Abstract
Free full text

Tristetraprolin regulates IL-8 mRNA stability in cystic fibrosis lung epithelial cells
Abstract
Cystic fibrosis (CF) is due to mutations in the CFTR gene and is characterized by hypersecretion of the proinflammatory chemokine IL-8 into the airway lumen. Consequently, this induces the highly inflammatory cellular phenotype typical of CF. Our initial studies revealed that IL-8 mRNA is relatively stable in CF cells compared with those that had been repaired with [WT]CFTR (wild-type CFTR). Relevantly, the 3′-UTR of IL-8 mRNA contains AU-rich sequences (AREs) that have been shown to mediate posttranscriptional regulation of proinflammatory genes upon binding to ARE-binding proteins including Tristetraprolin (TTP). We therefore hypothesized that very low endogenous levels of TTP in CF cells might be responsible for the relative stability of IL-8 mRNA. As predicted, increased expression of TTP in CF cells resulted in reduced stability of IL-8 mRNA. An in vitro analysis of IL-8 mRNA stability in CF cells also revealed a TTP-induced enhancement of deadenylation causing reduction of IL-8 mRNA stability. We conclude that enhanced stability of IL-8 mRNA in TTP-deficient CF lung epithelial cells serve to drive the proinflammatory cellular phenotype in the CF lung.
cystic fibrosis (CF) is the most common life-limiting autosomal recessive disease in the United States and Europe and is due to mutations in the CFTR gene (10, 20, 26, 30). CFTR mutations, of which the most common is [ΔF508]CFTR, cause a massive proinflammatory phenotype in the lung, which is chemically manifest in the airway by high levels of IL-8 and other proinflammatory cytokines and chemokines (1, 15, 31). IL-8 is the most potent known chemotactic agent for neutrophils (32) and is secreted from CF lung epithelial cells in a mutation-dependent fashion (4). The enhanced secretion of IL-8 seems to be an intrinsic property of the CF epithelium, since fetal CF lung epithelium spontaneously secretes high IL-8 levels into the airway as early as the 15th week of gestation (25, 37). However, the mechanism remains unknown by which a mutation in CFTR engenders massively upregulated levels of IL-8 expression.
The expression of inflammatory genes is known to be regulated by diverse processes. These processes include the rates of mRNA transcription and decay, and translation of the messages into cognate proteins (21, 38, 41). For example, the presence of cis-acting AU-rich sequences (AREs) in the 3′-UTR is known to confer instability upon mRNA, which prevents optimal response to extracellular stimuli (9, 33, 39). In fact, ~10% of all human genes contain ARE sequences in their 3′-UTR and are thus constrained to exhibit a broad range of decay rates (2, 18, 35). Many chemokine mRNAs, including IL-8, have short half-lives. However, the lifetimes of these messages can be increased by posttranscriptional mRNA stabilization mechanisms in response to a wide range of extracellular stimuli (12, 22, 24, 39). The types of transient extracellular stabilization activators include agents such as IL-1 and LPS (3, 11, 13, 24, 34–36). These stabilization mechanisms are mediated by specific transacting RNA binding proteins that specifically recognize the ARE motif (15–18). The best known of these ARE-binding proteins include 1) AUF-1/hnRNP D (42); 2) HuR, a member of the embryonic lethal abnormal visual (elav) family (5); 3) KSRP, the KH domain containing splicing factor (8); and 4) Tristetraprolin (TTP), the zinc finger protein (6, 7). Several studies have reported the role of the ARE-binding protein TTP in promoting enhanced destabilization of cytokine and chemokine genes (11, 17, 28, 29). We have therefore examined levels of TTP proteins in CF and [WT]CFTR (wild-type CFTR)-repaired lung epithelial cells to determine if differential levels of expression of TTP might characterize the inflammatory phenotype of CF cells.
We report here that TTP is expressed at very low levels in CF cells, both in CF cell lines and primary lung epithelial cells. Additionally, increases in TTP expression significantly lower IL-8 mRNA stability and IL-8 protein expression to levels observed in [WT]CFTR-repaired cells. We have also demonstrated that diminution of TTP in [WT]CFTR-repaired cells with siRNA targeting TTP stabilizes IL-8 mRNA to levels observed in the CF cells. We interpret these and other data to suggest that the mechanism by which CFTR mutations cause hyperinflammation in the CF airway may be due to aberrant regulation of IL-8 mRNA stability due to low levels of TTP.
