Abstract
Free full text

FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response
Associated Data
Abstract
The liver plays a crucial role in mobilizing energy during nutritional deprivation. During the early stages of fasting, hepatic glycogenolysis is a primary energy source. As fasting progresses and glycogen stores are depleted, hepatic gluconeogenesis and ketogenesis become major energy sources. Here, we show that fibroblast growth factor 21 (FGF21), a hormone that is induced in liver by fasting, induces hepatic expression of peroxisome proliferator-activated receptor γ coactivator protein-1α (PGC-1α), a key transcriptional regulator of energy homeostasis, and causes corresponding increases in fatty acid oxidation, tricarboxylic acid cycle flux, and gluconeogenesis without increasing glycogenolysis. Mice lacking FGF21 fail to fully induce PGC-1α expression in response to a prolonged fast and have impaired gluconeogenesis and ketogenesis. These results reveal an unexpected relationship between FGF21 and PGC-1α and demonstrate an important role for FGF21 in coordinately regulating carbohydrate and fatty acid metabolism during the progression from fasting to starvation.
In mammals, the liver plays a crucial role in maintaining systemic energy balance during fasting and starvation through coordinate effects on carbohydrate and lipid metabolism. During the early stages of fasting, the liver mobilizes glucose from its glycogen stores. As fasting progresses and glycogen reserves are depleted, the liver oxidizes fat to provide both energy for gluconeogenesis and substrate for ketogenesis. This synchronization of hepatic lipid and carbohydrate metabolism is critical for the normal fasting response; disruption of either one of these pathways has profound effects on the other (1–4).
Hormones such as glucagon, catecholamines, and glucocorticoids have important roles in controlling substrate utilization and maintaining energy balance during fasting. Recently, the hormone fibroblast growth factor 21 (FGF21) was shown to be induced in the liver during fasting (5–7). FGF21 is an unusual FGF family member in that it lacks the conventional heparin-binding domain (8) and thus can diffuse away from its tissue of origin and function as a hormone. FGF21 signals through cell-surface receptors composed of classic FGF receptors complexed with β-klotho, a membrane-spanning protein (9–14). Induction of FGF21 during fasting occurs through a mechanism that requires peroxisome proliferator-activated receptor α (PPARα) (5–7). FGF21 has diverse metabolic actions that include stimulating hepatic fatty acid oxidation and ketogenesis (5, 6, 15) and blocking the growth hormone signaling pathway (16). FGF21 also sensitizes mice to torpor, a short-term hibernation-like state of regulated hypothermia (6). Pharmacologic administration of FGF21 to insulin-resistant rodents and monkeys improves glucose tolerance and reduces plasma insulin and triglyceride concentrations (15, 17).
Peroxisome proliferator-activated receptor γ coactivator protein-1α (PGC-1α) is a transcriptional coactivator protein whose expression is induced in response to changes in nutritional status and other physiologic stimuli such as cold and exercise (18–21). PGC-1α is enriched in metabolic tissues, such as muscle and heart, where it interacts with multiple DNA-binding transcription factors to stimulate mitochondrial metabolic capacity. In liver, induction of PGC-1α by fasting stimulates the transcription of genes involved in fatty acid oxidation, tricarboxylic acid (TCA) cycle flux, mitochondrial oxidative phosphorylation, and gluconeogenesis (4, 22–24). Accordingly, these metabolic processes are impaired in mice in which PGC-1α function has been disrupted in liver (4, 25–28). In this article, we show that FGF21 induces PGC-1α and has marked effects on carbohydrate and lipid metabolism. Our findings reveal a prominent role for FGF21 in coordinating the adaptive metabolic response to starvation.
Results
FGF21 Stimulates a Fasting Metabolic State.
To examine how FGF21 affects liver metabolism, fatty acid and carbohydrate fluxes were measured by 2H/13C NMR isotopomer analysis in isolated liver from WT mice and transgenic mice overexpressing FGF21 in a liver-enriched manner (FGF21-TG) (6). As expected, hepatic oxygen consumption was increased in response to fasting in WT liver (Fig. 1A). Importantly, FGF21-TG liver from fed mice exhibited increased oxygen consumption rates that were equivalent to those observed in WT fasted liver (Fig. 1A). No further increase in oxygen consumption occurred in FGF21-TG liver in response to fasting (Fig. 1A). To characterize hepatic fat oxidation in more detail, we measured TCA cycle flux and ketogenesis. Ketogenesis was significantly increased, and there was a trend toward increased TCA cycle flux in fed FGF21-TG liver (Fig. 1A). Overall, hepatic β-oxidation nearly doubled in fed FGF21-TG liver (Fig. 1A). These data are consistent with the previous description of FGF21 as a ketogenic factor (5, 6) and provide additional evidence that FGF21 is critical for the induction of hepatic fat oxidation during fasting.
