Abstract
Free full text

Downregulating the sucrose transporter VpSUT1 in Verbascum phoeniceum does not inhibit phloem loading
Abstract
Sucrose is loaded into the phloem in the minor veins of leaves before export. Two active, species-specific loading mechanisms have been proposed. One involves transporter-mediated sucrose transfer from the apoplast into the sieve element-companion cell complex, so-called apoplastic loading. In the putative second mechanism, sucrose follows an entirely symplastic pathway, and the solute concentration is elevated by the synthesis of raffinose and stachyose in the phloem, not by transporter activity. Several sucrose-transporting plants have been shown to be apoplastic loaders by downregulating sucrose transporter 1 (SUT1), leading to accumulation of sugars and leaf chlorosis. In this study we compared the effect of downregulating SUT1 in Nicotiana tabacum, a sucrose transporter, and Verbascum phoeniceum, a species that transports raffinose and stachyose. To test the effectiveness of RNAi downregulation, we measured SUT1 mRNA levels and sucrose-H+ symport in leaf discs. Mild NtSUT1 downregulation in N. tabacum resulted in the pronounced phenotype associated with loading inhibition. In contrast, no such phenotype developed when VpSUT1 was downregulated in V. phoeniceum, leaving minimal sucrose transport activity. Only those plants with the most severe VpSUT1 downregulation accumulated more carbohydrate than usual and these plants were normal by other criteria: growth rate, photosynthesis, and ability to clear starch during the night. The results provide direct evidence that the mechanism of phloem loading in V. phoeniceum does not require active sucrose uptake from the apoplast and strongly supports the conclusion that the loading pathway is symplastic in this species.
Phloem loading provides the driving force for long-distance transport from leaves to sink organs in many plants by elevating the carbohydrate content and hydrostatic pressure in the sieve elements (SE) and companion cells (CC) of minor veins (1–3). In a large number of species, phloem loading is mediated by transporters on the plasma membranes of the SEs and CCs. These transporters drive sucrose, and in some cases sugar alcohol, from the apoplast into the SE-CC complex. The most direct proof of apoplastic loading has come from studies in which sucrose transporter 1 (SUT1/SUC2) is downregulated by antisense technology or DNA insertion (4–9). Such downregulation inhibits phloem loading and results in accumulation of sugars and starch, stunted growth, diminished photosynthesis, and leaf chlorosis. These experiments confirm the necessity of transmembrane transport of sucrose in these species and the indispensable role of SUT1 in apoplastic phloem loading.
In species that load from the apoplast, relatively few plasmodesmata connect the SE-CC complex to surrounding cells (10, 11). This is unsurprising given that extensive symplastic continuity would create a futile cycle of active loading from the apoplast and passive leakage back to mesophyll cells. However, in many other species plasmodesmata in the minor veins are numerous, sometimes extremely so (10–12). Plasmodesmata are especially numerous between bundle sheath cells and specialized companion cells, known as intermediary cells, in the minor veins of species that transport raffinose-family oligosaccharides (RFOs), raffinose and stachyose. The presence of these abundant plasmodesmata raises the possibility that, in RFO plants, sucrose stays within the symplast all the way from the mesophyll cells to the SEs, so-called symplastic loading.
The concept of apoplastic loading, mediated by transporters, is intuitively attractive because it employs the proton motive force to transfer sucrose into the phloem against a thermodynamic gradient. Symplastic loading, on the other hand, has been viewed with more caution because symplastic flux, through the cytoplasmic sleeve of plasmodesmata, is passive; there is no obvious direct mechanism for establishing an uphill concentration gradient (13–16). Yet, in RFO-transporting plants, the concentration of sugar in the phloem sap is comparable to that of apoplastic loaders and considerably higher than in bundle sheath and mesophyll cells (16, 17). This is a phenomenon that requires explanation: two adjacent plant cell types—bundle sheath and companion cell—linked by abundant plasmodesmata, yet with highly different solute concentrations and hydrostatic pressures.
