peak area stability. Despite all of the small sources of error in the measured ERETIC signal, the variability of the ERETIC signal was much less than the biological variability that exists between samples (3).
Although ERETIC conversion factors (ME) for the 20 and 35 μL rotors were similar, the invisible volumes for the rotors were very different. Since the sensitive volume of the RF coil in the nanoprobe is closely matched to the rotor’s sample chamber, any sample forced into the small space between inner walls of the rotor bottom and the plug will not be detected (Fig. 1b). Coincidentally, when a solution is inside of a spinning rotor, the solution is forced out towards the walls of the rotor and up along the plug. Thus, the MAS forces the solution into this space where it becomes invisible to the HR-MAS experiment. Because the surface area of this space is quite large, a small deviation in the thickness of this space produces a large change in its volume, which was the case for the 20 μL rotor relative to the 35 μL rotor. Although this demonstrates that the VI must be measured for each rotor bottom and plug, there was no need to recalibrate ME for a given rotor during the five-month time frame of the experiments presented here because of the relative long term stability of the ERETIC signal. Relative standard deviations < 3.0% and errors <4.2% were found using ME and the ERETIC peak for quantification of TSP in solution compared with values expected by weight. The fact that these precision and accuracy percentages are higher than the 0.5–3% range typically reported in 1H NMR (11) and the 0.25–1.3% previously reported for the ERETIC added to solution spectra (15) can be explained by the small errors described earlier, with the exception of the probe tuning variations. If necessary, the accuracy of quantifications based on ERETIC could be improved by increasing the signal to noise ratio.
The increase in the ERETIC peak area as a function of the ERETIC transmitter power was linear, but the rate of increase was 18.7% higher than the rate of increase expected for an ideal spectrometer. For the experiments included in this study, an ERETIC power of 0 dB produced a peak with an amplitude comparable to the metabolite peaks from the tissue samples. However, the optimal power depends on the sample size and the metabolite concentration in the tissue and would likely vary across different spectrometers and probes. Although the power can be changed easily, the deviation of the slope of the ERETIC peak area versus power from unity indicates that the ERETIC conversion factor needs to be calibrated at the desired power.
The reproducibility of the ERETIC peak area was comparable in tissue and solution, while the reproducibility of TSP corrected for mass was much poorer in tissue than in solution. Furthermore, a significant amount of the TSP added to the tissue samples was not observed in the spectra. The presence of this measurement error was confirmed and illustrated in Fig. 7 by comparing the TSP and ERETIC peaks from a prostate surgical sample and a solution sample acquired using the same HR-MAS procedure and the same amount of TSP. Although the increased coil loading created by the tissue sample would decrease the signal from the TSP, the observed decrease in the TSP peak area was much greater than the decrease in the ERETIC peak area and increase in the 90° pulse duration, which should be equivalently affected by the loading changes. The observed error in the TSP peak area would lead to an over-estimation of metabolite concentrations if used as a quantification reference as previously noted (5). Effects such as T1 saturation and overlap with spinning sidebands could have contributed to the errors observed in the tissue samples. However, the T1 of TSP when added to HR-MAS tissue experiments at 1°C is ~ 600 ms (5) leading to a signal loss of 0.13% with a 4 s repetition time. At a spin rate of 2.25 kHz, the ERETIC and TSP peaks would overlap with spinning sidebands from molecules resonating at 4.0 and 4.5 ppm, respectively. The spectral intensities at these frequencies are small (Fig. 3), rendering their spinning sidebands almost undetectable at 0 and −0.5 ppm (data not shown). As illustrated in Fig. 3, with adequate water suppression, spinning sidebands due to residual water were usually not observed. Thus, the tissue HR-MAS experimental design minimized the impact of T1 saturation and spinning sidebands on the results.
The large measurement error and variation in the TSP peak area may also be attributed to a variation in the amount of TSP forced into the invisible space in the rotor, a spatial variation in the RF coil sensitivity combined with an inhomogeneous distribution of TSP within the rotor, B0 inhomogeneity, and weighing errors, all of which are avoided with ERETIC. Additionally, the variability and error in the TSP signal observed in both tissue types is consistent with a previous study, which found that when comparable amounts of TSP were added to blood serum samples, the TSP had a very short T2 due to possible protein binding (12). Regardless of the cause of the TSP errors, the superior reproducibility of ERETIC would reduce the measurement error of metabolite concentrations in tissue, thereby providing values that are more reflective of true biological differences between tissues of different disease states.
