Abstract
Free full text

Neutrophil-Derived IL-6 Limits Alveolar Barrier Disruption in Experimental Ventilator-Induced Lung Injury1
Abstract
IL-6 is a biological marker of ventilator-associated lung injury that may contribute to alveolar barrier dysfunction in acute respiratory distress syndrome. To determine whether IL-6 affects alveolar barrier disruption in a model of ventilator-induced lung injury, we examined alveolar barrier albumin flux in wild-type (WT) mice given an IL-6-blocking Ab (IL6AB) and mice deficient in IL-6 (IL6KO). Albumin flux was significantly higher in mice given IL6AB compared with mice given a control Ab. Unexpectedly, albumin flux was similar in WT and IL6KO mice. To examine the mechanisms for these findings, lung neutrophil accumulation (myeloperoxidase activity) was compared, revealing a correlation between lung neutrophil accumulation and albumin flux. IL6AB mice had significantly more lung neutrophils than WT and IL6KO mice, which were similar. Therefore, to determine whether the cellular source of IL-6 influences neutrophil accumulation and alveolar barrier function, chimeric mice were compared. WT/KO chimeras (WT mice with IL6KO hematopoietic cells) showed significantly greater albumin flux and neutrophil accumulation with mechanical ventilation than WT/WT mice. Neutrophil depletion decreased albumin flux in WT and WT/KO mice. IL6KO neutrophils were more adherent in an in vitro assay compared with WT neutrophils. IL-6 from a hematopoietic cell source limits alveolar barrier disruption potentially by reducing neutrophil contact with the endothelium. Modulation of IL-6 signaling in a cell type-specific fashion may be a therapeutic target for patients with acute lung injury.
Acute lung injury, including the acute respiratory distress syndrome (ARDS),3 is a common cause of respiratory failure characterized by alveolar barrier dysfunction (1). Although treatment options for patients with acute lung injury are limited, one intervention associated with decreased mortality in patients requiring mechanical ventilation is tidal volume reduction (2). The mechanisms for the beneficial effects of lower tidal volume breathing in this patient population are incompletely understood, but previous clinical trails have identified associations between plasma IL-6 levels and ventilator-associated lung injury (3).
Several clinical studies have reported an association between plasma IL-6 levels and adverse outcomes, including mortality and organ failure, in patients with acute lung injury (2–4). In the ARDS clinical trials network study of lower tidal volume compared with higher tidal volume ventilation in patients with acute lung injury, plasma IL-6 levels positively correlated with mortality (2). Lower tidal volume ventilation was associated with a decrease in IL-6 consistent with a causal role for IL-6 in ventilator-associated lung injury (3). In contrast, another clinical study of trauma patients with acute lung injury found that higher plasma IL-6 levels were associated with lower mortality (5). It remains uncertain whether IL-6 levels are merely a marker of lung injury or whether IL-6 contributes to the pathogenesis of ventilator-associated lung injury.
Experimental studies suggest a complex role for IL-6 in lung injury. In some experimental models, IL-6 prevents lung injury. For example, in an aerosolized endotoxin model of lung injury, IL-6 decreased levels of TNF-α, MIP-2, and airspace neutrophils (6). Similarly, IL-6-blocking Abs increased lung protein permeability in a model of IgG-mediated lung injury (7). In another study, transgenic mice overexpressing IL-6 in the lung were protected from hyperoxia-mediated lung injury (8). However, the effect of IL-6 on alveolar barrier function may be dependent on the mechanism of lung injury. For example, in a mouse model of lipoteichoic acid-induced lung injury, IL-6 attenuated lung inflammation, but in the same study IL-6 null mice were protected from peptidoglycan-mediated lung injury (9). Other experimental studies support a deleterious role for IL-6 in acute lung injury. In a mouse sepsis model, IL-6 signaling was associated with increased mortality and increased lung complement 5a receptor expression (10), an effect expected to increase lung neutrophil recruitment. A possible explanation for these conflicting results is that the cellular source of IL-6 may influence measures of injury. For example, IL-6 knockout (IL6KO) mice are highly susceptible to Listeria monocytogenes infection and infusion of rIL-6 reverses this susceptibility (11, 12). Because neutrophil-depleted wild-type (WT) and IL6KO mice have similar susceptibility to L. monocytogenes, IL-6 signaling in neutrophils may be critical to infection prevention (11).
This study was undertaken to test the hypothesis that IL-6 is a mediator of alveolar barrier dysfunction in a mouse model of ventilator-induced lung injury (VILI). When initial studies revealed that mice treated with IL-6-blocking Ab were more susceptible to VILI, we investigated the effect of IL-6 on lung neutrophil recruitment and tested the hypothesis that neutrophil-derived IL-6 limits alveolar barrier dysfunction in this neutrophil-dependent model of lung injury using WT and IL6KO chimera mice.