A “polymer trap” mechanism (18–20) has been proposed to explain how sucrose is loaded through plasmodesmata in RFO plants. According to this model, sucrose diffuses through plasmodesmata from mesophyll cells to intermediary cells, where it is converted into RFOs. These larger sugars cannot diffuse back again due to their size. If this hypothesis is correct, sucrose transporters do not play a direct role in loading and their downregulation should have little, if any, effect on translocation.
Although the polymer trap hypothesis provides a theoretical framework for symplastic loading, it has been suggested that loading in RFO plants is nonetheless apoplastic and transporter driven (13, 14, 21). Indeed, sucrose transporters are found in the leaves of plants with abundant plasmodesmata, and SUT1 has been localized to the minor veins of an RFO-transporting species (13).
Until recently it has not been possible to test the concept of symplastic loading directly by downregulating SUT1, due to the lack of a readily transformable model plant. However, Verbascum phoeniceum, an RFO-transporting species, has been found to transform with ease (18). Earlier experiments demonstrated that RFO synthesis is required for efficient phloem loading in V. phoeniceum (18) but these experiments did not address the fundamental question of how sucrose enters the minor veins. The results presented here indicate that severe downregulation of SUT1, leading to almost complete loss of sucrose transporter activity, has little effect on long-distance transport.
Results
SUT1 in V. phoeniceum.
Degenerate primers were designed based on the alignment of SUT1 sequences from five species, including AmSUT1 from Alonsoa meridionalis, an RFO plant. An 816-bp fragment amplified from V. phoeniceum leaf cDNA with these primers was used as a probe to screen a cDNA library of mature V. phoeniceum leaves. Numerous screenings were carried out with different stringencies to identify members of the SUT1 gene family in V. phoeniceum. All identified clones encoded the same sequence with an ORF of 511 amino acids. The deduced amino acid sequence is highly homologous to SUT1 from A. meridionalis (80%), Asarina barclaiana (79%), Nicotiana tabacum (74%), Solanum tuberosum (73%), and Plantago major (75%) (13, 14, 22–24). The identified sucrose transporter gene from V. phoeniceum was named VpSUT1 and the sequence deposited in GenBank (accession FJ797307).
Transgenic Plants.
RNAi constructs were designed for the downregulation of SUT1 in V. phoeniceum and N. tabacum. Over 100 independent transgenic lines of each species were generated. In tobacco, visible chlorosis developed on the leaves of approximately 90% of the transgenic plants in a manner similar to that described when NtSUT1 is downregulated by antisense technology (4). The severity of the phenotype differed considerably in the various lines. In the weak phenotype tobacco line NtT1.27.2, one area approximately 2 cm in diameter between the third and fourth major vein on each mature leaf became chlorotic, while the rest of the leaf was healthy. The heights of the wild-type and transgenic plants with weak phenotypes were indistinguishable. However, transgenic tobacco plants with a more severe phenotype were noticeably stunted and the leaves were more chlorotic (Fig. 1A). Chlorosis developed in a basipetal pattern that paralleled the sink-source transition and became progressively more acute with leaf age. This chlorosis was more evenly distributed throughout the lamina than in plants with a weak phenotype and the leaves were smaller than in wild-type plants. Transgenic plants with very severe chlorosis grew to a height of less than 15 cm and did not produce seeds, even after 12 months in the greenhouse.

Representative wild-type and transgenic Nicotiana tabacum (A) and Verbascum phoeniceum (B) plants. Left to right (A): wild-type, weak, moderate and severely downregulated NtSUT 1 RNAi lines; (B): wild-type (left) and three significantly downregulated VpSUT1 RNA lines. Note that the degree of downregulation of NtT1.19.7 and VpT2.27.2 is similar. (Scale bars, A = 10 cm and B = 4 cm.)
Transgenic V. phoeniceum plants grew and developed normally (Fig. 1B) in growth chambers and in a greenhouse with supplemental lighting (600–800 μmol photons m−2 s−1). With the exception of a single individual, apparently suffering from an unrelated problem sometimes noted in wild-type plants, none of the more than 100 transgenic plants displayed evidence of chlorosis and there was no distinguishable difference in the size of leaves of transgenics and wild-type plants.