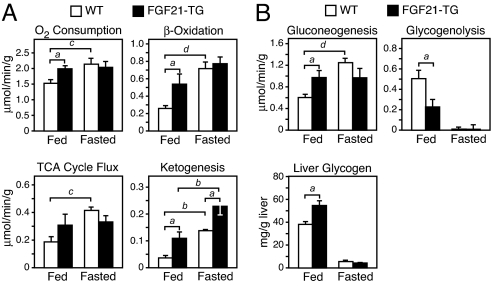
FGF21 regulates hepatic lipid and glucose metabolism. (A and B) Metabolic parameters of energy expenditure and lipid and glucose metabolism were determined by NMR in livers from fed and fasted 16- to 20-week-old WT and FGF21 transgenic (FGF21-TG) male mice (n = 5 per group). Livers were perfused with nonrecirculating media, and absolute fluxes were determined from the NMR data and rate of glucose production. Liver glycogen content (B) was analyzed in fed and fasted WT and FGF21-TG liver samples (n = 6 per group) independent of those analyzed by NMR. Data are presented as mean ± SEM (a, P < 0.05; b, P < 0.01; c, P < 0.005; d, P < 0.001).
Overexpression of FGF21 also affected carbohydrate metabolism. Notably, gluconeogenesis was increased 60% in fed FGF21-TG liver compared with that of fed WT liver (Fig. 1B) and was not induced any further by fasting (Fig. 1B). These data demonstrate that overexpression of FGF21 is sufficient to induce gluconeogenesis in the fed state to levels normally attained during prolonged fasting. The concept that FGF21 expression induces a metabolic state in liver that mimics long-term fasting was reinforced by impaired glycogenolysis (Fig. 1B) and an accompanying 50% increase in glycogen content in FGF21-TG liver (Fig. 1B). There was no difference in plasma glucagon concentrations between FGF21-TG and WT mice (Fig. S1A). These data are consistent with an increased reliance on gluconeogenesis and an autoregulatory sparing of glycogen (29). Taken together, our findings reveal an important role for FGF21 in regulating changes in both fatty acid and carbohydrate metabolism during a prolonged fast.
FGF21 Induces PGC-1α.
FGF21 was previously shown to induce hepatic expression of genes involved in fatty acid metabolism, including lipoprotein lipase (Lpl), pancreatic lipase (Pnlip), pancreatic lipase-related protein 2 (Pnliprp2), and carboxyl ester lipase (Cel) (5, 6). To gain additional insight into how FGF21 exerts its effects on hepatic metabolism, we performed microarray studies using liver from WT and FGF21-TG mice. Among the most strongly induced genes in FGF21-TG liver was peroxisome proliferator-activated receptor-gamma coactivator 1α (Pgc1α), which encodes a transcriptional coactivator that is crucial for coordinating gluconeogenesis and fatty acid oxidation in liver (18–20). Real-time quantitative PCR (QPCR) confirmed that Pgc1α mRNA was induced 5-fold in FGF21-TG liver compared with that in WT liver (Fig. 2A), and PGC-1α protein expression also was elevated in FGF21-TG liver compared with that in WT liver (Fig. 2B). By contrast, mRNA levels of the related factor, PGC-1β, were unchanged in FGF21-TG liver (Fig. S1B). The PGC-1α target genes glucose-6-phosphatase (G6pase) and phosphoenolpyruvate carboxykinase (Pepck), which encode key gluconeogenic enzymes, and the β-subunit of ATP synthase (Atp5b), cytochrome c (Cytc), and isocitrate dehydrogenase 3a (Idh3a), which encode proteins involved in mitochondrial oxidative phosphorylation and TCA cycle flux, were induced significantly in FGF21-TG liver (Fig. 2A).