Materials and Methods
Lung injury model and study groups
These protocols were approved by the University of California, San Francisco and San Francisco Veterans Affairs Medical Center Institutional Animal Car and Use committee and are consistent with National Institutes of Health guidelines on the use of research animals. C57BL/6 mice (20–25 g) were anesthetized with ketamine/xylazine following induction anesthesia with 4% isoflurane. Lung injury was induced using a previously described protocol (13). Briefly, the trachea was cannulated and mechanical ventilation was initiated with a peak airway pressure of 20 cm of H2O and an end expiratory pressure of 0 cm of H2O. Ventilation was continued for 3 h. Anesthesia was maintained with ketamine/xylazine, muscle relaxation was maintained with pancuronium, and mice received 0.25 ml of saline i.p. every hour. For these studies, mice were given IL-6-blocking Ab (2.5 mg/kg, i.v.) or a control Ab of the same isotype immediately before mechanical ventilation, or recombinant mouse IL-6 (rmIL-6; 4 μg/kg, i.v.) 2 h and immediately before mechanical ventilation. Additional groups of ventilated and spontaneously breathing control WT mice and IL-6-deficient mice (IL6KO) were also compared.
Alveolar barrier protein flux
Alveolar endothelial and epithelial permeability to protein were measured as the flux of radiolabeled albumin from the circulation to the interstitium and airspaces as previously described (13, 14). Briefly, mice were given 0.5 μCi of 125I-labeled albumin i.p. 2 h before the ventilation protocol. At the end of the experiments, a plasma sample was collected and the ratio of radioactivity in the blood-free lung to the plasma normalized to plasma volume is reported as extravascular plasma equivalents (EVP%).
Plasma IL-6 levels, cell counts, myeloperoxidase (MPO) assay, and histology
IL-6 levels were measured using a species-specific ELISA (Pierce Bio-technology) in plasma collected at the conclusion of select experiments. At the conclusion of select experiments, bronchoalveolar lavage (BAL) was done using 0.5 ml of PBS and recovered fluid used to determine differential cell counts. In separate studies, whole lung MPO activity was measured as a marker of neutrophil accumulation in the lung as previously reported (15, 16). For histology, lungs were fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with H&E.
Chimeric mice
Lethally irradiated WT and IL6KO mice were given bone marrow-derived WT or IL6KO hematopoietic cells to generate WT mice transplanted with WT bone marrow, (WT/WT), IL6KO mice transplanted with IL6KO bone marrow (KO/KO), WT mice transplanted with IL6KO bone marrow (WT/KO), and IL6KO mice transplanted with WT bone marrow (KO/WT). Briefly, bone marrow cells were collected from femurs of WT or IL6KO mice, washed in PBS, and RBC lysed with Red Blood Cell Lysing Buffer (Sigma-Aldrich) for 10 min. The remaining bone marrow cells were then centrifuged at 1500 × g and cell pellets were recovered in PBS. Anesthetized mice, irradiated with a divided dose of 14 Gy, were injected i.v. with 4 × 106 bone marrow cells suspended in 200 μl of PBS. Using this method, 85–95% of circulating hematopoietic cells were from donor marrow when mice were used in experiments 4–6 wk after transplantation. For neutrophil reconstitution studies, 106 bone marrow-derived neutrophils isolated from bone marrow by Percoll centrifugation (17) were injected via the internal jugular vein at the start of mechanical ventilation. In neutrophil depletion studies, mice were given either anti-Gr-1 Ab or an isotype control IgG Ab. In separate studies, WT mice were reconstituted with bone marrow from GFP-expressing transgenic mice and BAL was done 4 wk later. The percent GFP-positive macrophages in the BAL fluid was determined by FACS sorting.
Neutrophil adhesion and migration assays
Bone marrow-derived neutrophils from WT or IL6KO mice were isolated by Percoll gradient centrifugation (17) and suspended at 3.5 × 104 cells/ml in RPMI 1640 and plated on low-adhesion plastic dishes as previously described (18). After 2 h at 37°C and 5% CO2, plates were washed with PBS and the average number of cells in five ×40 fields per well was determined. Some cells were incubated for 30 min with rmIL-6 (10 ng/ml) before plating.
The movement of neutrophils through cultured human pulmonary microvascular endothelial cells (ScienCell) was measured as previously described, with a slight modification (19). Briefly, 106 neutrophils isolated from bone marrow as above were labeled with calcein-AM (Invitrogen) and plated in the apical chamber of Transwells with confluent monolayers of human pulmonary microvascular endothelial cells in serum-free conditions. Endothelial cells were plated at 105 cells per well and grown to confluence in complete growth medium for 3 days before migration studies. FMLP (10−5 M) (Sigma-Aldrich) was added to the basolateral compartment and the number of neutrophils in the basolateral compartment was determined as 480 nm fluorescence compared with a standard curve 1 h later. Data are expressed as an index of the ratio to unstimulated control wells. In some wells, rmIL6 (10 ng/ml) was added to both the apical and basolateral chambers just before the migration experiment. In separate wells, polymorphonuclear leukocytes of each genotype were incubated with rmIL6 (10 ng/ml) for 35 min and then washed twice in PBS-calcium and magnesium free before the migration experiments.
Effect of IL-6 on epithelial albumin flux and transepithelial electrical resistance
The effect of IL-6 on albumin permeability (flux) in primary rat alveolar type II cells was measured following 3 h of IL-6 exposure (1–100 ng/ml). Primary rat cells were isolated as previously reported and grown to confluence in Transwells. Following exposure to IL-6 in serum-containing medium, transepithelial electrical resistance was measured with a voltohm meter and the flux of FITC-labeled albumin was determined by adding 100 μg/ml albumin to the basolateral compartment of the Transwell and measuring fluorescence in the apical chamber 1 h later.