Although ERETIC shows great promise as a quantitation reference for HR-MAS spectroscopy of tissue, the method has a few limitations. First, the ERETIC phase must be matched to that of the metabolite signals on a per sample basis prior to the data acquisition or else the resulting signal may be out of phase from the metabolite signals. However, within the same day these phase adjustments were often small or negligible when the same rotor was used. In addition, this limitation can be overcome by using a spectral quantification tool that has the ability to quantify peaks with different phases. Second, the ERETIC frequency was offset by a constant amount from the center frequency, chosen to be water. Because of small frequency shifts in the water peak caused by minor temperature fluctuations and variations in determining the center frequency for the relatively broad water peak, the ERETIC frequency will vary slightly from sample to sample.
In this study, the ERETIC method provided an electronic reference that was stable, accurate, and precise compared to TSP under HR-MAS conditions. In prostate tissue, the ERETIC peak was also shown to have a reproducible area and a shape that was independent of B0 homogeneity. The ERETIC signal’s frequency, linewidth, and amplitude can be optimized for each application; however, the signal must be calibrated at the desired amplitude or power as the system does not behave as an ideal one-to-one system. In addition, the wide-mouth zirconia rotors possessed a rotor-dependent, HR-MAS invisible volume. When calibrating the ERETIC signal with reference solutions, it was essential to correct for this volume. Once the ERETIC signal was properly calibrated, its variability and accuracy as a quantification reference in HR-MAS spectra from prostate tissue was superior to that of TSP. The enhanced quantification accuracy and precision provided by ERETIC will improve the quantification of absolute metabolite concentrations in individual tissue samples and facilitate comparisons between tissue populations representing various biological conditions and diseases. Beyond quantifying spectra from prostate surgical and biopsy samples, the benefits afforded by ERETIC will be useful for quantifying HR-MAS spectra from other organs, such as brain, breast, liver, or kidney, and other sample sources, such as cell cultures, tissue cultures, or biological fluids.
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Daina Avizonis for her assistance in configuring our spectrometer to generate the ERETIC signal. We are grateful to Drs. Mark Dixon, Andy Zektzer, and Ralph Hurd for their helpful discussion regarding the implementation of the ERETIC method.
Grant sponsor: National Institutes of Health; Grant numbers: R01 CA102751, K01 CA096618; Grant sponsor: American Cancer Society; Grant number: RSG-05-241-01-CCE; Grant sponsor: University of California Discovery; Grant number: ITL-BIO04-10148.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1002/mrm.21808
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2879886?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Cellular Lactate Spectroscopy Using 1.5 Tesla Clinical Apparatus.
Int J Mol Sci, 23(19):11355, 26 Sep 2022
Cited by: 2 articles | PMID: 36232656 | PMCID: PMC9570142
Development of a Nuclear Magnetic Resonance Method and a Near Infrared Calibration Model for the Rapid Determination of Lipid Content in the Field Pea (Pisum sativum).
Molecules, 27(5):1642, 02 Mar 2022
Cited by: 0 articles | PMID: 35268743 | PMCID: PMC8911919
Research Progress of NMR in Natural Product Quantification.
Molecules, 26(20):6308, 19 Oct 2021
Cited by: 9 articles | PMID: 34684890 | PMCID: PMC8541192
Review Free full text in Europe PMC
A comparison of high-throughput plasma NMR protocols for comparative untargeted metabolomics.
Metabolomics, 16(5):64, 01 May 2020
Cited by: 14 articles | PMID: 32358672 | PMCID: PMC7196944
Quantitative NMR-Based Biomedical Metabolomics: Current Status and Applications.
Molecules, 25(21):E5128, 04 Nov 2020
Cited by: 51 articles | PMID: 33158172 | PMCID: PMC7662776
Review Free full text in Europe PMC
Go to all (41) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Determination of metabolite concentrations in human brain tumour biopsy samples using HR-MAS and ERETIC measurements.
NMR Biomed, 22(2):199-206, 01 Feb 2009
Cited by: 25 articles | PMID: 18833546
Optically transmitted and inductively coupled electric reference to access in vivo concentrations for quantitative proton-decoupled ¹³C magnetic resonance spectroscopy.
Magn Reson Med, 67(1):1-7, 14 Nov 2011
Cited by: 6 articles | PMID: 22084025
In-vivo assessment of tissue metabolite levels using 1H MRS and the Electric REference To access In vivo Concentrations (ERETIC) method.
NMR Biomed, 23(4):406-413, 25 Jan 2010
Cited by: 27 articles | PMID: 20101606
Electronic reference for absolute quantification of brain metabolites by 1H-MRS on clinical whole-body imaging.
J Neuroradiol, 37(5):292-297, 23 Mar 2010
Cited by: 7 articles | PMID: 20334920
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R01 CA102751-01A2
Grant ID: K01 CA096618
Grant ID: R01 CA102751




