Abstract
Background/objectives
Leprosy prominently involves both the skin and peripheral neural tissues and some symptoms persist after microbial cure. Because alterations in the dermis also occur in leprosy, we assessed here whether there were changes in cutaneous resonance running time (CRRT), a parameter that is influenced by collagen properties, in cured leprosy subjects.Methods
A reviscometer was used to measure the CRRT at various directions on the dorsal hand and the flexural forearms of 76 cured leprosy subjects aged 50-85 years and 68 age-matched normal subjects.Results
In comparison to normal subjects, CRRTs on the hands and the forearms were significantly reduced in all directions in cured leprosy, except at the 1-7, 2-8 and 3-9 o'clock directions on the forearms. CRRTs were reduced significantly at both the 4-10 and 5-11 o'clock directions on the forearm in lepromatous (73.33 +/- 4.19 at 4-10 o'clock and 67.44 +/- 2.71 at 5-11 o'clock direction) and borderline lepromatous types (77.58 +/- 5.84 at 4-10 o'clock and 79.85 +/- 6.81 at 5-11 o'clock direction) as compared with normal (143.10 +/- 7.75 at 4-10 o'clock and 125.18 +/- 8.14 at 5-11 o'clock direction). On the hand, CRRTs at all directions, except that at 4-10 o'clock direction, were also significantly reduced in lepromatous and borderline lepromatous types in comparison with normal. Significant differences in CRRT at some directions were found among the various subtypes of leprosy.Conclusion
CRRTs were abnormal in the cured leprosy subjects as a whole, but varied with leprosy subtypes, which suggested that the extent of reduction of CRRTs correlates with the severity of immune alteration. These results suggest that CRRT measurements could be a useful approach to quantify the extent of some residual abnormalities in cured leprosy and perhaps could also be used to evaluate the efficacy of treatment.Free full text

Decreased Cutaneous Resonance Running Time in Cured Leprosy Subjects
Abstract
Background/Objectives
Leprosy prominently involves both the skin and peripheral neural tissues and some symptoms persist after microbial cure. Because alterations in the dermis also occur in leprosy, we assessed here whether there were changes in cutaneous resonance running time (CRRT), a parameter that is influenced by collagen properties, in cured leprosy subjects.
Methods
A reviscometer was used to measure the CRRT at various directions on the dorsal hand and the flexural forearms of 76 cured leprosy subjects aged 50–85 years and 68 age-matched normal subjects.
Results
In comparison to normal subjects, CRRTs on the hands and the forearms were significantly reduced in all directions in cured leprosy, except at the 1–7, 2–8 and 3–9 o'clock directions on the forearms. CRRTs were reduced significantly at both the 4–10 and 5–11 o'clock directions on the forearm in lepromatous (73.33 ± 4.19 at 4–10 o'clock and 67.44 ± 2.71 at 5–11 o'clock direction) and borderline lepromatous types (77.58 ± 5.84 at 4–10 o'clock and 79.85 ± 6.81 at 5–11 o'clock direction) as compared with normal (143.10 ± 7.75 at 4–10 o'clock and 125.18 ± 8.14 at 5–11 o'clock direction). On the hand, CRRTs at all directions, except that at 4–10 o'clock direction, were also significantly reduced in lepromatous and borderline lepromatous types in comparison with normal. Significant differences in CRRT at some directions were found among the various subtypes of leprosy.
Conclusion
CRRTs were abnormal in the cured leprosy subjects as a whole, but varied with leprosy subtypes, which suggested that the extent of reduction of CRRTs correlates with the severity of immune alteration. These results suggest that CRRT measurements could be a useful approach to quantify the extent of some residual abnormalities in cured leprosy and perhaps could also be used to evaluate the efficacy of treatment.