Statistics
Data are reported as mean and SD or SE where indicated. Differences between groups were compared using ANOVA and post hoc the Student-Newman-Keuls test for multiple comparisons. Within-group differences were compared using paired t tests. Comparisons of nonparametric data were made using the Mann-Whitney U test. Values of p < 0.05 were considered significant.
Results
Effects of blocking IL-6 and IL-6 pretreatment on alveolar barrier disruption in VILI
To study the role of IL-6 in VILI, we measured alveolar barrier protein flux in C57BL/6 (WT) mice treated with an IL-6-blocking Ab. We found that mice treated with an IL-6-blocking Ab had higher alveolar barrier protein flux (Fig. 1). Pretreatment of mice with rmIL-6 decreased the severity of alveolar barrier disruption (Fig. 1). These findings suggest IL-6 limits alveolar barrier disruption in VILI.
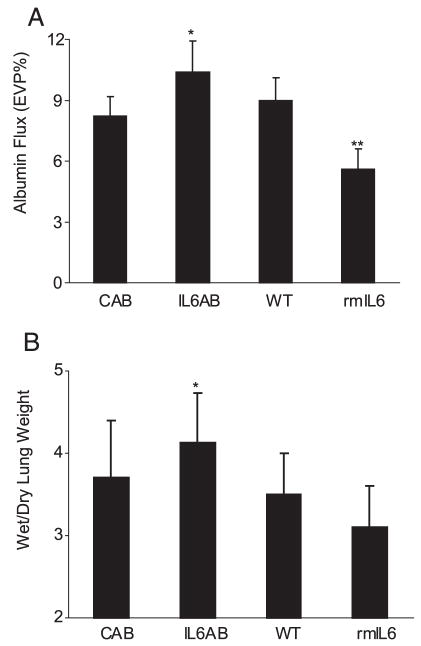
Effect of IL-6 blockade and IL-6 administration on lung alveolar barrier albumin flux in VILI. A, IL-6-blocking Ab (2.66 mg/kg i.v.) (IL6AB) resulted in significantly higher alveolar barrier protein permeability compared with isotype control Ab-treated mice (CAB) (*, p < 0.05; n = 6 in each group) as measured by albumin flux (expressed as the percentage of vascular volume in the extravascular spaces of the lung, EVP%) in the VILI model. Pretreatment with rmIL-6 significantly reduced albumin flux (n = 7; **, p < 0.05) compared with untreated WT controls (n = 9). B, The wet:dry lung weight ratio was significantly higher in IL-6-blocking Ab-treated mice compared with control Ab-treated mice (*, p < 0.05).
Effects of genetic deletion of IL-6 on alveolar barrier disruption in VILI
To confirm a role for IL-6 in VILI, we next compared alveolar barrier protein flux in WT and IL-6-deficient (IL6KO) mice. Baseline albumin flux without mechanical ventilation was higher in WT mice than in IL6KO mice (3.8 ± 0.9 vs 2.8 ± 1.2% (EVP%), p < 0.05, n = 9–11/group). Surprisingly, lung albumin flux was similarly increased in IL6KO and WT mice subjected to the VILI model (Fig. 2). Pretreatment with rmIL-6 decreased alveolar barrier albumin flux to a similar level in both WT and IL6KO mice. Histology showed similar changes in cellularity and edema in WT and IL6KO mice, but decreased cellularity and edema following rmIL-6 pretreatment (Fig. 3). Lung wet:dry weights were lower in mice treated with rmIL6 compared with IL6-deficient mice (p < 0.05; Fig. 2B). Lung compliance decreased more in IL-6-deficient mice compared with both WT mice and rmIL6-treated mice, which showed a significantly smaller decrease in compliance compared with the other two groups (compliance decreased 4.4 (1.4) μl/cm H2O in WT mice, 6.3 (2.5) μl/cm H2O in IL6KO mice, and 2.8 (1.6) μl/cm H2O in rmIL6-treated mice (p < 0.05 for WT and rmIL6 compared with IL6KO).
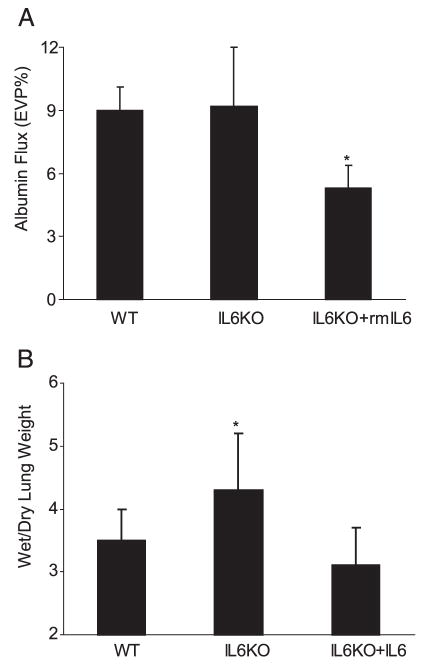
Lung alveolar barrier albumin flux in IL6KO mice. A, Protein permeability in IL-6-deficient mice (IL6KO) was similar to that of WT C57BL/6 mice (n = 9 in each group). Pretreatment of IL6KO mice with rmIL-6 before lung injury significantly reduced alveolar barrier protein flux (n = 7; *, p < 0.05). B, The lung wet:dry weight ratio was significantly higher in IL6KO mice compared with the other groups (p < 0.05).