Downregulation of Sucrose Uptake and SUT1 Expression.
The capacity of leaf tissue to take up exogenous sucrose, a measure of sucrose transporter activity (20), was measured by incubating leaf discs in Mes-buffered [14C]sucrose for 1 h before washing and scintillation counting. Preliminary experiments indicated that uptake was linear with time for more than 2 h. Autoradiographs of leaf discs demonstrated that radiolabel was localized primarily in the veins of wild-type plants of both species (Fig. 2 A and D).
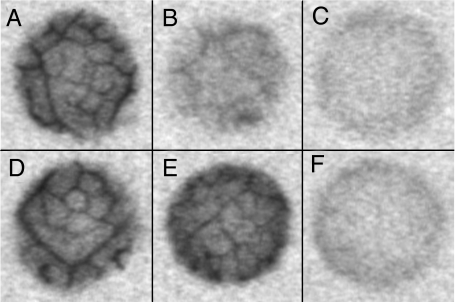
Autoradiographs of Nicotiana tabacum (A–C) and Verbascum phoeniceum (D–F) leaf discs exposed to [14C]sucrose. A and D are from wild-type plants, B and E from plants with moderate SUT1 downregulation (NtT1.19.5 and VpT2.27.10, respectively), and C and F from plants with severe SUT1 downregulation (NtT1.19.6 and VpT2.27.2, respectively). Diameter of leaf discs, 1.6 mm.
Quantitative uptake experiments were conducted with and without p-chloromercuribenzenesulfonic acid (PCMBS). PCMBS completely inhibits sucrose transporters but does not block uptake by a second, non-saturable transport mechanism (25–27). The second mechanism, constituting approximately 28% of total uptake in wild-type N. tabacum and V. phoeniceum, is not involved in phloem loading (see Discussion). The results reported here are for the PCMBS-sensitive phase only (see Materials and Methods).
Leaf tissue from phenotypic transgenic tobacco lines took up less [14C]sucrose than leaf tissue from wild-type plants (Fig. 3A). In the 20 independent tobacco lines tested, inhibition ranged from 23–93% and was most severe in transgenic plants that displayed the most severe chlorotic symptoms and retarded growth. Downregulation had no statistically-significant effect on PCMBS-insensitive uptake. Downregulation of SUT1 expression ranged from 4- to 7-fold in tobacco lines with moderate chlorosis to approximately 23-fold in plants with severe chlorosis (Fig. 4A).

PCMBS-sensitive [14C]sucrose uptake by leaf discs of (A) wild-type (NtWT) and transgenic Nicotiana tabacum and (B) wild-type (VpWT) and transgenic Verbascum phoeniceum lines. Results are from individual transgenics and from either two (A) or three (B) wild-type plants. PCMBS-sensitive (transporter driven) uptake values were obtained by subtracting uptake in the presence of PCMBS from uptake without PCMBS. Error bars, SE (n = 6 for NtWT, n = 9 for VpWT, n = 3 for transgenics). Uptake rates for discs from wild-type plants were 135 nmol cm−2 h−1 and 229 nmol cm−2 h−1 for tobacco and V. phoeniceum, respectively.

Real-time PCR expression patterns of SUT1 in Nicotiana tabacum (A) and Verbascum phoeniceum (B) leaves. Results are from individual transgenics and from either two (A) or three (B) wild-type (WT) plants. Expression levels are normalized to wild-type. Error bars, SE (n = 6 for NtWT, n = 9 for VpWT, n = 3 for transgenics).
Veins were visible in autoradiographs of wild-type (Fig. 2A) and most transgenic tobacco lines, although the intensity of the vein images declined with the degree of downregulation (Fig. 2B). No veins were visible in the autoradiographs from the most severely downregulated lines (Fig. 2C).