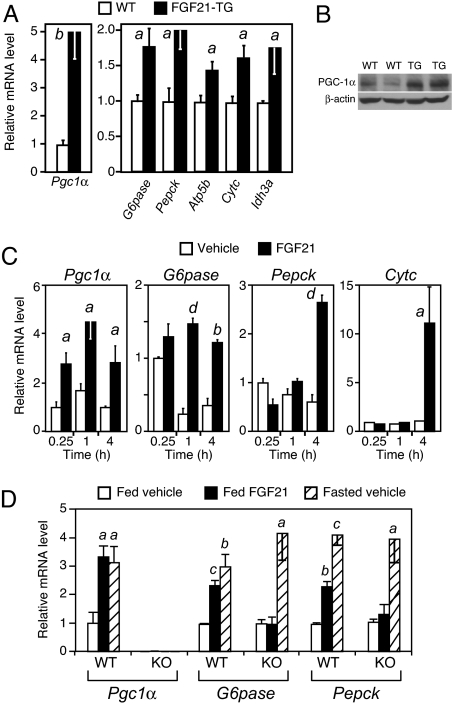
FGF21 induces gluconeogenic gene expression. (A) Hepatic metabolic gene expression in fed WT and FGF21-TG male mice (n = 5 per group). (B) Western blot analysis of PGC-1α protein in fed WT and FGF21-TG mouse liver. (C) Hepatic metabolic gene expression after injection of vehicle or FGF21 into fed WT mice for the indicated times (n = 4 per group). (D) Groups of WT and PGC-1α-KO mice (n = 4 per group except for WT/fasted vehicle group, where n = 3) were injected with FGF21 in the fed or fasted states as indicated. Hepatic gene expression was analyzed by real-time QPCR. Data are presented as mean ± SEM (a, P < 0.05; b, P < 0.01; c, P < 0.005; d, P < 0.001).
To determine whether acute administration of FGF21 is sufficient to induce PGC-1α and its target genes, we performed an FGF21 time course study. Pgc1α was induced in liver as early as 15 min after FGF21 injection (Fig. 2C). G6pase was induced at the 1-h time point, and Pepck and Cytc were induced at 4 h postinjection (Fig. 2C). Thus, acute administration of FGF21 is sufficient to induce PGC-1α and a subset of its target genes. The high basal expression of G6pase at the 15-min time point may reflect stress caused by injection.
To test whether the effects of FGF21 on gluconeogenic gene expression require PGC-1α, WT and PGC-1α-KO mice were injected with FGF21, and G6pase and Pepck mRNA levels were measured in liver. FGF21 injection into fed WT mice increased Pgc1α, G6pase, and Pepck mRNA to levels that mimicked those observed during fasting (Fig. 2D). Importantly, FGF21 injection did not induce G6pase and Pepck in PGC-1α-KO mice. In agreement with results in ref. 4, G6pase and Pepck were induced by fasting in PGC-1α-KO mice, indicating either redundant mechanisms for induction of gluconeogenic gene expression or a compensatory response in the knockout mice. Nevertheless, these data demonstrate that the coordinate effects of FGF21 on gluconeogenic gene expression require PGC-1α.
FGF21-KO Mice Have Metabolic Defects.
To complement the FGF21 gain-of-function studies, we examined the consequences of eliminating FGF21 in mice. A null allele of the Fgf21 gene was generated by introducing loxP sites upstream of exon 1 and downstream of exon 3 through homologous recombination in embryonic stem cells (Fig. S2). Mice heterozygous for the Fgf21loxP allele were bred with mice expressing a Meox-cre transgene, which deletes in the germ line, to generate Fgf21+/− animals. Fgf21+/− mice were then intercrossed to generate Fgf21−/− (FGF21-KO) mice (Fig. 3A). FGF21-KO mice were born at the expected Mendelian ratio and were viable. As expected, Fgf21 transcripts were dramatically elevated in liver during fasting in WT animals but were undetectable in FGF21-KO mice by RT-PCR (Fig. 3B) or QPCR (Fig. 3C). Commensurate with mRNA levels, plasma FGF21 was markedly elevated by fasting in WT mice but absent in FGF21-KO mice (Fig. 3D).
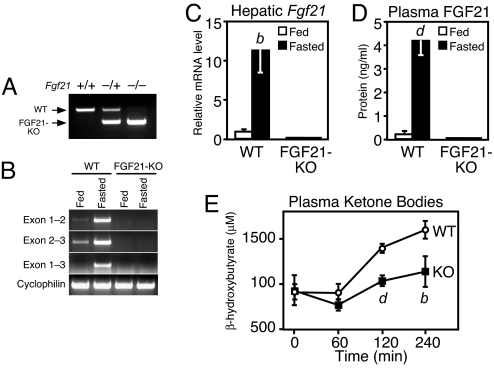
Generation and characterization of FGF21-KO mice. (A) Genotyping of Fgf21−/− mice by genomic PCR. Primer triplex includes one set flanking the 5′ loxP site and a third primer downstream of the 3′ loxP site. (B) Global deletion in the germ line by Meox-cre removes all three Fgf21 exons as demonstrated by semiquantitative RT-PCR using RNA prepared from liver of fed or 24-h-fasted WT or FGF21-KO mice (n = 5 per group). Primers are as indicated in Fig. S2. Cyclophilin served as a loading control. (C) FGF21 mRNA levels were measured by real-time QPCR using RNA prepared from liver of fed or 24-h-fasted WT or FGF21-KO mice (n = 5 per group). (D) FGF21 protein levels were measured by RIA in the plasma of fed and fasted WT and FGF21-KO mice (n = 5 per group). (E) Rate of ketone body production was measured in 24-h-fasted, 18- to 20-week-old WT and FGF21-KO male mice challenged with sodium octanoate injection. Tail blood was collected at the indicated times for analysis of plasma β-hydroxybutyrate concentrations. (C–E) Data are presented as mean ± SEM (a, P < 0.05; b, P < 0.01; c, P < 0.005; d, P < 0.001).