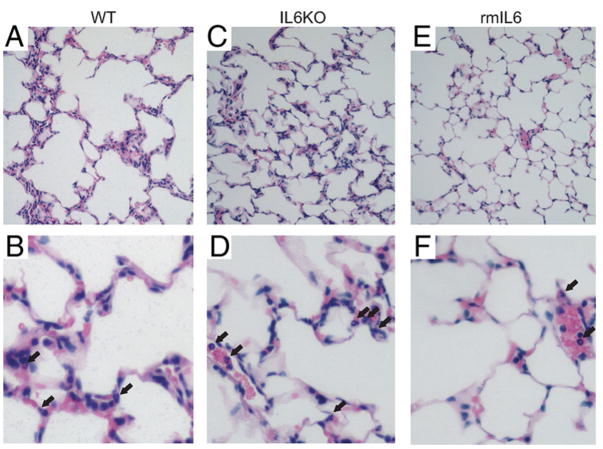
Histology from ventilator-injured lungs. Low (×10 objective, A, C, and E) and high-power (×40 objective, B, D, and F) views of mouse lungs following VILI. Pulmonary edema and increased cellularity including neutrophils (arrows) are apparent in lungs from both WT and IL6KO mice. IL-6 pretreatment (rmIL-6) decreased edema and neutrophil infiltration in IL6KO (E and F) and WT mice (data not shown).
Airspace neutrophil counts and lung MPO activity in WT and IL6KO mice
BAL fluid neutrophil counts were lower in IL6KO mice compared with WT mice; however, lung MPO activity was similar in both groups (Fig. 4). These data suggest that although the total number of neutrophils in the airspace and lung parenchyma was similar in the two groups, more neutrophils remained sequestered or adherent in the IL6KO mice. IL-6 pretreatment decreased BAL cell counts and MPO activity. IL6AB resulted in increased lung neutrophil accumulation, but similar to IL6KO mice, IL6AB decreased BAL neutrophil counts. These findings are consistent with IL-6 acting to limit neutrophil adhesion or promote neutrophil migration.
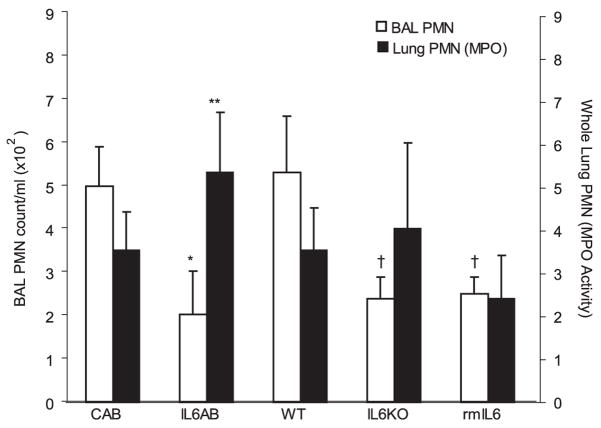
Effect of IL-6 on lung neutrophil accumulation in VILI. VILI resulted in a significant increase in lung MPO activity and airspace neutrophils (PMN). Whole lung MPO activity, a measure of total neutrophil sequestration in the lung (■), was increased in IL-6-blocking Ab-treated (IL6AB) mice compared with control Ab-treated (CAB) mice, but similar in WT and IL6KO mice (n = 6 in each group; **, p < 0.05). BAL fluid neutrophil counts were significantly lower in IL6AB mice than in control Ab-treated mice (□; *, p < 0.05, n = 5 in each group) and lower in IL6KO mice and IL6-treated mice than in WT mice (†, p < 0.05, n = 5 in each group). Therefore, the ratio of total lung neutrophils to airspace lavage neutrophils was higher in IL6AB mice and IL6KO mice than in control Ab-treated and WT mice.
Alveolar barrier protein flux in WT/IL6KO chimera mice in VILI
To further explore whether the cellular source of IL-6 influences the effect on alveolar barrier disruption in VILI, WT mice were irradiated and reconstituted with bone marrow from IL6KO mice (WT/KO) or bone marrow from WT mice (WT/WT). WT/KO mice showed significantly greater alveolar barrier protein leak than WT/WT mice, which were comparable to nonirradiated WT mice, KO/WT, and KO/KO. (Fig. 5A). Baseline EVP% in WT/WT mice was 3.8 ± 0.8 and 3.0 ± 0.6 in WT/KO mice (p > 0.05, n = 6 in each group). Therefore, the absence of IL-6 exclusively in hematopoietic cells resulted in increased barrier leak.

Neutrophils and alveolar barrier protein permeability. A, WT mice lethally irradiated and reconstituted with bone marrow-derived hematopoietic cells from IL-6 null mice (WT/KO) had significantly higher lung albumin flux (expressed as EVP%) than WT mice reconstituted with WT bone marrow (WT/WT); *, p < 0.05, n = 8–9 in each group. KO/KO and KO/WT were similar to WT/WT mice. Therefore, the absence of IL-6 in hematopoietic cells only resulted in greater alveolar barrier disruption. B, Infusion of 106 WT bone marrow-derived neutrophils into WT/WT mice had no effect on lung protein permeability measured as albumin flux across the alveolar barrier compared with WT/WT mice. However, infusion of WT neutrophils into WT/KO mice resulted in a decrease in lung protein permeability to a level comparable to that of WT/WT mice. C, Depletion of neutrophils with Gr-1 Ab significantly decreased albumin flux in both WT and WT/KO mice. These data support a role for differences in neutrophil-mediated alveolar barrier dysfunction in the chimeric mice.