MATERIALS AND METHODS
Reagents.
LHC-8 media, trypsin-EDTA (0.05%), and Lipofectamine transfection reagent were purchased from Invitrogen (New York). Bronchial Epithelial Growth Media (BEGM) and the normal human bronchial epithelial cells (NHBE) were purchased from Lonza. Actinomycin D (ActD) and protease inhibitor mixture were purchased from Sigma Chemical (St. Louis, MO). RNA aqueous kit for isolation of RNA from CF cells, Maxiscript in vitro transcription kit, cap analog (7meGpppG), and poly(A) tailing kit were obtained from Ambion (Austin, TX). IL-8 ELISA kit and antibody against GAPDH were purchased from R&D Systems (Minneapolis, MN). Antibody against TTP was purchased from Abcam (Cambridge, MA). Dupont-New England Nuclear (Boston, MA) was the source of [α-32P]ATP and [α-32P]UTP. Protogel, Sequagel (acrylamide, N,N-methylene bis-acrylamide, urea), and related buffers were obtained from National Diagnostics (Atlanta, GA). Protein assay reagents were purchased from Bio-Rad Laboratories (Richmond, CA).
Plasmids.
The plasmid encoding IL-8 cDNA was obtained from Origene Technologies (Rockville, MD). The pGem vector (pGEM-A0) was a generous gift from Jeffrey Wilusz (Colorado State Univ., Fort Collins, CO). The 3′-UTR of IL-8 (residues 1017-1076) was PCR amplified from the IL-8 cDNA and subcloned into pGEM-A0. The primers used to PCR amplify this fragment from IL-8 cDNA were GCGAATTCATAAATTATTTTCAAGTG (forward) and CGACAGATCTTGATGCTTAAATAC (reverse). A plasmid encoding the full-length human TTP cDNA containing a hemagglutinin epitope tag under control of the CMV promoter (pCMV.hTTP.tagHA) was generously provided by Perry Blackshear (National Institute of Environmental Health Sciences, Durham, NC).
Cell culture and transfection.
IB3-1 CF lung epithelial cells and the control CFTR-repaired IB3-1/S9 cells were maintained in LHC-8 serum-free medium in humidified 5% CO2 as previously described (16). Transfections were done using Lipofectamine transfection reagent. The magnitude of TTP expression was modulated by varying the amount of plasmid DNA for transfection. Bronchial brush biopsies were collected under an Institutional Review Board-approved protocol. Brushes were placed in L15 medium (Invitrogen) and were transported to the lab at 4°C. Cells were mechanically removed from the brushes and grown at 37°C, 5% CO2 in fibronectin-coated tissue culture plates, and BEGM (Lonza) supplemented with l-glutamine, 0.25 μg/ml Fungizone (Invitrogen), and 50 μg/ml gentamicin. The non-CF primary NHBE cells were also maintained in BEGM on fibronectin-coated plates in humidified 5% CO2.
Measurement of mRNA stability by qRT-PCR method.
RNA was isolated from the cells with RNA aqueous kit according to the manufacturer's instructions (Ambion). Biopsy samples from two CF patients were used for measuring IL-8 mRNA stability. First-strand cDNA synthesis and subsequent qPCR using LUX primers (Invitrogen) on ABI real-time instruments (ABI Prism 7500) was carried out using Superscript III Platinum Two step qRT-PCR kit (Invitrogen, according to the manufacturer's instructions). Briefly, 250 ng of high quality, intact RNA was used to synthesize first-strand cDNA synthesis in 20 μl of reaction volume containing 2× RT Reaction Mix (10 μl) and RT enzyme mix (2 μl). The reaction mix was incubated at 25°C for 10 min and the cDNA synthesis at 42°C for 50 min. The reaction was terminated at 85°C for 5 min and then chilled on ice. Escherichia coli RNase H (2 U) was added and incubated at 37°C for 20 min. qPCR was carried out in a volume of 25 μl using a final concentration of 1× Platinum quantitative PCR superMix-UDG, 200 nM each of LUX-labeled and unlabeled primers, 50 nM Rox reference dye, and 2.5 μl of cDNA from the first-strand synthesis reaction as mentioned above. Relative quantification of gene expression was performed by real-time quantitative PCR analysis as described by the manufacturer (Applied Biosystems User Bulletin 2). Cycling reaction program was followed as: 50°C for 2 min (UDG incubation); 95°C for 2 min; and 40 cycles of 95°C for 15 s and 60°C for 1 min. The real-time PCRs were performed in triplicate for both target and normalizer gene. For the primary cells, each of the two samples from CF patient lung biopsies was also analyzed in triplicate. Changes in mRNA expression level were calculated following normalization to human β-actin (acc. no. NM_001101). LUX fluorogenic primers and corresponding unlabeled primers for IL-8 gene (acc. no. NM_000584.2) were designed using D-LUX designer software. The sequence used for the reverse primer was CGCCTTTACAATAATTTCTGTGTTGGCG and that of the forward primer was CTTGGCAGCCTTCCTGATTTCT. The corresponding half-life (t1/2) values of IL-8 mRNA are indicated for all individual experiments.