FGF21-KO and WT mice showed no differences in body weight or plasma glucose, triglyceride, nonesterified fatty acid (NEFA), insulin, and glucagon concentrations in the fed state (Table 1). Plasma glucose levels, however, were significantly reduced in fasted FGF21-KO mice. Plasma ketone levels were significantly reduced in fed mice and trended lower in fasted FGF21-KO mice (Table 1). FGF21-KO mice also showed trends toward increased plasma NEFA and triglyceride concentrations during fasting (Table 1), suggesting that FGF21-KO mice may avoid overt hypoketosis during fasting by increasing lipid delivery to the liver. To further assess whether ketogenic potential is impaired in the absence of FGF21, we compared plasma ketone concentrations in WT and FGF21-KO mice after injection with octanoate, a medium-chain fatty acid that rapidly enters mitochondria in a carnitine-independent manner and is converted to ketones at a rate sensitive to the fasting state of the liver (30). Octanoate administration to fasted mice caused the plasma β-hydroxybutyrate concentration to double in WT mice compared with only a 20% increase in FGF21-KO mice (Fig. 3E). This result demonstrates that under conditions of identical nutritional state and substrate availability ketogenesis is markedly reduced in FGF21-KO mice.
Table 1.
Body weight and plasma parameters in WT and FGF21-KO mice
| Parameter | Fed | Fasted | ||
|---|---|---|---|---|
| WT | FGF21-KO | WT | FGF21-KO | |
| Body weight, g | 21.2 ± 2.14 | 21.0 ± 2.56 | 20.3 ± 2.20 | 19.8 ± 1.28 |
| Glucose, mg/dL | 140 ± 8.94 | 136 ± 11.4 | 76.4 ± 2.64 | 64.1 ± 1.90** |
| β-Hydroxybutyrate, μM | 35.9 ± 6.58 | 24.5 ± 8.23* | 857 ± 93.1 | 792 ± 125 |
| NEFA, meq/L | 0.627 ± 0.063 | 0.624 ± 0.060 | 3.07 ± 0.172 | 3.96 ± 0.294 |
| Insulin, ng/mL | 0.467 ± 0.107 | 0.440 ± 0.161 | 0.163 ± 0.089 | 0.115 ± 0.056 |
| Glucagon, pg/ml | 40.0 ± 7.96 | 39.1 ± 6.10 | 102 ± 13.9 | 105 ± 14.7 |
| Triglyceride, mg/dL | 140 ± 4.11 | 135 ± 15.6 | 42.7 ± 6.17 | 52.3 ± 14.1 |
*, P < 0.05;
**, P < 0.01.
The role of FGF21 in regulating hepatic gene expression was investigated by using WT and FGF21-KO mice in either the fed or fasted state. Under fasted conditions, Cel, Pnlip, Pnliprp2, Pgc1α, G6pase, and Pepck mRNAs were induced in WT mice (Fig. 4). With the exception of Pepck, induction of all of these genes was significantly attenuated in liver of FGF21-KO mice. The Pepck data indicate that either FGF21 is not involved in regulating Pepck in response to fasting or that other mechanisms compensate for FGF21's absence. Lpl mRNA was also significantly reduced in FGF21-KO liver in the fasted state (Fig. 4). Overall, these loss-of-function data demonstrate that FGF21 plays an important role in inducing PGC-1α and other genes involved in regulating carbohydrate and lipid metabolism during fasting.
