Abstract
Free full text

Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression
Abstract
Emerging evidence indicates that bone marrow (BM)-derived endothelial progenitor cells (EPCs) contribute to angiogenesis-mediated growth of certain tumors in mice and human. EPCs regulate the angiogenic switch via paracrine secretion of proangiogeneic growth factors and by direct luminal incorporation into sprouting nascent vessels. While the contributions of EPCs to neovessel formation in spontaneous and transplanted tumors and to the metastatic transition have been reported to be relatively low, remarkably, specific EPC ablation in vivo has resulted in severe angiogenesis inhibition and impaired primary and metastatic tumor growth. The existence of a BM reservoir of EPCs, and the selective involvement of EPCs in neovascularization, have attracted considerable interest because these cells represent novel target for therapeutic intervention. In addition, EPCs are also being used as pharmacodynamic surrogate markers for monitoring cancer progression, as well as for optimizing efficacy of anti-angiogenic therapies in the clinic. This review will focus primarily on recent advances and emerging concepts in the field of EPC biology and discuss ongoing debates involving the role of EPCs in tumor neovascularization. For detailed information on the in vitro characterization of EPCs contribution to non-tumor pathologies, the reader is directed towards several excellent reviews and publications [1] [2] [3] [4–6] and reviews by Bertolini, Voest and Yoder in this issue.
Introduction
Recent studies suggest that the BM-derived components of the tumor microenvironment are just not merely passive bystanders, but rather serve critical roles in regulating tumor growth and metastasis [7–9]. Tumor-derived paracrine signals instigate the BM compartment resulting in the mobilization and recruitment of discrete subsets of BM-derived cells to the tumor bed. Recruited proangiogenic BM-derived cells contribute significantly to neovasculature formation and tumor growth in adults [4, 10]. In addition to the perivascular contribution of BM-derived hematopoietic cells, the BM-derived EPCs provide an additional alternative source of endothelial cells that contribute to neovessel formation [3, 4, 11, 12]. In response to tumor cytokines, including VEGF [13], putative VEGFR2-positive EPCs mobilize into the peripheral circulation, and move to the tumor bed where they incorporate into sprouting neovessels [11, 14].
More recent investigations have shown that EPCs participate in neovascularization during acute ischemic injury in both human and mouse. For example, Minami et al. have shown that circulating endothelial cells engraft luminally into 15 to 29% of the vessels of the transplanted human heart [15]. BM-derived endothelial cells have also been shown to give rise to up to 16% of the neovasculature in spontaneous tumors growing in transgenic mice [16], and also contribute to human tumor vessels [17]. However, since the first description of EPCs [18], their identity and relative contribution to neovasculature formation has often been debated. Much confusion has prevailed due to the extensive variability in EPC contribution to vessel formation in a variety of tumor model systems [11, 19, 20] [17, 21–29]. The recent controversy notwithstanding, the existence of a BM reservoir of EPCs and their selective involvement in neovascularization, has attracted considerable interest because these cells not only represent a novel target for therapeutic intervention [14], but also are being successfully used as surrogate markers for monitoring cancer progression, as well as for optimizing efficacy of anti-angiogenic therapies such as anti-VEGFR2 antibody therapy [30, 31].
1. Bone-marrow derived hematopoietic cells support angiogenesis perivascularly
The BM compartment comprises the osteoblastic (or endosteal) and the vascular niches [32, 33]. The osteoblastic niche provides a quiescent microenvironment for stem cell maintenance, and the resident hematotpietic cells (HSCs) are anchored to the endosteal surface by calcium sensing receptors present on the HSC [34]. Growing tumors secrete soluble factors including VEGF, FGF, GM-CSF, osteopontin etc into the circulation that switches the marrow microenvironment from a quiescent state to a highly pro-angiogenic and pro-tumorigenic environment. This in turn, promotes the mobilization of both vascular and hematopoietic progenitors to the peripheral circulation which are recruited to primary tumors or metastatic lesions (Fig. 1) [35]. In the tumor bed, the BM recruited cells and other stromal cells (adipocytes, fibroblasts etc) constitute a unique microenvironment that can modify the neoplastic properties of the malignant tumor cells. Adult BM contributes significantly to endothelial and lymphatic neovessel formation and tumor growth and invasion [4, 10]. Among the BM-derived cells, much focus has been directed toward the proangiogenic hematopoietic mural cells that are recruited to the tumor bed where they exert their functions perivascularly via paracrine release of proangiogenic cytokines [4, 10, 36, 37]. Several populations of BM-derived hematopoietic cells have been reported to contribute to tumor angiogenesis and invasion. These include GR1+CD11b+ myeloid progenitors [38, 39], F4/80+ CD11b+ tumor-associated macrophages (TAMs) [37, 40], Tie2-expressing monocytes (TEM s) [23], CXCR4+ VEGFR1+ hemangiocytes [41], recruited BM-derived circulating cells comprising of a heterogenous population predominantly CD45+ /CD11b+ myeloid cells [42], PDGFR+ pericyte progenitors [43], VE-cadherin+ CD45+ leukocytes vascular leukocytes [44], and infiltrating mast cells and neutrophils [45, 46]. Despite the general importance of these cells in tumor angiogenesis, the precise contribution and biological function of specific lineages remains poorly understood.