Analysis of TTP mRNA levels by RT-PCR.
The reverse transcription PCR reaction was used to analyze TTP mRNA levels in the cells transfected or not with siRNA against TTP. The primers used were forward TCTCTGCCATCTACGAGAGCCTC and reverse GCTGATGCTTTGTCGCAGCACATG. The siRNA against TTP was obtained from Ambion (siRNA ID# s14978).
Cell-free mRNA decay.
Cell-free mRNA decay assays were performed as described previously (19). A 59-nucleotide fragment from the 3′-UTR of the IL-8 mRNA nucleotides 1017-1076 (GenBank acc. no. NM_000584) composed of overlapping as well as isolated AUUUA pentamers was cloned in pGem-A0 to generate pGemIL-8wtARE-A0. The plasmid was linearized with HindIII, and the RNA substrate was generated by in vitro transcription driven by SP6 polymerase. The poly(A) tail [α-32P]ATP radiolabeled substrate RNA was generated using the Ambion poly(A) tailing kit (according to the manufacturer's protocol) from a cold in vitro transcribed IL-8 substrate RNA transcribed from pGem-IL-81017-1076A0. The internally radiolabeled substrate RNA lacking a poly(A) tail was generated by in vitro transcription with [α-32P]UTP from HindIII-linearized pGem-IL-81017-1076A0 driven by SP6 polymerase. The radiolabeled RNA substrates were purified from a 6% polyacrylamide urea gel, and the specific activity of the substrates was ~0.2 × 106 cpm/ng. For the in vitro decay assay, 32P-labeled RNA (2 × 104 cpm) was incubated with S100 extract (16 μg of protein) in a 30-μl reaction volume containing 10 mM HEPES (pH 7.9), 50 mM KCl, 1 mM MgCl2, 1 mM DTT, 0.6 mM ATP, 1.0 μg free (poly)A, and 15 mM creatine phosphate for the indicated times. The products were resolved on a 5% polyacrylamide-urea gel and analyzed by autoradiography.
Statistical analysis.
Statistical analysis was performed using SAS version 9 for Windows. Significance values (P ≤ 0.05) were determined by a two-way ANOVA. Error bars on images represent SD.
RESULTS
IL-8 gene expression in CF lung epithelial cells is modulated by regulation of mRNA stability.
To determine the relative amount of IL-8 mRNA in the CF lung epithelial cell line (IB3-1) compared with CFTR-repaired cell line (IB3-1/S9), total RNA was isolated from both population of cells. Analyses of IL-8 mRNA by RT-PCR (Fig. 1A) using IL-8 gene-specific primer and IL-8 protein by ELISA (Fig. 1B) indicate that both IL-8 mRNA and protein levels are high in IB3-1 cells compared with the control IB3-1/S9 cells. Thus, upregulation of either synthesis or transcription of IL-8 mRNA appears to contribute to hyperexpression of IL-8 in CF cells. To examine whether posttranscriptional mechanisms were involved in this process, we therefore analyzed the stability of IL-8 mRNA in IB3-1 and IB3-1/S9 cells. Total RNA was isolated from these cells after actinomycin D treatment for various time intervals. The IL-8 mRNA levels were analyzed by qRT-PCR. As shown in Fig. 1C, the data indicate that IL-8 mRNA is degraded at a slower rate with ~1.5-fold difference in half-life in CF cells (t1/2 = 166 min) compared with CFTR-repaired control cells (t1/2 = 109 min). Thus, both potentially elevated mRNA synthesis and reduced mRNA decay contribute to high IL-8 expression in CF lung epithelial cells.