Introduction
Skin can be considered as a window to the internal status of a human, exhibiting both localized skin changes and other alterations that are indicators of systemic conditions. In traditional Chinese medicine, skin color, shine, smoothness or skin temperature provide important information in diagnosis of skin and/or systemic diseases. Current parameters of skin biophysical properties, such as stratum corneum (SC) hydration, sebum content, transepidermal water loss (TEWL), and skin surface pH, are utilized as important indicators of cutaneous and systemic function. For example, both age and sex significantly impact SC hydration, sebum content, barrier function and skin surface pH [1,2,3,4,5,6]. SC hydration is negatively correlated with serum thyroid-stimulating hormone levels [7]. Disorders that alter keratinocyte proliferation, differentiation and epidermal lipid metabolism, in turn, can affect SC properties, such as SC hydration and barrier function [8,9,10,11]. Moreover, these biophysical properties can have some effects on each other. For example, external reductions in environmental humidity decrease SC hydration and improve basal barrier function [12]. Elevation of skin surface pH could alter epidermal permeability barrier homeostasis [13]. Measurements of these skin biophysical parameters also have been used to evaluate the efficacy of skincare products [14,15,16,17]. Cutaneous resonance running time (CRRT), which measures the acoustical shockwave running time through skin between two sensors, is a potentially important indicator of cutaneous functions that is influenced in part by the skin biophysical properties. For instance, skin stiffness correlates negatively with CRRT [18]. Furthermore, aging not only affects CRRT, but also influences the angular anisotropy of CRRT [19]. In addition to SC hydration and age, CRRT also varies with skin thickness and elastic fiber properties [19,20,21].
Skin and peripheral nerves are the major organs that are involved in leprosy. Depending upon the leprosy subtype, clinical manifestations include sensory deficit, nerve thickening, hypo- or hyperpigmentation, dry skin, erythema, papules, nodules, and ulceration [22,23,24]. Some of these abnormalities remain after leprosy is cured microbiologically [25,26,27]. Further secondary complications, such as ulceration and extremity deformation, may also occur in cured leprosy subjects [28, 29]. Although little is known about skin biophysical properties in cured leprosy, there is substantial evidence that alterations occur frequently. In addition to clinical observations of excessive dry skin in leprosy, previous studies demonstrated that SC hydration power in leprosy lesions was significantly decreased [30, 31]. SC cholesterol sulfate and triglycerides levels were higher in lesions with sensory deficits in leprosy, and both sphingolipid and cholesterol esters were lower than in normal subjects [32]. Not only lipids were abnormal, epidermal proliferation also declined significantly in lepromatous leprosy (LL), as compared with borderline (BB) and tuberculoid leprosy (TT) [33]. Recent studies from our group have demonstrated that skin surface pH is increased and SC hydration is decreased in cured leprosy subjects, although there are no alterations in basal TEWL [34]. Since the dermis is thinner and dermal elastic fibers in leprosy are arranged irregularly in leprosy subjects [35], features that can influence CRRT [18,19,20,21], we postulated that there could be alterations in CRRT in cured leprosy. In the present study, we assessed CRRT at various directions on both the forearm and the hand in normal and cured leprosy subjects.
Materials and Methods
Subjects
In total, 76 cured leprosy subjects aged 50–85 years old (mean 67.21 ± 1.23) and 68 normal control volunteers aged 50–85 years old (mean 74.65 ± 1.71) were enrolled in this study (table (table1).1). The cured leprosy subjects were recovered from leprosy 19–51 years ago (mean 36.76 ± 1.48). All subjects have hand deformation and increased sensory threshold on the hands and the forearms without other skin disorders on either the hand or the forearm. All normal volunteers have no skin disorders on the hand and the forearm. No skincare products were applied to the measured sites within 24 h and no wash within 4 h prior to the measurement. All subjects participating in this study are Chinese located in northern China.