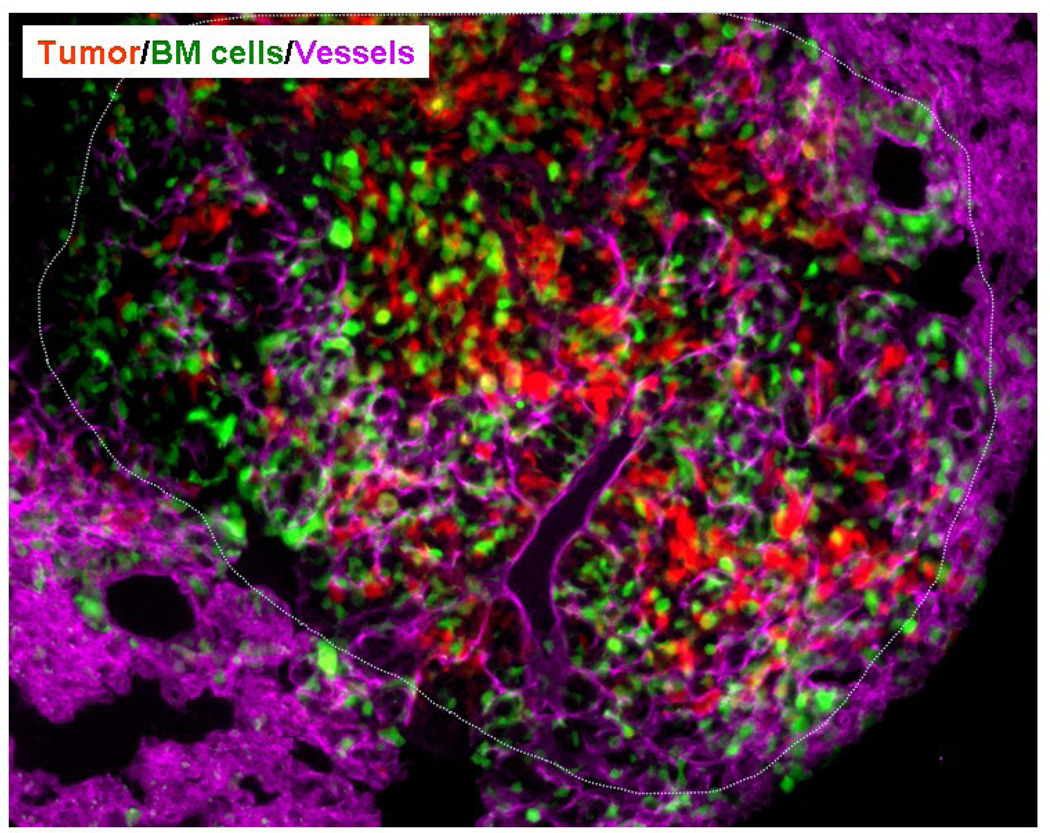
Wild type mice were lethally irradiated and transplanted with GFP+ BM. Following stable BM engraftment RFP expressing LLC cells were implanted in the flank of these mice. Following primary tumor growth, LLCs metastasized to the lungs. Immunohistochemical evaluation of the lung sections showed presence of BM-derived GFP+ cells, CD31+ mature vessels (magenta) in the RFP+ metastatic lesions. The dotted line separates the host tissue from the metastasis. DAPI was used to stain the nucleus of all cells. (Image courtesy of Dr. Dingcheng Gao, Weill Cornell University Medical Center)
2. BM-derived endothelial progenitor cells contribute to the angiogenic switch
In addition to the perivascular contribution of BM-derived hematopoietic cells, the BM-derived endothelial progenitor cells (EPCs) provide an additional source of endothelial cells that contribute to neovessel formation. Circulating EPCs in the peripheral blood of the adult human were originally identified in 1997 by Ashara as CD34+ VEGFR2+ mononuclear cells. These cells differentiated into an endothelial phenotype, expressed endothelial markers, and incorporated into neovessels at sites of ischemia [18]. Following Ashara’s observations, several other studies demonstrated that EPCs contribute to processes such as myocardial ischemia and infarction, limb ischemia, wound healing, atherosclerosis, endogenous endothelial repair, and tumor neovascularization in mice and human [4, 8, 11, 15, 17, 20, 47–49]. The initial demonstration that EPCs contribute to tumor angiogenesis was first demonstrated by Lyden et al. [11].