Since transgenic V. phoeniceum plants did not exhibit chlorotic symptoms, all of the more than 100 independently derived lines were screened for [14C]sucrose uptake capacity. Inhibition of PCMBS-sensitive [14C]sucrose uptake ranged from 0–93% in the transgenics, compared to wild-type plants (Fig. 3B). Quantitative RT-PCR on RNA of leaf tissue from representative plants indicated that, as in tobacco, plants with diminished [14C]sucrose uptake exhibited significant SUT1 downregulation. Downregulation in most of the lines with moderate reduction of [14C]sucrose uptake ranged from 6- to 10-fold while in lines VpT2.27.4 and VpT2.27.2, with the most severe repression of [14C]sucrose uptake, SUT1 was downregulated 17- and 24-fold, respectively (Fig. 4B). It is possible that there are other sucrose transporters, in addition to SUT1, in the V. phoeniceum leaf. However, if this is so, they must not play an appreciable role in sucrose uptake from the apoplast since downregulation of SUT1 alone results in almost complete inhibition of [14C]sucrose uptake. As with tobacco, SUT1 downregulation had no statistically-significant effect on PCMBS-insensitive uptake.
Veins were visible in autoradiographs of leaf discs from wild-type V. phoeniceum plants (Fig. 2D). Unlike tobacco, the intensity of vein images was not noticeably diminished in moderately downregulated lines (Fig. 2E). In the most severely downregulated line (VpT2.27.2), veins were apparent in autoradiographs of approximately 20% of the discs; no veins were apparent in the remaining 80% (Fig. 2F)
Carbohydrate Accumulation and Photosynthesis.
Soluble carbohydrates accumulated in the mature leaves of tobacco transgenics, as expected when phloem transport is inhibited (4–9). In transgenics grown in the relatively low light of the growth chamber (350 μmol of photons m−2 s−1), the sum of sucrose, glucose and fructose levels ranged from 5-fold to 28-fold in excess of those found in wild-type plants (Fig. 5A). To determine whether transgenic plants are able to export carbohydrate stored as starch, plants were kept in the dark for periods of 12–72 h. Although the leaves of wild-type tobacco plants lost their starch within 12 h, starch was readily apparent in the mesophyll cells of transgenic tobacco plants with mild to severe downregulation of SUT1, even after 72 h, as demonstrated by I/KI staining. In plants with mild or moderate downregulation of SUT1, starch accumulated in some areas of the lamina but not in others. Chlorosis was also patchy in these leaves, whereas in leaves with severe downregulation chlorosis developed more evenly throughout the lamina. As previously described (4) downregulation of NtSUT1 led to pronounced inhibition of photosynthesis (Fig. 6A). This was the case in all tested tobacco transgenic lines, including the plant with the weakest phenotype (NtT1.27.2) (Fig. 6A).
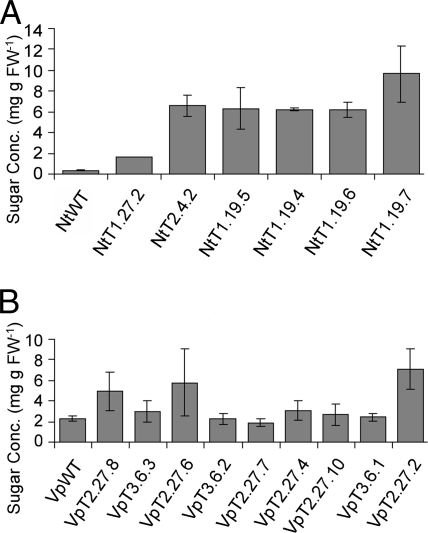
Soluble sugars in mature leaves of Nicotiana tabacum (A) and Verbascum phoeniceum (B). Results are from individual transgenics and from either two (A) or three (B) wild-type (WT) plants. Error bars, SE (n = 6 for NtWT, n = 9 for VpWT, n = 3 for transgenics) except N. tabacum line NtT1.27.2. Only one sample was analyzed for this line due to the limited amount of plant material.