Reconstitution of WT/KO chimera mice with WT neutrophils
Infusion of 106 bone marrow-derived neutrophils into WT/WT mice (WT/WT/WT) had no effect on alveolar barrier albumin flux. However, the infusion of WT neutrophils to WT/KO mice (WT/KO/WT) significantly decreased alveolar barrier albumin flux to a level comparable to that of WT/WT mice (Fig. 5B). Infusion of WT neutrophils into nonirradiated WT mice did not affect alveolar barrier permeability (data not shown). Therefore, infusion of neutrophils expressing IL-6 is sufficient to restore the WT phenotype in WT/KO chimeric mice.
Effect of neutrophil depletion on lung injury in WT and WT/KO chimera
Anti Gr-1 Ab decreased peripheral neutrophils >95%. WT mice given Gr-1 Ab showed decreased lung albumin flux with VILI compared with isotype control Ab-treated mice (n = 4 in each group; Fig. 5C). WT/KO mice given the Gr-1 Ab showed a proportional decrease in alveolar barrier albumin flux compared with control Ab-treated chimeras (n = 3 in each group; Fig. 5C). These data show that neutrophils are the major mediators of injury in this VILI model.
Airspace cell counts and lung MPO activity in WT/WT and WT/KO chimeras
Total lung neutrophil accumulation as measured by lung MPO activity was significantly higher in WT/KO than in WT/WT mice (Fig. 6). Similar to IL6KO mice, WT/KO mice had fewer airspace neutrophils than WT/WT mice. This finding supports the hypothesis that hematopoietic cell IL-6 limits neutrophil adhesion or promotes migration in the lung. Airspace neutrophil counts in KO/KO mice were comparable to those of IL6KO mice (2.6 ± 0.8 vs 2.4 ± 0.5, n = 5 in each group). Airspace neutrophil counts in KO/WT mice were comparable to those of WT mice (4.8 ± 2.2 vs 5.3 ± 1.3, p > 0.05, n = 5 in each group). In nonventilated WT mice reconstituted with GFP-positive bone marrow, 85 ± 5% of BAL cells were GFP positive, indicating that airspace macrophages were not entirely replaced with the protocol used in this study.

Lung neutrophil accumulation in chimera mice. Whole lung MPO activity, a measure of neutrophil sequestration in the lung, was significantly higher in WT/KO mice than in WT/WT mice (■; *, p < 0.05, n = 4–5 in each group). BAL fluid neutrophil counts following VILI were significantly lower in WT/KO mice than in WT/WT mice (□; **, p < 0.05, n = 6–7 in each group). Accordingly, the ratio of total lung neutrophils to airspace lavage neutrophils was higher in WT/KO mice.
Neutrophil adhesion and migration
To determine whether there are differences in neutrophil adhesion between WT and IL6KO neutrophils, adhesion to plastic was compared in a widely used assay (18). IL6KO neutrophils were more adherent to plastic than WT neutrophils (Fig. 7A). Coincubation with rmIL-6 decreased adhesion of neutrophils isolated from IL6KO mice. Incubation of WT neutrophils with rmIL-6 had no significant effect on adhesion to plastic in this assay. In a migration assay, fewer IL6KO than WT neutrophils moved through the lung microvascular endothelial cell monolayer in response to FMLP (Fig. 7B) and incubation of neutrophils with rmIL-6 restored IL6KO neutrophil migration to a level comparable to that of WT neutrophils. The addition of rmIL6 to the apical and basolateral chambers just before the migration experiment resulted in decreased neutrophil migration in both WT and KO neutrophils.