Transplantation of donor β-galactosidase-positive (β-gal+) BM from Rosa-26 mice into lethally irradiated angiogenesis-defective Id1-mutant mice revealed the presence of donor-derived LacZ+ BM cells in tumor vessels. Similarly, in humans previously transplanted with HSCs from a sex-mismatched donor, examination of secondary tumors revealed that 0.5–12% of tumor endothelial cells were donor-derived as determined by sex chromosome FISH analysis [17] [Fig.2C–E]
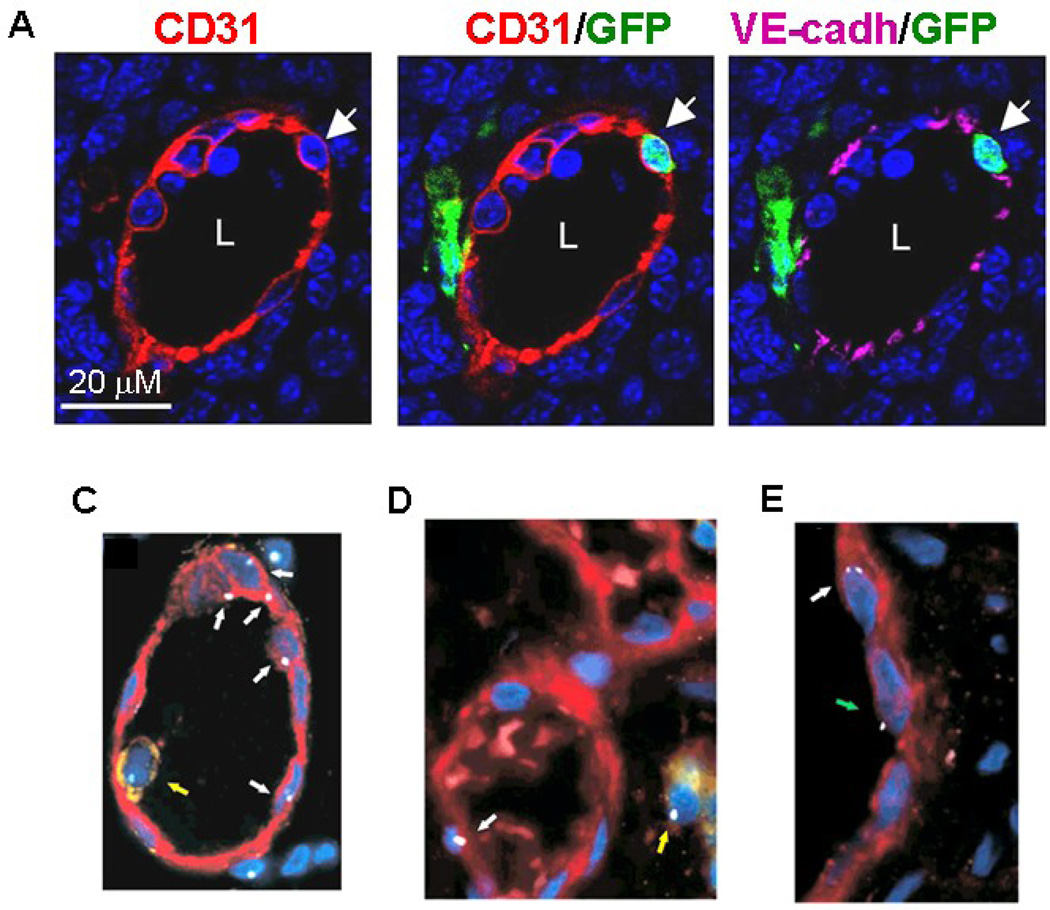
(A) High resolution image of a representative nascent CD31+ blood vessel in LLC tumor (day 6–8) showing a luminally incorporated BM-derived GFP+ CD31+ VE-cadherin+ co-expressing endothelial cell (arrow). The BM-derived endothelial cells had a single nucleus, GFP and CD31 signals were localized to the same individual cell, and VE-cadherin was localized to the endothelial cell junction. Scale bar, 20 µM. L, Lumen (Image courtesy of Nolan et al. Genes and Dev 2007).
(B–D) Analysis of secondary tumors that developed in humans previously transplanted with HSCs from a sex-mismatched donor show that tumor endothelial cells are donor-derived as determined by sex chromosome FISH analysis. C: Blood vessel of a male with colon cancer who had not undergone bone marrow transplantation showing endothelial cells with Y chromosome FISH signal (white arrows). D: Section of a thyroid cancer in a female after male bone marrow transplant. Blood vessel with one endothelial cell showing a Y chromosome FISH signal (white arrow). E: Section of male glossal mucoepidermoid carcinoma after female bone marrow transplant. Endothelial cell with two X chromosome FISH signals (white arrow). A single X chromosome (green arrow) positive endothelial cell is presumably derived from male recipient cells.
Note that yellow arrows point to CD45-positive cells with a Y chromosome signal. Endothelial cells were stained with von Willebrand Factor (red) and leukocytes with anti-CD45 antibody (yellow). (Image from Peters et al. Nature Med. 2005).
3. Id1 and endothelial progenitor cells
Id1 belong to the helix-loop-helix (HLH) fa mily of transcription factors [50]. Unlike positively acting factors in the HLH family that bind DNA to regulate transcription, the Id proteins, which lack a DNA binding domain, associate with other members of the family and prevent them from binding DNA or forming active heterodimers. The Id1 knockout mice were critical in demonstrating that BM derived progenitors are the source of tumor endothelium in some tumor types and grades since Id1 knockout mice failed to mobilize these progenitors and BM transplantation of Id1 knockout mice with wild type BM was shown to rescue the observed vascular defects [11, 51]. The importance of Id1+ progenitor cells was confirmed recently in vascular rebound that results from vascular disrupting therapies [52]. How Id controls the generation of EPCs is also beginning to be explored. In recent studies, Id1 was shown to be expressed in the long term repopulating hematopoietic stem cells (lin− Sca+ kit+ CD34−) [53, 54] in the BM and Id1 loss was shown to lead to an upregulation of the expression of the cyclin-dependent kinase inhibitor p21. The expression of p21 in turn drives the stem cells towards a more committed myeloid state, as assessed by gene expression profiling, an event that is associated with the depletion of cells capable of endothelial cell fate commitment. These results suggest that Id1 is required in early hematopoietic stem cells to restrain the commitment to the myeloid lineage and preserve a pool of cells that give rise to endothelial progenitors in response to vasculogenic growth signals. Although Id1 and Id3 are thought to be functionally redundant in many cells types, whether this is true in EPCs has not yet been established.