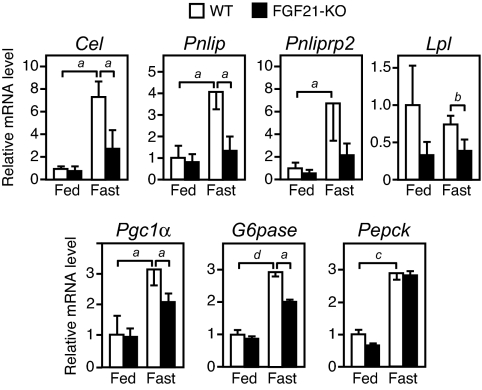
FGF21 regulates genes involved in hepatic carbohydrate and lipid metabolism during fasting. Shown is real-time QPCR analysis of gene expression in WT or FGF21-KO liver under fed or fasted conditions (n = 5 per group). Data are presented as mean ± SEM. (a, P < 0.05; b, P < 0.01; c, P < 0.005; d, P < 0.001).
To determine the effect of eliminating FGF21 on the regulation of hepatic glucose and fat oxidation under uniform metabolic conditions, we measured flux through these pathways in isolated liver perfused with equal concentrations of NEFA and gluconeogenic substrates. In agreement with the octanoate challenge data, fasted FGF21-KO liver had diminished oxygen consumption, β-oxidation, TCA cycle flux, and ketogenesis compared with those of fasted WT liver (Fig. 5A). These fluxes in FGF21-KO liver failed to respond to fasting. Remarkably, there was also no induction of gluconeogenesis in FGF21-KO liver in response to fasting (Fig. 5B). Neither glycogenolysis nor liver glycogen concentrations were significantly different between WT and FGF21-KO liver under fed or fasted conditions (Fig. 5B). The loss of fasting-induced gluconeogenesis in FGF21-KO mice is consistent with the hypoglycemia that occurs in these animals (Table 1) and the findings in the gain-of-function perfusion experiments (Fig. 1B). Thus, FGF21 is crucial for the liver's adaptive metabolic response to prolonged fasting.
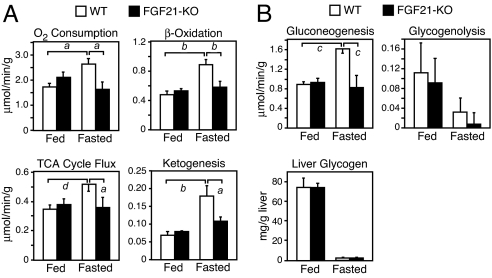
FGF21 is required for inducing hepatic lipid and glucose metabolism during fasting. (A and B) Metabolic parameters of energy expenditure and lipid and glucose metabolism were determined by NMR in livers from fed and fasted 16- to 20-week-old WT and FGF21-KO male mice (n = 6 per group). Livers were perfused with nonrecirculating media, and absolute fluxes were determined from the NMR data and rate of glucose production. Liver glycogen content (B) was analyzed in fed and fasted WT and FGF21-KO liver samples (n = 6 per group) independent of those analyzed by NMR. Data are presented as mean ± SEM (a, P < 0.05; b, P < 0.01; c, P < 0.005; d, P < 0.001).
Discussion
FGF21 was shown previously to be a ketogenic factor induced by PPARα in response to fasting (5, 6). Using both gain-of-function and loss-of-function approaches, we now show that the metabolic actions of FGF21 extend well beyond fatty acid oxidation and ketogenesis to include striking effects on TCA cycle flux and carbohydrate metabolism. Total flux through the gluconeogenic pathway was increased in fed FGF21-TG liver to levels comparable to those seen in fasted WT liver. Remarkably, there was no induction of gluconeogenesis in the fasted FGF21-KO liver. Consistent with these findings, plasma glucose concentrations were significantly reduced during fasting in FGF21-KO mice. These results demonstrate that this hormone plays a crucial role in mediating the liver's adaptive response to nutritional deprivation.
FGF21 is similar to the fasting hormone glucagon in that it induces hepatic gluconeogenesis, fatty acid oxidation, and ketogenesis. However, in marked contrast to glucagon, FGF21 does not promote glycogenolysis. Indeed, FGF21-TG mice accumulate significantly more hepatic glycogen than WT mice in the fed state. Interestingly, in humans, circulating glucagon concentrations spike after 3–5 days of fasting and then decline, whereas plasma FGF21 levels increase only after a 7-day fast (31–33). These findings raise the possibility that FGF21 maintains gluconeogenesis and ketogenesis in the context of prolonged fasting and starvation, when glycogen stores are depleted and glucagon levels have fallen. In this model, glucagon and FGF21 are not redundant but rather sequential in their actions. Several properties of FGF21 are consistent with this hypothesis. First, circulating FGF21 concentrations are induced in rodents and humans only after prolonged fasting (5, 6, 33). Second, FGF21 sensitizes mice to torpor, a hibernation-like state that occurs only under severe nutritional stress (6). Third, FGF21 causes growth hormone resistance and blunts growth in mice, a phenomenon that occurs during starvation (34). On the basis of these findings, we propose that FGF21 is a bona fide starvation hormone rather than a mediator of shorter-term fasting responses.