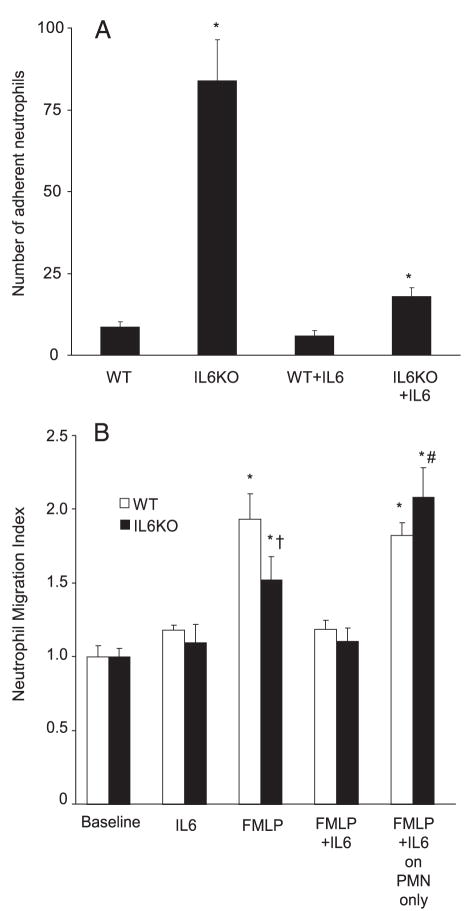
Adhesion and transendothelial migration in IL6KO bone marrow-derived neutrophils in vitro. A, Neutrophils from IL6KO mice were significantly more adherent to low-adhesion plastic than WT neutrophils (*, p < 0.05 compared with all other groups; nine replicates of eight wells each per condition. Data are mean ± SEM of the mean number of cells per ×40 field). Note that incubation with rmIL-6 before plating resulted in a significant decrease in adhesion in IL6KO neutrophils, but not WT neutrophils. B, FMLP increased neutrophil migration through primary human pulmonary microvascular endothelial cells (*, p < 0.05 compared with baseline). IL-6 did not affect neutrophil migration in the absence of FMLP. However, fewer IL6KO neutrophils migrated through human primary pulmonary microvascular endothelial cell monolayers in response to FMLP stimulation (†, p < 0.05 compared with FMLP-stimulated WT neutrophils; three to six replicates of three wells each). Incubation of IL6KO neutrophils with IL6 before migration (10 ng/ml) increased the number of migrating neutrophils to a level comparable to that of WT neutrophils (far right). The addition of IL-6 to endothelial cells just before and during neutrophil migration decreased migration in response to FMLP in neutrophils of both genotypes (*, p < 0.05 compared with baseline and #, p < 0.05 compared with FMLP only with IL6KO neutrophils).
Effect of IL-6 on epithelial permeability
The effect of IL-6 on albumin permeability (flux) in primary rat alveolar type II cells was measured following 3 h of IL-6 exposure. IL-6 had no significant effect on alveolar epithelial albumin flux. Albumin flux normalized to control (no IL-6) was 1.0 ± 0.1, 1.2 ± 0.25, and 1.4 ± 0.1 for a dose range of 1, 10, and 100 ng/ml, respectively (mean ± SEM, p > 0.05, n = three replicates of three wells each). Similarly, IL-6 had no effect on transepithelial electrical resistance in primary rat alveolar type II epithelial cells over 3 h. Transepithelial electrical resistance was 1434 ± 309 Ohm · cm2 in control cells and 1363 ± 245 Ohm · cm2 in IL-6-treated cells (p > 0.05). These data indicate that IL-6 does not directly influence permeability of the epithelial cell barrier.
IL-6 plasma levels
Circulating IL-6 levels were not different in WT, WT/WT, WT/KO, or rmIL-6-pretreated IL6KO mice (Table I). IL-6 levels were lower in KO/WT mice compared with WT/WT and WT/KO mice (p < 0.05). WT/WT/WT mice and WT/KO/WT mice had similar IL-6 plasma levels (Table I). IL-6 levels were undetectable in IL-6 KO mice (data not shown). Baseline IL-6 levels (mean 95% confidence interval) in unventilated WT mice (156 (112) pg/ml) were not significantly different compared with unventilated WT/WT mice or WT/KO mice (17 (34) and 194 (173) pg/ml, respectively, n = 3–6 in each group).
Table I
Plasma IL-6 levels in VILIa
| WT | WT/WT | WT/KO | KO/WT | KO + rmIL-6 | WT/WT/WT | WT/KO/WT | |
|---|---|---|---|---|---|---|---|
| Mean (95% CI) (pg/ml) | 3034 (2008) | 1222 (597) | 1590 (952) | 156b (83) | 3277 (3183) | 2558 (2216) | 1577 (1204) |
Discussion
The primary objective of this study was to determine the role of IL-6 in the pathogenesis of VILI with a focus on alveolar barrier dysfunction, a hallmark of ARDS and an important determinant of outcome in ARDS patients (1, 20). Our initial hypothesis was that IL-6 blockade would prevent VILI. Contrary to this hypothesis, we found that mice treated with IL-6-blocking Ab were more susceptible to alveolar barrier disruption during high tidal volume ventilation and that pretreatment with IL-6 reduced lung protein permeability. These data support a protective role for IL-6 in VILI.
To confirm a role for IL-6 in VILI, we subjected WT and IL6KO mice to the VILI model and found that lung protein permeability was similar. To explore the mechanisms for this unexpected finding, we examined lung MPO activity as a measure of lung neutrophil accumulation as well as BAL neutrophil counts. Total lung neutrophil accumulation was the same in the WT and IL6KO mice, but airspace neutrophil counts were lower in IL6KO mice, indicating a greater proportion of lung neutrophils were sequestered in vasculature, adherent, or had impaired migration in IL6KO mice. IL-6-blocking Ab-treated mice had greater lung neutrophil accumulation, but airspace neutrophil counts were comparable to IL6KO mice, indicating more neutrophils were in the lung, but fewer entered the airspace. Because the IL-6-blocking Ab dose was selected to completely suppress IL-6 levels and the KO mice lacked IL-6 expression, the effect of partial IL-6 inhibition in this model is uncertain and may differ from the effect of complete blockade as has been observed in other models (10). Although it is possible that the IL-6-blocking Ab may have effects not controlled for by the isotype-specific Ab control, together these findings point to a role for IL-6 in limiting alveolar barrier neutrophil migration during VILI.