4. Controversy surrounding endothelial progenitor cells
Controversy exists about the identity and function of BM-derived EPCs, and many studies have not only questioned their relatively low contribution to tumor vasculature, but also their functional significance in tumor growth. Extensive variability ranging from a major contribution [11, 19, 20] to a minor contribution [17, 21, 22], and in some cases no contribution [23–29] has been reported. Such conflicting reports can be attributed to a limited analysis of the EPC phenotype in each study, and a lack of more definitive methods for distinguishing vessel incorporated BM-derived endothelial cells and intimately associated perivascular cells. Yet another source of variability may arise from the differences in tumor types [16, 51], and stage and failure to examine functional roles by performing their specific ablation. Below, we address some of these specific issues.
4A. Conflicting assessment of the EPC phenotype
There is a lack of consensus regarding the precise panel of cell surface markers that uniquely define EPCs. Attempts to characterize accurately circulating EPCs have been confounded by the dominant presence of cells of the hematopoietic lineages and circulating endothelial cells that have sloughed off from the mature vasculature [1]. Considerable overlap between cell surface markers such as CD31, CD34, and VEGFR-2/KDR that are expressed on EPCs and on the cells of hematopoietic lineages has further exacerbated the confusion. In addition, nonendothelial cells which exhibit some functional characteristics of endothelial cells, such as uptake of acetylated low-density lipoprotein (LDL), binding to specific lectins, and transdifferentiation into endothelial lineages in culture have been used in many studies of EPCs. Notably, no study has addressed the possible heterogeneity of the circulating EPCs representing differing stages of maturity. The first description of EPCs by Ashara and colleagues [18], relied on cells from human peripheral blood mononuclear cells (PBMC) that expressed VEGFR-2 and CD34. These markers are expressed on some hematopoietic stem cells (HSCs) as well as mature endothelial cells; therefore more specific markers are essential to distinguish EPCs from mature endothelial cells or HSCs. Other studies have shown that subsets of CD34+ VEGFR-2+ cells that coexpress the human progenitor marker CD133 have high proliferative capacity and comprise endothelial precursors [55], a notion challenged by others [56]. However, more recent studies have suggested that CD133 expression is not limited to EPCs, but is broadly expressed on a variety of other cells such as HSCs, non-HSCs including neural stem cells and embryonic stem cell lines [57].
Indeed, recent studies, including some of our own, have attempted to address this issue, at least in part, by phenotyping murine EPCs with a comprehensive set of endothelial, hematopoietic and progenitor markers [1, 8, 30, 48]. Analysis of EPCs in the BM, peripheral blood and tumors grown in mice that were previously transplanted with GFP+ BM required the use of a distinct combination of cell-surface markers. In early tumors, BM-derived EPCs were identified as GFP+ cells expressing VE-cadherin (uniformly on cell surface), CD31dim, and Prominin I/AC133. These cells also expressed VEGFR2 and lacked hematopoietic markers including CD11b, CD45B220, CD41 [48]. Notably, whether EPCs are CD45− [58] or CD45dim[1, 59], remains to be clarified [60]. In this context, it may be important to determine the status of CD45 on EPCs in the context of multiple CD45 isoforms, CD45RA, CD45RB, CD45RO, and CD45B220. Regardless, the ability of isolated EPCs to differentiate into mature endothelial cells and luminally incorporate into sprouting neovessels in vitro was used as a functional readout [48]. Vessel-incorporated BM-derived endothelial cells were detected by the expression of intracellular GFP, and these incorporated endothelial cells exhibited hallmarks of a mature endothelial cell such as cell-surface expression of CD31 and VE-cadherin restricted to intracellular adherens junctions. High resolution microscopy showed that vessel-incorporated GFP+ endothelial cells maintained colocalization of these markers in all serial sections of the confocal plane. Vessel-incorporated BM-derived endothelial cells were interrogated by administration of fluorescent isolectin IB4 and analysis of Isolectin+ CD31+ GFP+ CD11b− cells [8, 48]. Isolectin ensured luminal incorporation; GFP validated BM-derivation; CD31 confirmed endothelial cells; and CD11b gated out any hematopoietic contamination in the CD31 channel. This was critical because CD31 is expressed by a subset of hematopoietic cells [61]. Similarly, circulating EPCs, in the peripheral blood were detected as GFP+ c-Kit+ VEGFR2+ VE-cadherin+ CD11b− cells. In this scenario, c-kit (progenitor marker) distinguished BM-derived EPCs from circulating endothelial cells that have sloughed off from mature vessels. Similar schemes have been used by other investigators for the analysis of circulating EPCs [1, 30]. While a consensus on markers to enumerate EPCs is gradually evolving, there is a necessity for novel and unique EPC-specific markers.