Net photosynthesis in wild-type (WT) and transgenic lines of Nicotiana tabacum (A) and Verbascum phoeniceum (B). Results are from individual transgenics and from either two (A) or three (B) wild-type plants. Error bars indicate SE (n = 6 for NtWT, n = 9 for VpWT, n = 3 for transgenics).
The leaves of most tested V. phoeniceum transgenic plants with significant down regulation of SUT1 did not accumulate soluble carbohydrates in excess of amounts in wild-type plants (Fig. 5B). Line VpT2.27.2, with the most severe downregulation of RNA and sucrose transport activity, accumulated 2.8-fold more soluble carbohydrate than wild-type (Fig. 5B).
The amount of soluble carbohydrate accumulation in transgenic V. phoeniceum leaves was negligible compared to the amount exported. Given the rate of net photosynthesis, the fresh weight of mature leaves (32.4 mg cm−2), and an assumed export rate of 80% of carbon fixed daily (which is typical for herbaceous plants), the leaves exported approximately 154 mg carbon g−1 fresh weight from the time they reached full size to harvest, approximately 8 days later. Since the leaves of line VpT2.27.2. accumulated only 2 mg carbon g−1 fresh weight more than wild-type leaves during this period, this constituted less than 2% of the amount exported.
Transgenic V. phoeniceum plants exported carbon from starch normally. Even in line VpT2.27.2, starch was no longer visible after the plants had been kept in the dark for 12 h. Rates of net photosynthesis were indistinguishable from wild-type plants (Fig. 6B).
Discussion
Downregulating sucrose transporters is the most compelling approach found to date to test models of phloem loading. Downregulation of SUT1 by antisense technology or DNA insertion results in severe inhibition of phloem loading as demonstrated by accumulation of soluble carbohydrates in leaves, reduced photosynthesis, stunted growth and leaf chlorosis (4–6, 8, 9). According to these results, SUT1 appears to be the primary, if not the only, sucrose transporter responsible for apoplastic phloem loading.
In our experiments, a 393-bp fragment based on tobacco SUT1 cDNA sequence CAA57727 was amplified to make an RNAi construct. The amplified partial sequence shared 98 and 95% similarity at the nucleic acid level with CAQ58422 and FM164638, two identified NtSUT1 genes, respectively (24). Considering that downregulation of NtSUT1 resulted in virtually identical symptoms of loading inhibition described for NtSUT1 antisense plants (4), and did so in approximately 90% of the independently-derived transgenic lines, the results support the conclusion that NtSUT1 is primarily responsible for phloem loading in tobacco leaves.
To determine if downregulating NtSUT1 inhibits sucrose uptake from the apoplast, we measured uptake of [14C]sucrose into leaf discs from wild-type and transgenic tobacco plants. The leaf disc technique has been widely used to characterize the in vivo properties of mono- and disaccharide transporters (28–33) and to correlate rates of phloem loading with photosynthetic capacity (34). Sucrose uptake into leaf discs is biphasic (35). A saturable, PCMBS-sensitive phase displaying Michaelis-Menten kinetics is superimposed on a linear phase that is insensitive to PCMBS. The linear phase does not drive phloem loading (36, 37). The PCMBS-sensitive portion of total uptake represents sucrose-transporter-mediated uptake activity (25–27). In transgenic tobacco lines, PCMBS-sensitive uptake into leaf discs declined with increasing downregulation of NtSUT1 expression. It is apparent that if all three members of the NtSUT1 family are active, they were all downregulated by the partial sequence from CAA57727 because the sucrose uptake ability in severe phenotype lines almost disappeared. Autoradiographic analysis indicated that exogenous [14C]sucrose was primarily taken up by the veins, consistent with SUT1 localization in the phloem (23), and that the intensity of the vein images declined with downregulation of SUT1.
The phenotype of transgenic tobacco plants in this study is essentially the same as that described for plants in which SUT1 is downregulated by antisense technology. We noted, as did Kühn et al. (38), that some areas of affected leaves accumulate starch while others do not. Therefore, quantitative measurement of starch in whole transgenic leaves may not accurately reflect processes occurring within localized affected regions.