Table 1
Subjects' characteristics
| Leprosy | Normal | |||||
|---|---|---|---|---|---|---|
| LL | BL | BB | TT | BT | ||
| Female | 6 | 4 | 2 | 2 | 0 | 30 |
| Male | 18 | 16 | 4 | 16 | 8 | 38 |
| Total | 24 | 20 | 6 | 18 | 8 | 68 |
Measured Sites and Methods
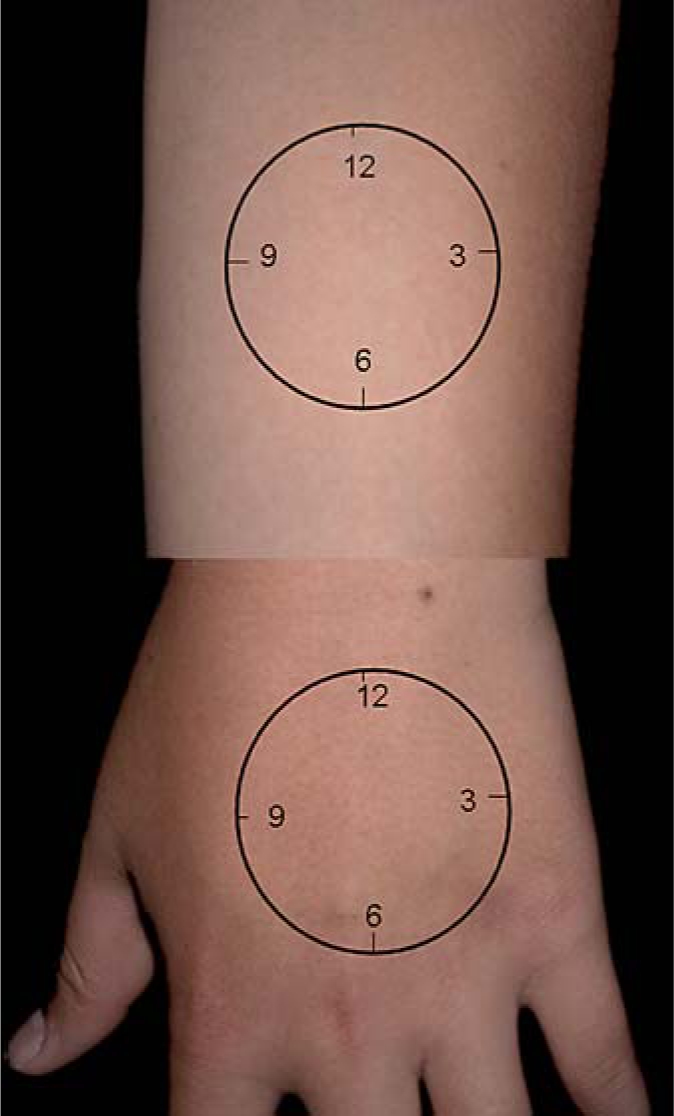
A Courage-Khazaka Reviscometer RVM600 was used to measure the CRRT on the central dorsal of the left hand and the flexor site of the left forearm 10 cm above the wrist [19]. Measurements were begun at the 12 o'clock position, which were determined with the forearms and hands in the upside-down position (fig. (fig.1).1). Both arm and hand were laid on a table horizontally during the measurement. Measurements then were taken clockwise at every 1-hour interval or at every 15 degrees. These measurements provide the CRRTs at the directions of 12–6 o'clock, 1–7, 2–8, and so on. All subjects were resting at 24–26°C at a relative humidity of 46–55% for 30 min before the measurements were taken. All studies were completed in the Summer of 2008.
Statistics
Data were expressed as mean ± SEM. GraphPad Prism 4 software was used for all statistical analysis. Unpaired two-tailed t test with Welch's correction was used to determine the significance between two groups. The one-way ANOVA test with Tukey correction was used when three or more groups were compared. p < 0.05 was considered significant.
All human research protocols were approved by the Human Research Subcommittee of the Dalian Skin Disease Hospital.
Results
Anisotropy Changes in CRRTs in Both Normal and Cured Leprosy Subjects
We first assessed the CRRTs in both normal and cured leprosy subjects. As seen in figure figure2,2, there are no differences in the pattern of CRRTs at various directions on either the hands or the forearms between the cured leprosy and normal subjects as a whole group (fig. (fig.2).2). Moreover, the maximum CRRTs on the forearms in both the cured leprosy and normal controls are at the 1–7 o'clock direction (fig. (fig.2;2; table table2),2), while the minimum CRRTs on the forearms in the normal controls are at the 3–9 o'clock direction (p < 0.001, vs. 1–7 o'clock directions). In contrast, in the cured leprosy subjects, the minimum CRRTs on the forearm are at the direction of 5–11 o'clock (p < 0.001, vs. 1–7 and 2–8 o'clock directions). On the hands, however, the maximum CRRTs are at the 5–11 o'clock direction in the normal controls (p < 0.001, vs. 1–7 and 2–8; p < 0.05, vs. 3–9 o'clock directions), while the maximum CRRTs on the hands in the cured leprosy subjects are at the 12–6 o'clock direction (p < 0.01, vs. 1–7; p < 0.001, vs. 2–8; p < 0.05, vs. 3–9 o'clock directions). Finally, the minimum CRRTs on the hands in both normal and cured leprosy subjects are at the direction of 2–8 o'clock. Thus, in either normal or cured leprosy subjects, CRRTs display anisotropy.