4B. Lack of definitive methods for distinguishing vessel incorporated BM-derived endothelial cells and intimately associated perivascular cells
A reliable criterion to determine the existence of EPCs is the ability to reliably identify luminally incorporated BM-derived endothelial cells in nascent tumor vessels. Previous studies reported that greater than 50 – 90% of the CD31+ vessels in large, established tumors were BM-derived as determined by X-gal staining in LacZ+ BM transplants [11, 20]. There is a possibility that diffused LacZ expression in BM-derived cells within the tumors makes it difficult to distinguish authentic vessel incorporated BM-derived endothelial cells from other closely associated BM cells such as leukocytes, platelets, pericytes, macrophages occupying a perivascular location. This may have resulted in an overestimation of incorporated BM-derived endothelial cells. Since these reports, utility of high resolution fluorescent confocal microscopy has been advocated for the accurate determination of vessel incorporated endothelial cells [24, 62]. Such high resolution stereo-confocal microscopy is required to accurately discern luminally incorporated endothelial cells from perivascular cells that are intimately associated with tumor vessels. Indeed, luminally incorporated BM-derived endothelial cell determined by high resolution microscopic analysis of multiple Z-stacks (resolution of at least 0.275–0.35 µm, depth of 30µm), have shown that the BM-derived endothelial cells have a single nucleus, and that the GFP and CD31 signals are localized to the same individual cell indicating that the incorporated endothelial cell is derived from the BM [Fig.2A]. Notably, the vessel incorporated BM-derived endothelial cells exhibits hallmarks of a typical mature endothelial cell such as, uniform surface expression of CD31, a characteristic VE-cadherin staining at the intercellular adherens junctions, and a lack of hematopoietic markers [8, 48]. However, such high resolution microscopic analysis failed to detect vessel incorporated BM-derived endothelial cells in mice whose marrow expressed a GFP reporter driven by the endothelial-specific Tie2 promoter [23, 24]. Notably, in these studies Tie2 promoter marked non-endothelial cells referred to as Tie2+ monocytes and pericyte progenitors and not all the Tie2+ mature endothelial cells.
4C. Analysis of specific tumor types, mouse genetic models and tumor stage
Previous studies have shown that in spontaneous tumors, contribution of BM-derived cells to tumor neovessels differs depending on the tumor type. Mice heterozygous for the tumor suppressor Pten (Pten+/−), that display a spectrum of tumors including lymph hyperplasia, uterine carcinomas, prostate intraepithelial neoplasias and pheochromocytomas were used in determining EPC contribution. BM-derived EPCs contributed to 16% of neovessels in Pten+/− uterine carcinomas, while EPC contribution to vasculature of lymph hyperplasia was undetectable [16]. Importantly, the effect of loss of the transcription factor Id1 was much more profound on the viability of uterine carcinomas than the lymph hyperplasias, which may be due to the dependence on EPCs in the former case and cooption and sprouting of local vessels, less affected by Id1 loss, in the latter. Recently, a systematic analysis of EPC contribution to implanted tumors (Lewis lung carcinoma, B6RV2 lymphoma, melanoma), spontaneous breast tumors arising in MMTV-PyMT mice and pulmonary metastases that develop in these mice [8, 48], has shown that approximately 10–20 % of the early tumor vessels were BM-derived. So how does one explain the extreme variability in EPC contribution to tumor vessels in various published studies? A possible explanation is that in addition to tumor-type dependence it is possible that EPC contribution could be tumor stage specific. Indeed, a kinetic analysis of EPC contribution as a function of tumor growth showed that EPCs are recruited to early tumors preceding vessel formation, followed by differentiation into endothelial cells and luminal incorporation into a subset of sprouting tumor neovessels [48]. Noticeably, in growing tumors these chimeric BM-derived vessels were eventually diluted/replaced with non-BM derived host vessels [48], thereby explaining the low contribution observed in large established tumors in various studies [21, 23–25, 47, 62]. However, using transgenic models of de novo tumorigenesis (RIP-Tag5 that develop islet carcinomas and Alb-Tag that develop hepatocellular carcinoma) Spring et al. have shown that advanced tumors recruit and incorporate BM-derived EPC into neovessels [49]. Thus, it appears that stage specific recruitment of EPCs may be dependent on the tumor type, and more model systems need to be examined. Variability in the observed EPC contribution may also relate to whether sufficient BM progenitor cells are transplanted into irradiated hosts, so they can reconstitute vascular progenitors in the recipient mice. This can be achieved by determining that bona fide colony forming units of vascular progenitors are also engrafted into the marrow [63].
The selection of specific mouse cancer models is important in the analysis of EPC contribution to neovascularization. In some studies, use of a murine parabiosis model where two animals share anastomosed circulatory system has been advocated to avoid the adverse effects myeloablation often performed during BM transplantation. Notably, GFP+ EPCs were not observed in adenomas developing in a parabiotic APCmin mice surgically joined to GFP+ transgenic mice [29]. While the parabiotic model system presents an elegant experimental system, it is associated with certain deficiencies. For example, the lack of contribution of the GFP+ EPCs to the adenomas could have been diluted by BM contributed by the wild type mice. This aspect is further exacerbated by the fact that adenomas developing into APCmin mice should not recruit EPCs since in this model EPCs are mobilized and incorporate into vessels only during transformation of adenomas into carcinomas [49].
4D. Insufficient functional analysis
The contribution of EPCs to neovessel formation of both spontaneous, and transplanted tumors and metastatic transition has been observed in numerous studies [8, 11, 16, 17, 20, 47, 48, 64]. However, luminally incorporated BM-derived endothelial cells represent a minor fraction of the total tumor vasculature questioning the biological significance of this contribution to tumor growth. More, recent studies have begun to directly address their functional role in supporting tumor angiogenesis in vivo. For example, Id1 knockout mice or shRNA-mediated silencing of Id1 in vivo exhibit impaired EPC mobilization, severe angiogenesis inhibition and impaired tumor growth [8, 11, 52]. Acute and conditional shRNA-mediated silencing of Id1 mRNA in the adult BM resulted in EPC mobilization defects associated with severe angiogenesis inhibition and impaired primary tumor growth and progression of micrometastasis to macrometastasis, suggesting a critical role for these cells in angiogenesis-mediated tumor growth [8]. Notably, germline Id1 deficiency also compromises the engrafting potential of long term repopulating HSCs [53, 54]. In another study, targeting transiently expressed monomeric VE-cadherin specifically on EPCs using radiolabeled anti-VE-cadherin antibody resulted in severe angiogenesis inhibition and impaired tumor growth [65] [48]. So, why is this relatively minor contribution so critical for tumor growth. Notably, in addition to vessel incorporation, tumor recruited EPCs have been shown to secrete proangiogenic growth factors [8], also observed in cultured EPCs [66], suggesting that in addition to providing stability to nascent vessels EPCs have a paracrine role in vessel recruitment at a critical early stage of tumor growth.