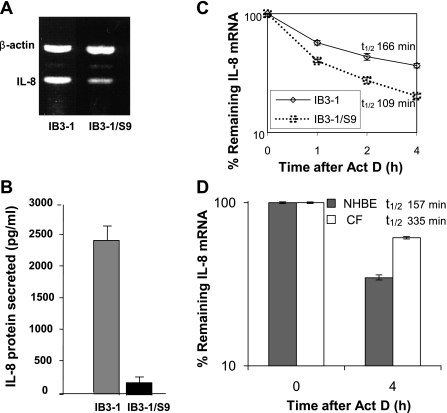
Stability and levels of IL-8 mRNA in cystic fibrosis (CF) cells. RNA was isolated from CF lung epithelial cells (IB3-1) and CFTR-repaired cells (IB3-1/S9). A: IL-8 mRNA and control β-actin mRNA levels were analyzed by RT-PCR using specific primers. B: the corresponding level of IL-8 protein secreted was determined by ELISA. C: the RNA was isolated from IB3-1 and IB3-1/S9 cells after treatment with actinomycin D (5 μg/ml) for the indicated time intervals (0, 1, 2, and 4 h). The remaining RNA was analyzed by quantitative real-time PCR and normalized against that of control β-actin mRNA. The data (P < 0.01) reflect averages of at least 3 independent experiments. D: the RNA was isolated from primary lung epithelial cells from CF patients (CF) or non-CF controls (NHBE) after treatment with actinomycin D (5 μg/ml) for 0 and 4 h. The data show averages from 2 patient samples (n = 2) and a non-CF control, each of which were repeated in triplicate (P < 0.01).
Moreover, examination of the stability of the IL-8 mRNA in primary bronchial epithelial cells obtained from CF lung brush biopsy strongly supports the cell culture model. As depicted in Fig. 1D, the half-life of IL-8 mRNA (t1/2 = 335 min) appears to be ~2.0-fold higher than that observed in the non-CF primary NHBE control cells (t1/2 = 157 min). A similar trend in enhanced IL-8 mRNA stability was thus observed in both the CF cell lines and primary cells compared with respective controls. These data strongly support our hypothesis that mRNA stability contributes to the hyperexpression of IL-8 gene in CF cells. We have therefore focused on the role of posttranscriptional mechanisms operational in IB3-1 cells in regulating IL-8 gene expression.
Analyses of proteins associated with ARE-mRNA decay in CF lung epithelial cells.
Based on the fact that IL-8 mRNA is degraded at a relatively slower rate in CF cells, we analyzed protein S100 extracts from these cells for the expression of protein factors known to cause enhanced decay of ARE-containing mRNAs. We examined the expression of poly(A)-specific ribonuclease (PARN), an enzyme responsible for initiating ARE-mRNA decay, by removing the poly(A) tail from the 3′-end of the RNA as well as the ARE-binding protein TTP. Figure 2A depicts that both PARN and TTP protein levels are significantly diminished in IB3-1 cells. The reduced expression of PARN and TTP proteins in CF cells is in agreement with our observation that IL-8 mRNA degrades at a relatively slower rate in these cells compared with CFTR-repaired control cells. However, we found that modulation of PARN levels in CF cells was ineffective in changing IL-8 mRNA expression (data not shown). We therefore turned our attention to TTP. We also examined the levels of TTP mRNA in the IB3-1 CF cells, the IB3-1/S9 CFTR-repaired control cells, the primary CF cells and the non-CF primary NHBE cells (Fig. 2B). The mRNA levels of TTP are barely detectable in IB3-1 cells as well as in the primary CF cells.