We then tested the hypothesis that the difference between the blocking Ab data and the IL6KO data may be attributable to differences in cellular sources of IL-6. Using WT and KO chimeric mice, we found that WT mice transplanted with IL6KO hematopoietic cells (WT/KO) were more susceptible to alveolar barrier disruption than WT mice transplanted with WT hematopoietic cells (WT/WT), which did not differ from nonirradiated WT mice. These observations indicate a hematopoietic cell source of IL-6 limits alveolar barrier disruption in VILI. However, because the alveolar macrophage replacement was 85% in the chimeras, an effect attributable to a small residual population of IL-6-expressing macrophages cannot be excluded. To further delineate the cellular source of IL-6 important to limiting alveolar barrier disruption in VILI, we infused WT neutrophils into WT/WT and WT/KO mice immediately before mechanical ventilation. Infusion of WT neutrophils decreased alveolar barrier protein permeability in WT/KO mice to a level comparable to that of WT/WT chimera mice. Therefore, an infusion of WT neutrophils was sufficient to restore the WT phenotype to WT/KO mice, implicating neutrophils the source of IL-6 important to limiting alveolar barrier disruption in VILI.
We then examined lung and airspace neutrophil accumulation in the chimera mice. Similar to IL-6-blocking Ab-treated mice, WT/KO mice also had greater lung neutrophil accumulation compared with WT/WT mice. However, as was observed in IL6KO mice, WT/KO mice had fewer airspace neutrophils than WT/WT mice, suggesting increased neutrophil sequestration, impaired adhesion, or decreased migration in the WT/KO mice. Because WT/KO mice lack IL-6 expression only in hematopoietic cells, these data support the hypothesis that hematopoietic cell IL-6 limits neutrophil contact with the endothelium in VILI and this results in a smaller increase in alveolar barrier protein permeability. Because VILI is a neutrophil-dependent form of lung injury and because WT/KO mice had more lung neutrophils and higher lung protein permeability, it is likely the differences in alveolar barrier permeability were attributable to neutrophil-dependent injury. When neutrophils were depleted in with Gr-1 Ab in WT mice, ventilator-induced alveolar barrier dysfunction was reduced. Neutrophil depletion resulted in a similar decrease in lung injury in WT/KO chimeric mice that lacked IL-6 expression in hematopoietic cells. Therefore, the majority of the effect of the lack of IL-6 in hematopoietic cells appears to be attributable to differences in neutrophil-dependent injury.
To determine whether there were differences in WT and IL6KO neutrophil adhesion, we compared neutrophil adhesion in an in vitro assay. Consistent with the in vivo studies, IL6KO neutrophils were more adherent than WT neutrophils and adding IL-6 decreased adhesion in IL6KO neutrophils. In addition, we assessed the effect of IL-6 expression on neutrophil migration through endothelial layers in response to FMLP. IL-6KO neutrophils showed a small but significant decrease in migration across endothelial cells compared with WT cells and the addition of IL-6 to neutrophils before the migration assay increased the number of migrating IL6KO neutrophils to a level comparable to WT cells. Coincubation of endothelial cells and neutrophils with rmIL6 prevented migration in neutrophils of both genotypes. These observations suggest that neutrophil IL-6 can regulate neutrophil adherence and migration through the endothelium. However, the effect of neutrophil IL-6 on migration in this assay was small and of uncertain biological importance. The net effect of IL-6 from all sources on neutrophil migration in vivo may not be modeled by this assay. Neutrophil adhesion to the endothelium and migration through the alveolar barrier is a complex, multistep process; the effect of IL-6 on adhesion molecules potentially important to the appearance of neutrophils in the airspaces was not addressed in this study. However, the absence of neutrophil IL-6 potentially results in prolonged neutrophil-endothelial contact and greater lung injury. This possibility is consistent with a recent study reporting that prolonged neutrophil-endothelial contact promotes lung injury in neutrophil elastase-deficient mice following mechanical ventilation (21). Further supporting an effect of IL-6 on neutrophils in this model is the finding that IL-6 alone did not affect alveolar epithelial albumin flux of transepithelial electrical resistance in vitro, suggesting that the effect of IL-6 on alveolar barrier protein permeability is not primarily due to a direct effect on epithelial cells.
Although these data indicate that IL-6 from hematopoietic cells limits lung neutrophil accumulation, they are also consistent with the possibility that IL-6 from nonhematopoietic cell sources plays a detrimental role in VILI. Specifically, lung neutrophil accumulation was lower in IL6KO mice compared with WT/KO mice. IL-6-blocking Ab as used in this study resulted in conditions more comparable to WT/KO mice than IL6KO mice. A potential explanation for these findings is that IL-6 may up-regulate proteins important to endothelial cell activation and neutrophil-endothelial cell adhesion in this model and that IL-6 from nonhematopoietic cells is sufficient for this effect (22). It is noteworthy that the specific proteins involved in transmigration of neutrophils across the alveolar barrier are somewhat specific to the mode of lung injury (23) and neutrophil transmigration was not specifically examined in vivo in this study.