5. EPCs in tumor growth and metastasis- Clinical translation
EPCs provide both instructive (release of proangiogenic cytokines) and structural (vessel incorporation and stabilization) functions that contribute to the initiation of tumor neoangiogenesis. Thus selective targeting of EPCs has been heralded as a promising avenue for antiangiogenic cancer therapy. EPCs are being considered as useful surrogate markers for monitoring cancer progression, as well as for optimizing the efficacy of anti-angiogenic therapies, such as anti-VEGFR2 antibody therapy [1]. There are several lines of investigation underway to elucidate the role of EPCs in both early and late stage human breast cancer as well as the transition to metastatic progression. These include attempts to answer practical questions such as whether variations exist in EPCs according to tumor type, stage and response to therapy. The expectation is that EPCs will evolve into clinically useful prognostic and predictive tools as well as represent a valid target for treatment- either in the adjuvant or metastatic setting. Clinical trials are currently underway to understand its role and find its niche in the treatment of breast cancer.
A major stumbling block in finding its niche has been the lack of consensus as to the optimal measurement of EPCs. As clinical specimens need to be processed in real time and since the timing of these specimens can be unpredictable, this can make for subpar testing conditions. Recently, attempts have been made to standardize EPC quantitation in fresh as well as frozen tissue samples [67]. The other major barrier to defining the clinical utility of EPCs as well as their specific role in the metastatic cascade is the lack of models to predict which patients will relapse and which will remain in remission. Since the turning “on” of the angiogenic switch is probably not an event that happens over a prolonged period of time, observational studies in high risk cohorts are needed in order to better define the precise role of this and other cells in the metastatic cascade. Because of all of these challenges, it is difficult to pool data from existing studies in order to arrive at a consensus opinion about EPCs at the current time. With the above caveats, it is not unexpected that the data are mixed with regard to the role of EPCs with respect to stage and response to therapy. As shown in Table 2, in 2 of the 3 studies, there were no correlations of EPCs with stage of breast cancer. In the study by Naik et al, 25 patients with breast cancer were evaluated at baseline (prior to chemo but after primary surgery) and prior to administration of their second chemotherapy treatment. The median number of EPCs (CD45− CD133+ VEGFR2+) was 6,920 EPCs × 105 MNC for Stage 1 and 2 breast cancer patients as compared to 165,000 EPCs × 105 MNC for Stage 3 and 4 breast cancer patients [68]. In contrast, Goon et al. demonstrated exactly the opposite in a much larger cohort of 160 patients (Goon, personal communication). Another study found an elevated number of EPCs with increasing tumor size but only for those tumors over 2 cm [69]. Finally, Kim and colleagues failed to observe significant difference in EPCs among varying stages of breast cancer [70]. Some studies have examined the effects of surgery, chemotherapy and antiangiogenic therapy on EPCs. In the study by Mancuso et al. patients with advanced, measurable breast cancer receiving metronomic (low dose) chemotherapy with or without thalidomide, no correlation in the number of EPCs and response to therapy was observed [71]. This is in contrast to another report in which the number of EPCs positively correlated with response to chemotherapy in both stage 3 and stage 4 breast cancer patients receiving neoadjuvant chemotherapy [68], while no post-treatment effects were observed in the EPCs numbers of Stage 1 and 2 patients. What is particularly thought provoking about this observation is that the numbers of EPCs were similar for both Stage 3 and 4 breast cancer patients although their prognosis is different. Patients with Stage 3 breast cancer are potentially curable and those with Stage 4 are not. Surgery normalized levels of EPCs in one study perhaps suggesting an additional benefit of surgery other than the obvious removal of the primary tumor [69].
Recently, because of interest in the role of EPCs in turning on the “angiogenic switch”, strategies have been employed to keep this switch in the “off” position. An ongoing Phase II trial of a copper depletion compound, tetrathiomolybdate, in a high risk for relapse breast cancer population at Weill Cornell Medical College Breast Center is attempting to modulate this switch through copper dependent mechanisms. In this clinical trial, EPCs and other cells thought to be requisite for metastatic progression are being evaluated on a monthly basis. Preliminary results suggest that EPCs are maintained at a low level with copper depletion to 30% of normal. However in the 2 patients that have relapsed on study, a 5- to 7-fold rise in EPCs heralded an overt relapse ranging from 2 to 5 months prior to that relapse (Blinder, 2008). This suggests that similar to what has been observed in mouse models; there is a peak in VEGFR2+ cells as tumors transition from micrometastatic to macrometastatic. Further studies are underway to characterize this transition in patients and this trial continues to accrue.