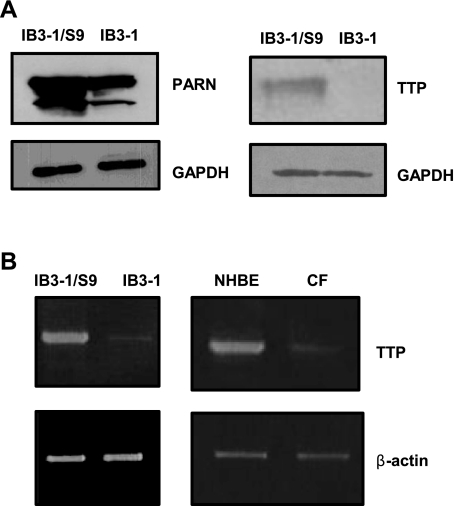
Expression of Tristetraprolin (TTP) and poly(A)-specific ribonuclease (PARN) in CF cells. A: S100 protein extracts prepared from IB3-1 and IB3-1/S9 were analyzed by Western blot using antibodies against the TTP and PARN proteins. The level of GAPDH protein was also simultaneously measured in the blots. B: the TTP mRNA levels in the IB3-1 and IB3-1/S9, as well as the primary CF cells and NHBE control cells, were also measured by RT-PCR using TTP-specific primers. The corresponding β-actin levels were used as endogenous control.
TTP promotes enhanced decay of IL-8 mRNA in CF lung epithelial cells.
We have used qRT-PCR to test the effect of elevation in TTP on the stability of IL-8 mRNA. As shown in Fig. 3A, high levels of TTP lead to a significantly more rapid degradation of IL-8 mRNA (~1.9-fold) than in the vector control. Specifically, the t1/2 of 157 min for the vector control is reduced to 84.5 min by transient transfection with the TTP expression vector. Additionally, Fig. 3B shows that concomitantly with elevated TTP expression, there is a corresponding decrease in secreted IL-8 protein. As shown in Fig. 3C, transient transfection of a plasmid encoding TTP results in substantially elevated expression of TTP protein in IB3-1 CF cells compared with untransfected control cells. TTP also appears to be phosphorylated in these cells as evident from the electrophoretic mobility shift of the protein upon treatment with alkaline phosphatase. The corresponding levels of TTP mRNA in the IB3-1 and IB3-1/TTP were also measured by RT-PCR (Fig. 3D).
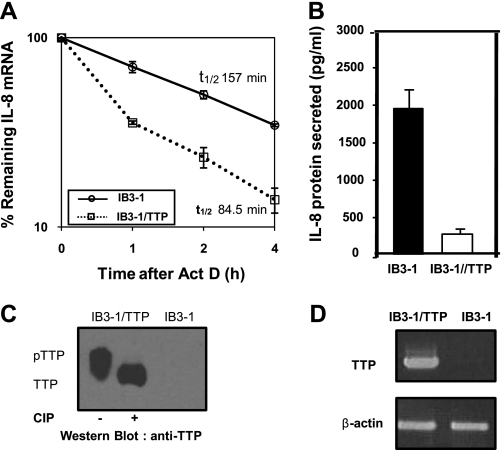
Effect of TTP on IL-8 mRNA stability in CF lung epithelial cells. A: IB3-1 cells were transiently transfected with TTP expression plasmid pCMV.hTTP.tagHA (0.5 μg/2 × 106 cells). RNA was isolated from IB3-1/TTP and IB3-1 cells after actinomycin D treatment for the indicated time intervals, and the remaining mRNA was analyzed by quantitative real-time PCR. The data (P < 0.001) reflects averages of at least 3 independent experiments. B: IL-8 protein secreted by IB3-1/TTP cells was analyzed by ELISA 16 h after transfection. C: S100 protein extracts prepared from the IB3-1 and IB3-1/TTP cells were analyzed by Western blot using anti-TTP antibody. The phosphorylation status of TTP in IB3-1/TTP was assessed by treatment of S100 extracts from these cells with alkaline phosphatase. D: the TTP mRNA levels in the IB3-1 and IB3-1/TTP cells were also analyzed by RT-PCR.
TTP directly impacts IL-8 mRNA stability in CF lung epithelial cells.