Our finding that FGF21 rapidly induces hepatic expression of PGC-1α, a prominent transcriptional regulator of metabolism (19–21, 25), suggests a mechanism for its metabolic actions. FGF21 may also modulate PGC-1α activity via posttranslational modifications. Notably, induction of PGC-1α and its downstream target, G6Pase, was significantly attenuated in liver of FGF21-KO mice after a 24-h fast. Moreover, the inductive effect of FGF21 on gluconeogenic gene expression was virtually eliminated in PGC-1α-KO mice. NMR isotopomer analyses show that PGC-1α-KO liver has reductions in fatty acid oxidation, ketogenesis, TCA flux, and gluconeogenesis that mirror those in the FGF21-KO liver (4). Because glucagon and its downstream effector, cAMP, induce PGC-1α and gluconeogenic gene expression during the early stages of the fasting response (24, 35), we propose that FGF21 maintains the expression of these genes during prolonged fasting and starvation.
How does FGF21 induce PGC-1α in liver? The rapidity with which FGF21 induces PGC-1α and the fact that FGF21 acts through receptors with tyrosine kinase activity suggest that FGF21 may affect the phosphorylation of transcription factors that bind to the Pgc1α promoter. However, an analysis of candidate transcription factors that regulate the Pgc1α promoter did not reveal changes in the phosphorylation of FOXO1, CREB, or TORC2 in livers of mice injected with FGF21 (Fig. S3A). Moreover, we have been unable to recapitulate FGF21-mediated induction of Pgc1α in either isolated, perfused mouse liver or primary cultures of rat or mouse hepatocytes, precluding the use of these models to elucidate how PGC-1α is induced (Fig. S3 B–D). These negative findings raise the interesting possibility that FGF21 might induce Pgc1α through an indirect mechanism involving the central nervous system.
FGF21 functions as a potent insulin sensitizer in various animal models of insulin resistance and diabetes (15, 17, 36, 37). Our data suggest that FGF21 does not improve glycemic control by suppressing hepatic gluconeogenesis per se. Rather, they indicate that FGF21 improves hepatic insulin action indirectly by stimulating hepatic fatty acid disposal. Decreasing hepatic fatty acid content has well-documented effects on liver insulin sensitivity in a variety of models and indirectly improves the responsiveness of hepatic glucose production, via either glycogenolysis or gluconeogenesis (38, 39). In this respect, the effects of FGF21 are analogous to those of PPARα agonists, which improve hepatic insulin action and overall glycemic control in diabetic rodents despite increasing hepatic gluconeogenesis (40, 41). Indeed, as previously shown, FGF21 expression is regulated directly by PPARα, which may explain many of the therapeutic effects of PPARα agonists. Because all of our studies were done in lean mice, additional experiments will have to be performed to determine whether FGF21 has similar effects on metabolism in insulin-resistant animals.
In summary, we demonstrate that the fasting-induced hormone FGF21 has pronounced effects on carbohydrate and fatty acid metabolism in liver. Mice lacking FGF21 have impaired hepatic gluconeogenesis and ketogenesis. We propose that FGF21 acts subsequent to glucagon during nutritional deprivation to elicit and coordinate diverse aspects of the adaptive starvation response.
Methods
Animal Experiments.
FGF21-TG, PGC-1α-KO, and Meox-cre mice were described previously (6, 28, 42, 43). Mice were fed standard chow containing 4% fat ad libitum. All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center.
Generation of FGF21-KO Mice.
The FGF21 targeting vector was constructed by using a conditional KO vector containing a neomycin-resistance gene flanked by Frt sites and two loxP sites flanking a multicloning sequence. A 1.5-kb short arm harboring the Fgf21 promoter, a 2-kb fragment harboring exons 1–3, and a 3.8-kb long arm harboring the 3′ end of the Fgf21 gene were generated by high-fidelity PCR amplification (Expand High-Fidelity Long Template; Roche). The FGF21 targeting vector was linearized and injected into ES cells. ES cell clones were isolated and analyzed for homologous recombination by long-range PCR and verified by Southern blot analysis. Clones properly targeted for FGF21 were injected into 3.5-day C57BL/6 blastocyts, and the resulting chimeras were crossed with C57BL/6 females to obtain germ-line transmission of the targeted Fgf21 allele (Fgf21loxP/+). The Fgf21 gene was deleted in the germ line by crossing Fgf21loxP/+ mice with Meox-cre mice (C57BL/6) to generate Fgf21+/− mice. Removal of the Meox-cre allele and deletion of the Fgf21 gene were confirmed by genotyping. Heterozygote breeding was performed for two additional generations, and WT (FGF21+/+) and homozygous (FGF21−/−) breeding lines were subsequently maintained.