Recent observations that EPCs are the main regulators of the angiogenic switch in progression from micrometastases to macrometastases suggest that their selective targeting may be a promising approach in cancer patients where metastatic colonization to the lung has already occurred. This represents a paradigm shift and a foundation for very exciting clinical applications [8]. Given that in many cancer patients, metastatic spread has already occurred by the time of primary diagnosis, this study suggests that selectively targeting the angiogenic switch via the endothelial progenitor cells may provide a clinically feasible approach to block metastasis progression and prevent death in cancer patients. For example, following resection of primary tumors, patients with Stage 3 breasts or colon cancers are often treated with chemotherapy to destroy dormant micrometastases. Nonetheless, frequently, many of these patients succumb to progression of the micrometastatic invasive tumors. Thus, combinations of adjuvant chemotherapy with anti-angiogenic agents to block recruitment of the endothelial progenitor cells provide a highly effective strategy to impair establishment of metastatic lesions. Indeed, EPC targeting enhanced the efficacy of other anticancer therapies, such as vascular disruptive agents and select chemotherapy drugs [52, 72, 73].
6. Emerging concepts and future directions
BM-derived EPCs contribute to angiogenesis-mediated tumor growth and metastasis, and recent studies have begun to recognize the biological significance of this contribution. Major efforts are geared towards interrogating mechanisms governing EPC activation and expansion in the BM compartment, and their subsequent mobilization and recruitment to the tumor bed leading to the initiation of the angiogenesis program. Parallel technological advancements pertaining to BM transplantation systems, informative mouse genetic models of tumor progression and cell-specific reporters have facilitated a more detailed investigation of these cells. Several key concepts have emerged from these studies. First, given that EPCs comprise 0.02% of the total BM contribution (compared to 4% by GR1+ myeloid cells) and that only about 10–20% of tumor vessels have incorporated BM-derived endothelial cells the question has arisen as to the relevance of this minor contribution in tumor growth. Conspicuously, EPC ablation resulted in severe angiogenesis inhibition and impaired tumor growth and metastasis [8, 46, 74]. Indeed, in their incorporated state tumor recruited EPCs secreted numerous proangiogenic factors [8], suggesting that in addition to providing structural support to nascent vessels, EPCs have paracrine roles that may be essential in endothelial cell migration and proliferation at an early stage of tumor growth. Indeed, recent studies have begun to set forward a notion that paracrine signaling by specific population of cells that comprise the tumor microenvironment have significant biological effects. For example, depletion of myeloid cells-derived VEGF caused vasculature normalization even when abundant sources of VEGF were present in the tumor microenvironment [75]. Similarly, endothelial cell-autonomous VEGF was shown to be critically required for the homeostasis of blood vessels and not the abundant extracellular VEGF (Lee et al., 2007). In other studies, blocking recruitment of specific subsets of BM-derived cells such as neutrophils, macrophages, pericyte progenitors, Tie2+ monocytes had dramatic effects in tumor progression [24, 40, 46].
Tumor promoting functions of EPCs have been inferred from several clinicopathological studies in which a high number of these cells in the peripheral blood correlated with increased angiogenesis and metastasis associated with reduced patient survival. Many studies provide insights into how mobilization and recruitment of BM-derived cells impact existing clinical practice. For example, there are preclinical and clinical data suggesting that administration of certain chemotherapy drugs is associated with an increase in levels of BM-derived EPCs and VEGFR1+ cells that stimulate tumor progression and metastasis [52, 76, 77]. Therefore, blocking recruitment of these cells may be essential to overcome their negative consequences in patient survival and thereby achieve the full anticancer potential of the chemotherapy drug. Often, recombinant granulocyte colony-stimulating factor (G-CSF) is used for cancer patients with myelosuppression induced by chemotherapy. However, G-CSF promotes tumor angiogenesis by increasing circulating EPCs and Gr1+CD11b+ cells [72, 78]. Thus, caution must be exercised in using G-CSF in cancer patients with residual tumors. In summary, new therapies aimed at targeting the recruitment of cells from the BM into the tumor bed has considerable benefits in combination with either chemotherapy or targeted therapeutics.
The provocative findings from these studies raise additional questions that need to be answered. First, what is the hierarchical organization of the EPC lineage in the context of the hematopoietic system in the adult? Second, can reliable culture and expansion methods be developed to address the origin and functional definition of EPCs. Third, do EPCs have the potential to engraft the bone marrow compartment following BMT, or are EPCs derived from a hematopoietic cell (or hemangioblast). Fourth, are EPCs necessary only for initiating neovessel formation or also for their maintenance? Finally, it is important to determine whether local organ-restricted resident progenitors also contribute to EPC population. To move forward, it is first necessary to identify EPC-specific markers and gene promoters so that direct single cell lineage tracing can be performed to unravel their true identity in a physiological setting.