Since overexpression of TTP in IB3-1 cells promotes significantly rapid decay of IL-8 mRNA, we investigated whether TTP is directly affecting IL-8 mRNA stability. Therefore, we analyzed IL-8 mRNA stability in the control CFTR-repaired cell line IB3-1/S9 by knocking down TTP expression to see if, in the absence of TTP, these cells now exhibit increased stability of IL-8 mRNA comparable to that observed in IB3-1 CF cells. As depicted in Fig. 4, A and B, the ablation of TTP expression in IB3-1/S9 cells causes significant increase in IL-8 mRNA stability (t1/2 = 303 min) compared with that in IB3-1/S9 cells (t1/2 = 118 min). However, the IB3-1 CF cells that already express low endogenous levels of TTP exhibit only a slight increase in the stability of IL-8 mRNA when transfected with siRNA against TTP (t1/2 = 198 min compared with t1/2 = 175 min). The diminution of TTP mRNA using siRNA against TTP was highly efficient as evident from RT-PCR analyses of the corresponding levels of TTP mRNA (Fig. 4C).
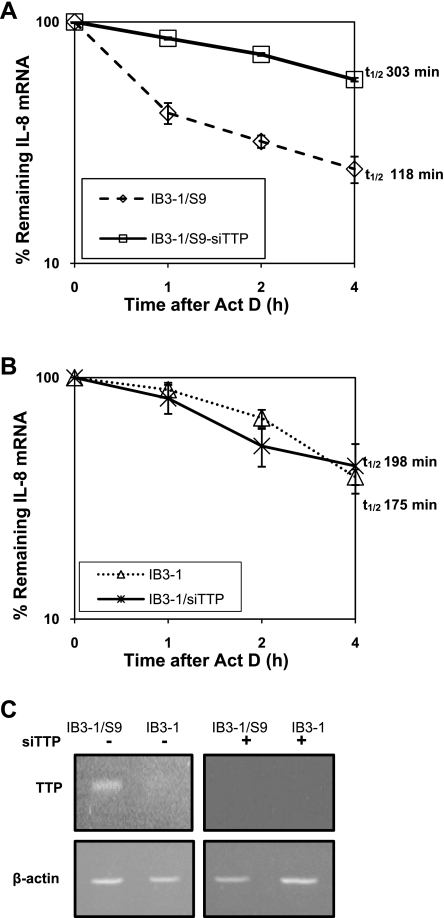
TTP directly impacts the stability of IL-8 mRNA in CF cells. A and B: IB3-1/S9 cells and IB3-1 cells were transfected with siRNA against TTP or a negative control siRNA. Subsequently, the IL-8 mRNA levels in these cells were determined by qRT-PCR. The data (P < 0.01) are the average of 3 independent experiments. C: the efficient knockdown of TTP expression using siRNA against TTP was analyzed by examining the level of TTP mRNA by RT-PCR.
TTP enhances degradation of IL-8 mRNA by promoting deadenylation.
As mentioned in the Introduction, TTP has been previously shown to enhance mRNA degradation by increased deadenylation of ARE-containing mRNAs (27). To further investigate whether TTP promotes enhanced decay of IL-8 mRNA by inducing increased deadenylation, we performed in vitro degradation assays using IL-8 mRNA 3′-UTR fragment containing ARE sequences. The IL-8 3′-UTR sequence used for the assays is depicted in Fig. 5A. The deadenylation-degradation assays were performed using a poly(A) tail-radiolabeled ([α-32P]ATP) substrate RNA in vitro transcribed from pGem-IL-8wtARE1017-1076A0. We compared the in vitro decay of the RNA substrate upon incubation with protein S100 extracts prepared from IB3-1, IB3-1/S9, and IB3-1/TTP cells. As shown in Fig. 5B, TTP expression in CF cells promotes enhanced in vitro degradation of the IL-8 RNA substrate at a near comparable level to that in CFTR-repaired cells. Since the radiolabel resides in the poly(A) tail, we could use this technique to assess in vitro deadenylation of the RNA substrate. The IB3-1 cells thus appear to be deficient in their ability to deadenylate IL-8 mRNA compared with the CFTR-repaired control IB3-1/S9 cells. The degradation process requires the presence of a poly(A) tail. As depicted in Fig. 5C, a RNA substrate that lacks a poly(A) tail exhibits negligible degradation. These data provide strong support for the concept that low levels of TTP in CF cells make a major contribution to the CFTR mutation dependence of IL-8 hyperexpression in the CF airway.