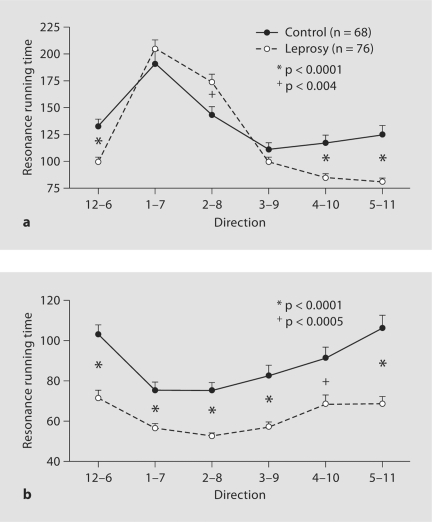
Decreased CRRTs in cured leprosy subjects. a CRRTs on the forearm (numbers of subjects are listed in table 1). b CRRTs on the hand. Significances between normal and cured leprosy subjects are indicated.
Table 2
Comparison of CRRTs at different directions
| Forearm | Hand | |||
|---|---|---|---|---|
| normal control | leprosy | normal control | leprosy | |
| 12–6 o'clock position | 132.80 ± 6.73 | 99.50 ± 4.18@ | 103.00 ± 6.46 | 71.53 ± 3.69 |
| 1–7 o'clock position | 191.00 ± 11.40 | 205.10 ± 8.14# | 75.38 ± 3.85&#, @ | 56.60 ± 1.98& |
| 2–8 o'clock position | 143.10 ± 7.75 | 174.40 ± 7.26 | 75.27 ± 3.78&#, @ | 52.65 ± 1.34@ |
| 3–9 o'clock position | 111.00 ± 6.04 | 99.61 ± 3.90@,  | 82.59 ± 5.05 | 57.11 ± 2.29 |
| 4–10 o'clock position | 117.20 ± 7.12 | 84.70 ± 3.87@,  | 91.54 ± 4.93 | 68.38 ± 4.44# |
| 5–11 o'clock position | 125.18 ± 8.14 | 80.91 ± 3.40@,  | 106.35 ± 6.15 | 69.65 ± 3.55# |
| Significance |  p < 0.001, vs. all p < 0.001, vs. all | @ p < 0.001, vs. 1–7 |  p < 0.05, vs. 5–11 p < 0.05, vs. 5–11 | # p < 0.01, vs. 2–8 |
 p < 0.001, vs. 2–8 p < 0.001, vs. 2–8 | # p < 0.01, vs. 12–6 |  p < 0.05, vs. 12–6 p < 0.05, vs. 12–6 | ||
| # p < 0.01, vs. 2–8 | @ p < 0.001, vs. 5–11 | @ p < 0.001, vs. 12–6 | ||
| & p < 0.01, vs. 12–6 | ||||
Decreased CRRTs in Cured Leprosy
We next analyzed the differences of CRRTs between normal and cured leprosy subjects. On the forearms, the CRRTs in the cured leprosy subjects are significantly reduced at the directions of 12–6, 4–10, and 5–11 o'clock in comparison with CRRTs at these sites in normal controls (fig. (fig.2a).2a). Yet, at the direction of 2–8 o'clock, the CRRT in the cured leprosy subjects is increased significantly as compared with normal controls (p < 0.004). On the hands, the CRRTs in the cured leprosy subjects are significantly reduced at all directions (fig. (fig.2b).2b). These results indicate that alterations in CRRTs in cured leprosy vary with both body sites and directions.
CRRTs Vary within Leprosy Subtypes
As seen in tables tables33 and and4,4, the CRRTs at some directions vary significantly with leprosy subtypes. On the forearms, the CRRT at the direction of 4–10 o'clock in borderline tuberculoid (BT) is significantly longer than either in LL or BL (p < 0.01, table table3);3); the CRRT at the direction of 5–11 o'clock in BT is also significantly longer than in LL (p < 0.001, table table3).3). On the hands, the CRRTs at the directions of 1–7 and 5–11 o'clock in BT is significantly increased as compared with LL (p < 0.05 and p < 0.01, respectively, table table4).4). In comparison with normal controls, the CRRTs on the forearms at directions of 4–10 and 5–11 o'clock in LL and BL are significantly reduced (table (table3).3). On the hands, the CRRTs at all directions except 4–10 o'clock are also reduced significantly in LL and BL as compared with normal control (table (table4).4). In addition, the CRRTs at the 2–8 o'clock direction in BT and 5–11 in TT are significantly shorter than in normal controls (table (table4).4). These results demonstrate that changes in CRRTs are also associated with leprosy subtypes.