FGF Injection Experiments.
Human FGF21 (residues 29–209) with a hexahistidine tag on the amino terminus was expressed in Escherichia coli and purified by sequential Ni-chelating and size-exclusion chromatography. FGF21 was injected into mice at a concentration of 0.75 μg/g body weight. For the in vivo time course studies, FGF21 was injected i.p. For experiments involving WT and PGC-1α-KO mice, mice receiving FGF21 were fed ad libitum and were injected i.p. at 5 PM on the first day and 7 AM the following day. Mice were euthanized 2 h after the second injection.
Liver Perfusion Experiments.
Livers from ad libitum fed and 24-h-fasted FGF21-TG, FGF21-KO, and WT littermates were isolated and perfused for 60 min in a nonrecirculating fashion at 8 mL/min with a Krebs–Henseleit-based perfusion medium containing 1.5 mM lactate, 0.15 mM pyruvate, 0.25 mM glycerol, 0.4 mM free fatty acid (algal mix bound to 3% albumin), 2% deuterated water, and 0.1 mM [U-13C3]propionate as described previously (3, 4, 44, 45).
Immunoblot Analysis.
Immunoblotting was performed using an anti-PGC-1α antibody (Cell Signaling Technology) and β-actin antibody (Sigma) as described in ref. 16.
RT-PCR and QPCR Analysis.
Total RNA was extracted from WT and FGF21-KO mouse livers with Stat 60 reagent (IsoTex Diagnostics). Four micrograms of RNA from each sample was then used to generate cDNA. RT-PCR for the deleted exons of the FGF21 gene was performed using the primer pairs: Exon 1 forward 5′-GCCTGAGCCCCAGTCTGAACCTGACCC-3′, Exon 2 reverse 5′-CCATAGAGAGCTCCATCTGGCTGTTGGC-3′, Exon 2 forward 5′-GCCAACAGCCAGATGGAGCTCTCTATGG-3′, Exon 3 reverse 5′-GAAGAGTCAGGACGCATAGCTGGGGCTTCGGC-3′.
QPCR was performed using SYBR green as described in ref. 46. The primer sequences used for gene expression analyses are as follows: Pgc-1α FWD 5′-AGACAAATGTGCTTCCAAAAAGAA-3′, REV 5′-GAAGAGATAAAGTTGTTGGTTTGGC-3′; G6pase FWD 5′-GTGGCAGTGGTCGGAGACT-3′, REV 5′-ACGGGCGTTGTCCAAAC-3′; Pepck FWD 5′-CACCATCACCTCCTGGAAGA-3′, REV 5′-GGGTGCAGAATCTCGAGTTG-3′; Lpl FWD 5′-GGCCAGATTCATCAACTGGAT-3′, REV 5′-GGCTGTCTCCCAAGAGATGGA-3′, Atp5b FWD 5′-AGGTGGCCCAGCATTTG-3′, REV 5′-GCCTTCAGTGCCATCCATAG-3′; Cytc FWD 5′-GAAAAGGGAGGCAAGCATAAG-3′, REV 5′-TGTCTTCCGCCCGAACA-3′; Idh3a FWD 5′-TCGTCACCATCCGAGAGAAC-3′, REV 5′-GCACAACCCCATCAACGAT-3′; Cyclophilin FWD 5′-GGAGATGGCACAGGAGGAA-3′, REV 5′-GCCCGTAGTGCTTCAGCTT-3′.
Metabolic Parameter Measurements.
Plasma glucose levels were measured by using the glucose autokit (Wako Chemicals). Plasma triglyceride concentrations were measured by using an L-type TG H triglyceride kit (Wako Chemicals). Plasma NEFAs were measured by using a NEFA C kit (Wako Chemicals). Plasma β-hydroxybutyrate concentrations were measured by using a d-3-hydroxybutyric acid kit (Wako Chemicals). Plasma glucagon levels were measured by using a glucagon ELISA kit (Wako Chemicals). Plasma insulin levels were measured by using the Ultra Sensitive Mouse Insulin ELISA kit (Crystal Chem). Plasma FGF21 protein concentration was determined by using the FGF21 RIA kit (Phoenix Pharmaceuticals). All measurements were performed following the manufacturer's instructions. Glycogen levels were determined as described in ref. 4.