Table 1
Contribution of BM-derived EC in tumor neovessels in various studies
| Ref. | Tumor model | Tumor stage | % of contribution | BM tracking | EC Marker | BM-EC detection | No. of BM cells transplanted | |
|---|---|---|---|---|---|---|---|---|
| Asahara et al 1999 | Colon Cancer (MCA 38) s.c. | 1–3 weeks | positive | Flk-lacZ or Tie2-LacZ | IHC | 2×106 BM mononuclear cells | ||
| Lyden et al. 2001 | Lymphoma cell (B6RV2) s.c. | 2 weeks | 90% | Rosa26-LacZ | vWF | IHC | 1×106 BM mononuclear cells | |
| Davidoff et al. 2001 | Neuroblasma cell (NXS2) s.c. | - | 5% | BM cells transduced with MSCV-tsFIk-l-I-GFP | CD31 and CD34 | IF | 2×106 modified BM cells without RBC | |
| Reyes et al. 2002 | Lewis Lung Carcinoma (LLC) s.c. | 2 weeks | 35% | Human β2-microglobulin | CD31 and vWF | IF | 0.25×106 human MAPC–derived endothelial cells | |
| De Palma et al. 2003 | LLC, s.c., Melanoma (B16) s.c. | Late stage | neg | Tie2-GFP | CD31 | IF, 3D microscopy | 1×106 Lin− BM cells | |
| Garcia-Barros et al. 2003 | Fibrosarcomas (MCA/129) s.c. | 2 weeks | 50% | Rosa26-LacZ | vWF | IHC | 1×107 BM cells | |
| Ruzinova et al. 2003 | Pten+/− mouse | Uterine carcinoma | spontaneous | 16% | Rosa26-LacZ | CD31 | IHC | 1×106 BM mononuclear cells |
| lymph hyperplasia | neg | |||||||
| Dwenger et al. 2004 | MMTV-PyMT mouse | 10–12 week s | 1.3% | Rosa26-LacZ | CD31 | IHC | 2–5×106 BM cells | |
| Rajantie et al. 2004 | B16F1 s.c. | 2–3 weeks | neg | Actb-GFP | CD31 and vWF | IF | 2×106 BM cells | |
| Goethert et al. 2004 | LLC s.c., B6RV2 s.c. | 2 weeks | neg | Endothelial-SCL-Cre-ER× R26R or R26R-EYFP | CD31 | IHC, FACS | 2×106 BM cells | |
| Spring et al. 2005 | RIP1-Tag5 mice AlbTag | 10–16 weeks | 3–38% | Tie2Cre×RAGE-EGFP | Lectin-perfusion, CD31 | IF, FACS | 2×106 BM cells | |
| Peters et al. 2005 | Various human tumors | - | 1–12% | X or Y Chromosome | vWF | FISH | ||
| Duda et al. 2006 | LLC s.c., B16 s.c., Breast cancer cell (MCa8) orth. | - | <1% | Actb-GFP, Tie2-GFP | CD31 | IF, FACS | 5×106 BM cells | |
| Nolan et al. 2007 | LLC s.c., B16F0 (ortho); MMTV-PYMT mouse | Several time points in first 2 weeks | 2–20% | Actb-GFP | CD31, VE-cadh, Lectin-perfusion | FACS, 3D microscopy | 1×107 BM cells | |
| Gao et al. 2008 | LLC s.c., MMTV-PYMT mouse | Lung metastasis | 12% | Actb-GFP | CD31 | IF, FACS | 1×107 BM cells | |
| Purhonen et al. 2008 | B16 s.c. | 2–3 weeks | neg | Actb-GFP, VEGFR2-LacZ | CD31, vWF | IF | 7×106 BM cells | |
s.c.: subcutaneous injection; ortho.: orthotropic injection; IF:immunofluorescence; IHC: immunohistochemistry; FACS: flouorescent activated cell sorting; neg: negative; RAGE: receptor for advanced glycated end products
Acknowledgements
The authors thank some investigators for sharing their unpublished work. We acknowledge that space limitations might have precluded the citation of some excellent published work. We acknowledge support from National Institute of Health and the Robert Goldman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.bbcan.2009.05.001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3649840?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.bbcan.2009.05.001
Article citations
The crosstalk between lung cancer and the bone marrow niche fuels emergency myelopoiesis.
Front Immunol, 15:1397469, 01 Aug 2024
Cited by: 0 articles | PMID: 39148724 | PMCID: PMC11324509
Review Free full text in Europe PMC
Angiogenesis and Ovarian Cancer: What Potential Do Different Subtypes of Circulating Endothelial Cells Have for Clinical Application?
Int J Mol Sci, 25(11):6283, 06 Jun 2024
Cited by: 0 articles | PMID: 38892471 | PMCID: PMC11172689
Review Free full text in Europe PMC
Aberrant tumor vasculature. Facts and pitfalls.
Front Pharmacol, 15:1384721, 21 Mar 2024
Cited by: 0 articles | PMID: 38576482 | PMCID: PMC10991687
Review Free full text in Europe PMC
Cell Therapy of Vascular and Neuropathic Complications of Diabetes: Can We Avoid Limb Amputation?
Int J Mol Sci, 24(24):17512, 15 Dec 2023
Cited by: 2 articles | PMID: 38139339 | PMCID: PMC10743405
Angiogenesis in Lung Cancer: Understanding the Roles of Growth Factors.
Cancers (Basel), 15(18):4648, 20 Sep 2023
Cited by: 7 articles | PMID: 37760616 | PMCID: PMC10526378
Review Free full text in Europe PMC
Go to all (108) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Using the transcription factor inhibitor of DNA binding 1 to selectively target endothelial progenitor cells offers novel strategies to inhibit tumor angiogenesis and growth.
Cancer Res, 70(18):7273-7282, 31 Aug 2010
Cited by: 31 articles | PMID: 20807818 | PMCID: PMC3058751
Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis.
Science, 319(5860):195-198, 01 Jan 2008
Cited by: 415 articles | PMID: 18187653
Decursin inhibits vasculogenesis in early tumor progression by suppression of endothelial progenitor cell differentiation and function.
J Cell Biochem, 113(5):1478-1487, 01 May 2012
Cited by: 12 articles | PMID: 22298358
EPCs and pathological angiogenesis: when good cells go bad.
Microvasc Res, 79(3):207-216, 25 Feb 2010
Cited by: 61 articles | PMID: 20188747 | PMCID: PMC3650470
Review Free full text in Europe PMC