Table 3
The CRRTs vary with leprosy subtypes on the forearm
| 12–6 o'clock position | 1–7 o'clock position | 2–8 o'clock position | 3–9 o'clock position | 4–10 o'clock position | 5–11 o'clock position | |
|---|---|---|---|---|---|---|
| Normal (n = 68) | 132.80 ± 6.73 | 191.00 ± 11.4 | 143.10 ± 7.75 | 111.00 ± 6.04 | 117.20 ± 7.12 | 125.18 ± 8.14 |
| LL (n = 24) | 102.46 ± 9.42 | 204.40 ± 13.64 | 175.21 ± 13.83 | 92.81 ± 4.59 | 73.33 ± 4.19b,d | 67.44 ± 2.71c |
| BL (n = 20) | 102.90 ± 8.25 | 223.03 ± 13.48 | 190.53 ± 13.33 | 97.68 ± 7.43 | 77.58 ± 6.84a,d | 79.85 ± 6.81a |
| BB (n = 6) | 82.64 ± 7.11 | 184.64 ± 19.98 | 177.64 ± 33.55 | 85.57 ± 4.77 | 75.29 ± 9.25 | 78.71 ± 9.49 |
| BT (n = 18) | 104.85 ± 7.88 | 178.82 ± 14.64 | 150.38 ± 12.78 | 114.15 ± 10.31 | 114.47 ± 11.76 | 104.50 ± 9.75e |
| TT (n = 8) | 85.75 ± 5.83 | 236.13 ± 43.7 | 179.81 ± 18.65 | 106.19 ± 18.24 | 81.63 ± 9.27 | 75.75 ± 10.34 |
Table 4
The CRRTs vary with leprosy subtypes on the hand
| 12–6 o'clock position | 1–7 o'clock position | 2–8 o'clock position | 3–9 o'clock position | 4–10 o'clock position | 5–11 o'clock position | |
|---|---|---|---|---|---|---|
| Normal (n = 68) | 103.00 ± 6.46 | 75.38 ± 3.85 | 75.27 ± 3.78 | 82.59 ± 5.05 | 91.54 ± 4.93 | 106.35 ± 6.15 |
| LL (n = 24) | 66.52 ± 5.79b | 49.10 ± 1.71c | 49.23 ± 1.78c | 55.33 ± 3.27b | 72.15 ± 11.25 | 57.06 ± 3.39c |
| BL (n = 20) | 67.05 ± 5.23a | 54.30 ± 2.76a | 50.35 ± 2.25c | 58.08 ± 4.17a | 66.45 ± 5.80 | 71.10 ± 6.77a |
| BB (n = 6) | 64.71 ± 4.95 | 58.14 ± 3.71 | 55.79 ± 2.52 | 56.43 ± 3.53 | 64.21 ± 7.48 | 63.21 ± 6.36 |
| BT (n = 18) | 90.26 ± 12.13 | 64.35 ± 4.61d | 56.65 ± 2.77a | 61.09 ± 7.52 | 75.15 ± 9.25 | 90.94 ± 11.77e |
| TT (n = 8) | 63.88 ± 5.86 | 67.00 ± 12.58 | 57.44 ± 7.83 | 52.13 ± 3.40 | 51.13 ± 2.66 | 54.69 ± 2.95a |
Discussion
Skin biophysical properties represent not only localized skin conditions, but also systemic conditions. Alterations in SC hydration, TEWL and skin surface pH have been found in some skin disorders [36,37,38,39,40]. Age-related changes in skin surface pH, TEWL, SC hydration, as well as sebum content have been well documented [3, 6,41,42,43]. In addition, SC hydration is decreased in hemodialysis patients and subjects with hypothyroidism [7,44,45,46]. CRRT represents another highly sensitive tool to assess skin biophysical properties. With age, CRRT changes with skin stiffness, firmness, thickness, as well as tissue composition [18, 19,47,48,49,50]. Skin firmness is positively correlated with CRRT, while skin stiffness is negatively correlated with CRRT [18]. In the present study, we first demonstrated that CRRTs in leprosy are shorter than normal at most directions. This observation suggests that the cured leprosy skin is stiffer. Secondly, the alterations of CRRT are associated with the severity of skin damage. The reduction of CRRTs at all directions occurs on the hand where deformation and sensory deficit are more severe than on the forearm. The greater reductions of CRRTs on the hand could reflect the increased skin tension caused by hand deformation, because the greater the tension, the lower the CRRT measurement [51]. However, reduction of CRRTs on the forearm in leprosy was only observed in some directions. The exact mechanisms underlying changes in CRRT in leprosy are not clear, but they are most likely due to the extent of damage in the dermis. The dermal connective tissue damage in leprosy includes degenerated collagen [52] and absence of elastic fibers is also found in infiltrated areas in leprosy lesions [35].