Octanoate Challenge.
Sodium octanoate (500 mM, in sterile H2O) was injected i.p. at 6 μL/g of body weight into WT and FGF21-KO mice fasted 24 h. Aliquots of tail blood were withdrawn for analysis of β-hydroxybutyrate concentrations.
Acknowledgments.
We thank D. Kelly (Burnham Institute for Medical Research, Orlando, FL) for PGC-1α-KO mice and discussing the manuscript, M. Tallquist (UT Southwestern, Dallas, TX) for Meox1-cre mice, M. Montminy (Salk Institute, La Jolla, CA) for CREB and TORC2 antibodies, L. Peng for technical assistance, N. Anderson and J. Horton for microarray analysis, and M. Kuro-o and members of the Mangelsdorf/Kliewer lab for helpful discussions. This work was supported by National Institutes of Health Grants DK067158, P20RR20691, 1RL1GM084436–01 (S.A.K. and D.J.M.); U19DK62434 (D.J.M.); DK078184, RR02584, and DK076269 (S.C.B.); and DE13686 (M.M.); the Robert A. Welch Foundation (D.J.M. and S.A.K.); and the Howard Hughes Medical Institute. D.J.M. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904187106/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0904187106
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/106/26/10853.full.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/full/106/26/10853
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/abstract/106/26/10853
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/reprint/106/26/10853.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches.
Int J Mol Sci, 25(16):8731, 10 Aug 2024
Cited by: 0 articles | PMID: 39201418 | PMCID: PMC11354927
Review Free full text in Europe PMC
Exploring endocrine FGFs - structures, functions and biomedical applications.
Int J Biochem Mol Biol, 15(4):68-99, 25 Aug 2024
Cited by: 0 articles | PMID: 39309613 | PMCID: PMC11411148
Review Free full text in Europe PMC
Weighing in on the role of brown adipose tissue for treatment of obesity.
J Pharm Pharm Sci, 27:13157, 17 Jul 2024
Cited by: 0 articles | PMID: 39087083 | PMCID: PMC11290130
Review Free full text in Europe PMC
New advances in drug development for metabolic dysfunction-associated diseases and alcohol-associated liver disease.
Cell Biosci, 14(1):90, 06 Jul 2024
Cited by: 1 article | PMID: 38971765 | PMCID: PMC11227172
Review Free full text in Europe PMC
Team players in the pathogenesis of metabolic dysfunctions-associated steatotic liver disease: The basis of development of pharmacotherapy.
World J Gastrointest Pathophysiol, 15(4):93606, 01 Aug 2024
Cited by: 0 articles | PMID: 39220834 | PMCID: PMC11362842
Review Free full text in Europe PMC
Go to all (458) article citations
Other citations
Wikipedia
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo.
Endocrinology, 152(8):2996-3004, 28 Jun 2011
Cited by: 156 articles | PMID: 21712364 | PMCID: PMC3138239
Fibroblast growth factor 21 increases hepatic oxidative capacity but not physical activity or energy expenditure in hepatic peroxisome proliferator-activated receptor γ coactivator-1α-deficient mice.
Exp Physiol, 103(3):408-418, 16 Jan 2018
Cited by: 12 articles | PMID: 29215172 | PMCID: PMC5832578
Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice.
J Biol Chem, 281(28):19000-19008, 02 May 2006
Cited by: 80 articles | PMID: 16670093 | PMCID: PMC3047410
Fibroblast growth factor 21: from pharmacology to physiology.
Am J Clin Nutr, 91(1):254S-257S, 11 Nov 2009
Cited by: 146 articles | PMID: 19906798 | PMCID: PMC2793111
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Howard Hughes Medical Institute
NCRR NIH HHS (4)
Grant ID: P41 RR002584
Grant ID: RR02584
Grant ID: P20 RR020691
Grant ID: P20RR20691
NIDCR NIH HHS (2)
Grant ID: DE13686
Grant ID: R01 DE013686
NIDDK NIH HHS (7)
Grant ID: DK078184
Grant ID: R01 DK067158
Grant ID: R01 DK078184
Grant ID: U19 DK062434
Grant ID: DK067158
Grant ID: DK076269
Grant ID: U19DK62434
NIGMS NIH HHS (2)
Grant ID: 1RL1GM084436-01
Grant ID: RL1 GM084436