The same experimental design used on tobacco was used to test the hypothesis that phloem loading is driven by sucrose transporter activity in V. phoeniceum. When VpSUT1 was downregulated in V. phoeniceum, PCMBS-sensitive [14C]sucrose uptake also declined, indicating that, as in tobacco, SUT1 activity is primarily, if not entirely, responsible for energized uptake of sucrose from the apoplast. We cannot exclude the possibility that other sucrose transporters are expressed in V. phoeniceum leaves, but if they are, they must share enough sequence similarity with the identified VpSUT1 to be downregulated simultaneously, or these sucrose transporters do not account for an appreciable amount of sucrose transporter activity, since severely downregulating VpSUT1 essentially eliminated all PCMBS-sensitive sucrose uptake into the leaf discs (Fig. 3B). It should be noted that, although SUT1 downregulation appears to be more uniformly severe in transgenic V. phoeniceum in comparison to tobacco in Figs. 3 and and4,4, this is due to the conscious choice of lines with these properties, not due to inherent differences in susceptibility.
From autoradiographs of leaf discs it is clear that exogenous [14C]sucrose accumulates in the veins of wild-type V. phoeniceum leaves, as in tobacco. However, downregulation of SUT1 did not appreciably modify the V. phoeniceum vein images, except in the most severely downregulated line, and then not consistently. These results are similar to those in which sucrose transporter activity in RFO-transporting plants is inhibited with PCMBS (19). Apparently, when sucrose transporter activity is blocked, [14C]sucrose enters the tissue by the non-saturable, PCMBS-insensitive uptake mechanism (36, 37) and is then sequestered in the veins by diffusion through plasmodesmata and polymer trapping in the intermediary cells (34). However, the genetic technique used here circumvents several objections raised against the pharmacological approach to blocking sucrose transporter activity with PCMBS, including lack of specificity and difficulties with tissue infiltration (39).
The major difference between the results on V. phoeniceum and tobacco is that downregulating SUT1 and reducing sucrose uptake capacity in V. phoeniceum did not have an appreciable effect on phloem loading in any of the more than 100 independently derived transgenic lines grown either in low (growth chamber) or high (greenhouse) light. It could be argued that V. phoeniceum does not display the same symptoms as tobacco when loading is inhibited, but this is clearly not the case since downregulating RFO synthesis in the same cultivar of this species, using RNAi technology as in the present study, results in the classic symptoms of transport inhibition: excessive accumulation of soluble carbohydrates, inability to transport starch during an extended dark period, stunted growth, and leaf chlorosis (18).
What is the function of VpSUT1, if not in phloem loading? Results from several types of experimentation indicate that sucrose leaks continuously from phloem cells into the apoplast and must be retrieved in order for long-distance transport to operate efficiently (13, 40–43). This may be the reason that sucrose transporters localize to the phloem in the petiole and stem, in addition to the minor veins, in many species. In A. meridionalis, SUT1 localizes to the ‘ordinary’ CCs that are present in the minor veins in addition to intermediary cells. A. meridionalis is in the same family as V. phoeniceum and its minor vein anatomy and transport sugars are essentially identical (13, 44).
The retrieval function could also explain why leaf discs of wild-type V. phoeniceum take up more exogenous sucrose than discs from wild-type N. tabacum (Fig. 3). If retrieval along the path phloem requires more transporter activity than loading itself, this could mask differences in the latter activity. Also, it should be kept in mind that rates of uptake from exogenous solutions in different species can differ due to dissimilarities in tissue thickness and geometry that affect diffusion rates.