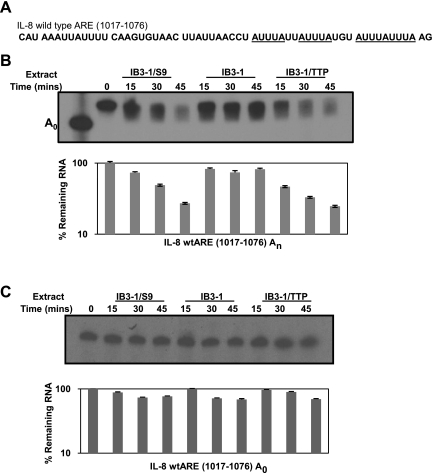
TTP modulates the stability of IL-8 mRNA in CF cells by promoting deadenylation. Cell-free mRNA decay assays were performed by incubating S100 extracts prepared from IB3-1/S9, IB3-1, and IB3-1/TTP cells with IL-8 mRNA substrate. A: the IL-8 sequence used for the assays included nucleotides 1017-1076 spanning the AU-rich 3′-UTR of IL-8 gene (GenBank acc. no. NM_000584). B: the IL-8-wtARE1017-1076-An RNA substrate was radiolabeled at the poly(A) tail using [α-32P]ATP. C: A non-adenylated [α-32P]UTP radiolabeled RNA substrate (IL-8-wtARE1017-1076 A0), lacking a poly(A) tail, was also assayed similarly. The remaining IL-8 mRNA levels for both the degradation assays are quantitatively depicted with the respective assays. Each assay is representative of 2 or more independent experiments.
DISCUSSION
An enduring mystery in the CF field has been the problem of how mutations in the CFTR gene result in massive hyperexpression of IL-8 and other proinflammatory cytokines in the CF airway. The data presented here show that reduced expression of TTP results in stabilization of the IL-8 mRNA in the CF lung IB3-1 epithelial cell and consequent hypersecretion of IL-8 protein. A similar phenomenon was also observed in the primary cells obtained from lung biopsies of CF patients compared with non-CF primary control cells. However, if IB3-1 cells are repaired by gene transfer with [WT]CFTR, or transfected with a TTP expression vector, IL-8 mRNA is destabilized by ~50%, and IL-8 protein secretion is substantially reduced. The data clearly show that the destabilization mechanism is due to deadenylation of the 3′-UTR of the IL-8 message. That a cause-effect relationship exists between CFTR mutation and TTP expression is revealed by experiments showing that gene transfer of [WT]CFTR into IB3-1 cells alone results in both elevation of TTP expression and reduction in IL-8 mRNA stability as well as IL-8 protein secretion. Moreover, ablation of TTP expression in the IB3-1/S9 CFTR-repaired cells leads to stabilization of IL-8 mRNA to a level comparable to the inherent stability of IL-8 mRNA in the IB3-1 cells. This demonstrates that TTP has a direct impact on IL-8 mRNA stability in CF cells, and the TTP deficit is clearly the dominant mechanism. These data suggest that CFTR-dependent regulation of TTP may therefore play an important role in the hitherto mysterious process of intrinsic proinflammatory signaling in the CF lung.
The effects of TTP on deadenylation are reported to involve cooperative interactions with the poly(A)-specific ribonuclease (PARN). However, no direct interactions between the two have been detected (27). Nonetheless, as shown in Fig. 2, lower levels of PARN were detected in IB3-1 cells compared with the repaired IB3-1/S9 cells. Furthermore, differential expression studies with recombinant PARN failed to affect IL-8 mRNA stability in contrast to the clearly shown effects of TTP. It is therefore possible that the levels of PARN in the CF cells, although lower than controls, may still be sufficient to help mediate TTP-dependent ARE-mRNA decay. Thus adding TTP to the CF cell might allow the system of TTP and PARN to act together to more efficiently destabilize IL-8 mRNA.
The stability of ARE-containing mRNAs is known to be enhanced by proinflammatory stimuli (14, 23, 40). However, the IB3-1 cells constitutively express high levels of IL-8 in the absence of extracellular stimuli. Although the CF-dependent mechanism is not yet known, it does appear to involve constitutive activation of the TNFα/NF-κB signaling pathway (16). Thus the stabilization of the IL-8 mRNA, engineered through the lower levels of TTP, may be a consequence of intrinsic, mutation-dependent activation of proinflammatory signaling occurring in spite of no objective evidence for infection or other conventional stimulus.