Differences among the groups in alveolar barrier disruption were not explained by differences in circulating IL-6 levels as plasma IL-6 levels were not different in WT, WT/WT, WT/KO, or rmIL6-treated mice. IL-6 levels were lower in KO/WT mice compared with these other groups, but their lung albumin flux was similar to that of WT/WT mice. IL-6 was not measured in the plasma of KO/KO chimeras after ventilation, but because these are essentially identical to IL-6 KO mice, the levels are almost certainly undetectable. Airspace IL-6 levels were not measured in this study; therefore, it remains unresolved whether there were differences in airspace IL-6 levels among the groups. These data suggest that plasma IL-6 levels may be a poor measure of the IL-6 that plays a critical role in VILI. In addition, they suggest that the majority of circulating IL-6 during VILI is released from nonhematopoietic cells and not the hematopoietic cell source (likely neutrophils) that is responsible for the differences in alveolar barrier permeability measured in this study. Therefore, locally released IL-6 may modulate barrier properties or neutrophil-mediated injury in VILI and it is possible that neutrophil-derived IL-6 signals a down-regulation of alveolar barrier disruption in a spatially restricted fashion.
Clinical data have suggested a role for IL-6 in the pathogenesis of ventilator-associated lung injury (2–4), but a causal role for IL-6 in alveolar barrier disruption has not been reported. Data from the present study contradict the hypothesis that IL-6 mediates alveolar barrier disruption and support a protective role for hematopoietic cell-derived IL-6, which acts to decrease neutrophil adhesion, sequestration, and possibly migration in this lung injury model. These data raise the possibility that modulation of IL-6 production in a cell-specific fashion may be a therapeutic target for patients at risk for VILI.
Footnotes
1This work was supported by funding from the National Institutes of Health and National Heart, Lung and Blood Institute Grants HL088440 (to J.A.F.) and HL075026 (to P.J.W.).
3Abbreviations used in this paper: ARDS, acute respiratory distress syndrome; rmIL-6, recombinant mouse IL-6; WT, wild type; EVP%, extravascular plasma equivalent; VILI, ventilator-induced lung injury; BAL, bronchoalveolar lavage; MPO, myeloperoxidase.
Disclosures
The authors have no financial conflict of interest.
References
Full text links
Read article at publisher's site: https://doi.org/10.4049/jimmunol.0801323
Read article for free, from open access legal sources, via Unpaywall:
http://www.jimmunol.org/content/182/12/8056.full.pdf
Free to read at www.jimmunol.org
http://www.jimmunol.org/cgi/content/abstract/182/12/8056
Free after 12 months at www.jimmunol.org
http://www.jimmunol.org/cgi/content/full/182/12/8056
Free after 12 months at www.jimmunol.org
http://www.jimmunol.org/cgi/reprint/182/12/8056
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4049/jimmunol.0801323
Article citations
Metagenomic next-generation sequencing of bronchoalveolar lavage fluid from children with severe pneumonia in pediatric intensive care unit.
Front Cell Infect Microbiol, 13:1082925, 16 Mar 2023
Cited by: 6 articles | PMID: 37009495 | PMCID: PMC10064343
Respiratory issues in patients with multiple sclerosis as a risk factor during SARS-CoV-2 infection: a potential role for exercise.
Mol Cell Biochem, 478(7):1533-1559, 21 Nov 2022
Cited by: 0 articles | PMID: 36411399 | PMCID: PMC9684932
Review Free full text in Europe PMC
TNF-α Induces Neutrophil Apoptosis Delay and Promotes Intestinal Ischemia-Reperfusion-Induced Lung Injury through Activating JNK/FoxO3a Pathway.
Oxid Med Cell Longev, 2021:8302831, 29 Dec 2021
Cited by: 10 articles | PMID: 35003520 | PMCID: PMC8731283
The Lung-Brain Axis in Ventilator-induced Brain Injury: Enter IL-6.
Am J Respir Cell Mol Biol, 65(4):339-340, 01 Oct 2021
Cited by: 5 articles | PMID: 34153209 | PMCID: PMC8525199
Tocilizumab in the Management of COVID-19: A Preliminary Report.
Am J Med Sci, 361(2):208-215, 09 Nov 2020
Cited by: 6 articles | PMID: 33358502 | PMCID: PMC7649658
Go to all (41) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
NF-κB activation in myeloid cells mediates ventilator-induced lung injury.
Respir Res, 14:69, 03 Jul 2013
Cited by: 28 articles | PMID: 23822633 | PMCID: PMC3708752
The Modulation of Interferon Regulatory Factor-1 via Caspase-1-Mediated Alveolar Macrophage Pyroptosis in Ventilator-Induced Lung Injury.
Mediators Inflamm, 2022:1002582, 15 Apr 2022
Cited by: 2 articles | PMID: 35462787 | PMCID: PMC9033353
Activation of Src-dependent Smad3 signaling mediates the neutrophilic inflammation and oxidative stress in hyperoxia-augmented ventilator-induced lung injury.
Respir Res, 16:112, 16 Sep 2015
Cited by: 13 articles | PMID: 26377087 | PMCID: PMC4574227
TLR2 deficiency aggravates lung injury caused by mechanical ventilation.
Shock, 42(1):60-64, 01 Jul 2014
Cited by: 2 articles | PMID: 24667617
Funding
Funders who supported this work.
NHLBI NIH HHS (6)
Grant ID: HL075026
Grant ID: R01 HL088440-02
Grant ID: R56 HL088440
Grant ID: R01 HL088440
Grant ID: HL088440
Grant ID: R01 HL075026