In the present study, we also demonstrated that changes in CRRTs, especially on the hands in cured leprosy subjects, are associated with leprosy subtypes. CRRTs on the hands are decreased at almost all directions in LL and BL, which have more severe alteration in immunity. Interestingly, SC hydration is also lower in LL and BL as compared with other subtypes of leprosy. A previous study showed that SC hydration positively correlates with CRRT [18]. Additionally, collagen I and III levels are higher in LL than in other subtypes of leprosy [35]. Thus, both decreased SC hydration and alteration of dermal extracellular matrix components could, at least in part, be attributable to the reduction of CRRT in LL and BL leprosy. However, there are directional differences in CRRTs between cured leprosy and normal subjects. These phenomena strongly suggest that there are other unknown factors, which also have an influence. Nevertheless, the present study clearly demonstrated that the CRRTs are altered in cured leprosy, and further that these alterations are associated with leprosy subtypes. Since CRRT measurements are straightforward and noninvasive, they could be an alternative approach to quantitatively assess both the extent of some cutaneous abnormality and the therapeutic efficacy in leprosy.
Acknowledgements
The authors thank Dr. Joachim W. Fluhr for his critical review and helpful advice. This work was supported by National Institutes of Health grants AR 19098 and the Medical Research Service, Department of Veterans Affairs Medical Center and Guizhou Governor's Foundation Qian 2006-48, the People's Republic of China.
References
Articles from Skin Pharmacology and Physiology are provided here courtesy of Karger Publishers
Full text links
Read article at publisher's site: https://doi.org/10.1159/000231527
Read article for free, from open access legal sources, via Unpaywall:
https://www.karger.com/Article/Pdf/231527
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1159/000231527
Article citations
Split-face comparison of hydroquinone 4% plus nitrogen plasma vs. hydroquinone 4% alone in the treatment of melasma.
Lasers Med Sci, 38(1):113, 27 Apr 2023
Cited by: 0 articles | PMID: 37103690
Evaluation of agreement between clinical and histopathological data for classifying leprosy.
Int J Infect Dis, 17(3):e189-92, 14 Nov 2012
Cited by: 13 articles | PMID: 23158973
Cutaneous resonance running time is decreased in psoriatic lesions.
Skin Res Technol, 18(2):232-237, 25 Aug 2011
Cited by: 4 articles | PMID: 22092918
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Cutaneous resonance running time is decreased in psoriatic lesions.
Skin Res Technol, 18(2):232-237, 25 Aug 2011
Cited by: 4 articles | PMID: 22092918
Cutaneous resonance running time varies with age, body site and gender in a normal Chinese population.
Skin Res Technol, 16(4):413-421, 01 Nov 2010
Cited by: 21 articles | PMID: 21039906 | PMCID: PMC3049161
Abnormalities in stratum corneum function in patients recovered from leprosy.
Skin Pharmacol Physiol, 22(3):131-136, 09 Jan 2009
Cited by: 7 articles | PMID: 19136834 | PMCID: PMC2790797
Scapular inclination and glenohumeral joint stability: a cadaveric study.
J Orthop Sci, 13(1):72-77, 01 Jan 2008
Cited by: 18 articles | PMID: 18274859
Funding
Funders who supported this work.
NIAMS NIH HHS (2)
Grant ID: R01 AR019098
Grant ID: AR 19098