The 3′-UTR of the IL-8 mRNA is characterized by both clustered and isolated AUUUA elements. Similar distributions of AUUUA elements have also been reported for TNFα mRNA, which has also been shown to be sensitive to TTP (7). Consistently, we find that a 59-bp ARE-containing fragment of the IL-8 3′-UTR, similar in many respects to 3′-UTR sequences in the TNFα message, is sensitive to TTP. While we have not analyzed TNFα expression in the present study, it is well known that the CF airway is highly populated by TNFα as well. Thus there may be clinical as well as mechanistic similarities manifest in the parallel effects of TTP on 3′-UTR sequences in both IL-8 and TNFα mRNAs. Further studies will be needed to investigate this interesting possibility.
GRANTS
This study was supported by USU-Intramural Funds (R. Biswas), National Institutes of Health Grants NO1-HV-28287 (H. B. Pollard) and RO1-DK-53051 (H. B. Pollard), and the Cystic Fibrosis Foundation (R. Biswas and H. B. Pollard).
DISCLOSURES
The views expressed are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, the Department of the Defense, or the United States government.
Acknowledgments
We thank Anders Virtanen (Uppsala Univ., Sweden) for antibody against PARN, Jeffrey Wilusz (Colorado State Univ.) for the plasmid pGem-A0, and Perry Blackshear (National Institute of Environmental Health Sciences, Durham, NC) for providing the pCMV.hTTP.tagHA plasmid.
REFERENCES
Articles from American Journal of Physiology - Lung Cellular and Molecular Physiology are provided here courtesy of American Physiological Society
Full text links
Read article at publisher's site: https://doi.org/10.1152/ajplung.90601.2008
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2692809
Free after 12 months at intl-ajplung.physiology.org
http://intl-ajplung.physiology.org/cgi/content/full/296/6/L1012
Free to read at intl-ajplung.physiology.org
http://intl-ajplung.physiology.org/cgi/content/abstract/296/6/L1012
Citations & impact
Impact metrics
Citations of article over time
Article citations
Transcriptional analysis of primary ciliary dyskinesia airway cells reveals a dedicated cilia glutathione pathway.
JCI Insight, 9(17):e180198, 23 Jul 2024
Cited by: 0 articles | PMID: 39042459 | PMCID: PMC11385084
Bacterial endotoxin lipopolysaccharides regulate gene expression in human colon cancer cells.
BMC Res Notes, 16(1):216, 13 Sep 2023
Cited by: 1 article | PMID: 37705049 | PMCID: PMC10500902
Bioinformatic Analysis of the CXCR2 Ligands in Cancer Processes.
Int J Mol Sci, 24(17):13287, 27 Aug 2023
Cited by: 2 articles | PMID: 37686093 | PMCID: PMC10487711
Targeting HuR-Vav3 mRNA interaction prevents Pseudomonas aeruginosa adhesion to the cystic fibrosis airway epithelium.
JCI Insight, 8(3):e161961, 08 Feb 2023
Cited by: 2 articles | PMID: 36602863 | PMCID: PMC9977432
Identification of Bcl2 as a Stably Expressed qPCR Reference Gene for Human Colon Cancer Cells Treated with Cottonseed-Derived Gossypol and Bioactive Extracts and Bacteria-Derived Lipopolysaccharides.
Molecules, 27(21):7560, 04 Nov 2022
Cited by: 6 articles | PMID: 36364387 | PMCID: PMC9655230
Go to all (31) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines.
Am J Physiol Lung Cell Mol Physiol, 300(1):L81-7, 15 Oct 2010
Cited by: 36 articles | PMID: 20952496 | PMCID: PMC3023294
Regulation of miR-155 biogenesis in cystic fibrosis lung epithelial cells: antagonistic role of two mRNA-destabilizing proteins, KSRP and TTP.
Biochem Biophys Res Commun, 433(4):484-488, 21 Mar 2013
Cited by: 27 articles | PMID: 23524258
Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8.
J Biol Chem, 286(13):11604-11615, 31 Jan 2011
Cited by: 143 articles | PMID: 21282106 | PMCID: PMC3064214
Regulation of mRNA turnover in cystic fibrosis lung disease.
Wiley Interdiscip Rev RNA, 8(4), 13 Nov 2016
Cited by: 1 article | PMID: 27863009
Review
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: N01-HV-28287
NIDDK NIH HHS (1)
Grant ID: R01-DK-53051