Inefficient retrieval may be the reason that soluble carbohydrates accumulate to a limited extent in the most severely downregulated V. phoeniceum transgenics. However, even in these severely affected plants, with almost no residual active sucrose uptake capacity, photosynthetic rates are normal and starch is cleared from mesophyll cells during the night period, indicating that there is little effect on the overall capacity of leaves to produce, load and transport soluble carbohydrates. These data provide molecular genetic evidence that, in species with abundant minor vein plasmodesmata, photosynthates are able to migrate from mesophyll cells to the phloem along an entirely symplastic pathway, and that differences in sugar concentration and hydrostatic pressure between mesophyll cells and the phloem, sufficient to drive long-distance transport, can be established and maintained without the need for active sucrose uptake from the apoplast. Since phloem unloading is often symplastic as well (3), it seems likely that, in many species, photoassimilate travels from the cytosol of mesophyll cells to sink cells without crossing a single plasma membrane, a finding with potentially important implications in the study of carbon partitioning.
Materials and Methods
Plant Material.
Seeds of V. phoeniceum L. cv. “Flush of White” (Garden Makers) and N. tabacum “Petite Havana SRI” were surface-sterilized and germinated as described (18). Plants were kept in a growth chamber with 14-h day/10-h night cycles at approximately 100 μmol photons m−2 s−1 for plant transformation. Transgenic plants of both species were grown either in a growth chamber on a 14-h day/10-h night cycle with 350 μmol photons m−2 s−1, or in a greenhouse with 600–800 μmol photons m−2 s−1.
Gene Cloning.
Degenerate primers for sucrose transporter 1 (SUT1) were designed based on the amino acid sequence alignment of AmSUT1 (13), AbSUT1 (14), StSUT1 (23), PmSUC2 (22), and AtSUC1 and AtSUC2 (45). Primers used for the amplification of the V. phoeniceum SUT1 fragment were 5′-GGSGTGCARTTCGGNTGGGCNYT-3 (forward) and 5′-GTAMACCTCYTTNCCCATCCARTC-3′(reverse). RNA was isolated from mature leaves of V. phoeniceum using the Plant RNA Mini Kit (Omega Bio-Tek). cDNA was synthesized using oligo(dT) primer and SuperScript RT (Invitrogen). The 816-bp fragment amplified from V. phoeniceum was used as a 32P labeled probe to screen a cDNA library from mature leaves with different stringencies (18). Clones were sequenced by Cornell Bioresource Center and the homology analysis of derived sequences was conducted with BLASTx.
RNAi Vector Construction and Plant Transformation.
For tobacco, based on GenBank sequence CAA57727, a 393-bp sense fragment, with XhoI and EcoRI at the two ends, was amplified by forward primer 5′-AATGCCTCGAGGGCGGAAAAGCGAGGATGA-3′ and reverse primer 5′AATGCGAATTCCGGAAACCTCGCAATCCAG-3′. A 393-bp anti-sense fragment, with XbaI and HindIII at the two ends, was amplified by forward primer 5′-AATGCTCTAGAGGCGGAAAAGCGAGGATGA-3′ and reverse primer 5′-AATGCAAGCTTCGGAAACCTCGCAATCCAG-3′. For V. phoeniceum, a 352-bp sense fragment, with XhoI and KpnI at the two ends, was amplified by forward primer 5′-AATGCCTCGAGCGATTGGATGGGTCGGGA-3′ and reverse primer 5′-AATGCGGTACCTGCCGCGAGAGGGATACC-3′. A 352-bp anti-sense fragment, with XbaI and ClaI at the two ends, was amplified by forward primer 5′-AATGCTCTAGACGATTGGATGGGTCGGGA-3′ and reverse primer 5′-AATGCATCGATTGCCGCGAGAGGGATACC-3′. The two hairpin cassettes were released from pHANNIBAL by restriction digestion and subcloned into the NotI site of binary vector pART27 for Agrobacterium (GV3101)-mediate transformation in the two species. Transformation of tobacco followed standard methods. Transformation of V. phoeniceum followed the procedure previously described (18), but with revisions as detailed in SI Text.
Quantitative RT-PCR Expression Analysis.
To prepare template for quantitative RT-PCR, 0.5 μg total RNA was used to synthesize cDNA using the iScript cDNA synthesis kit (Bio-Rad). Quantitative RT-PCR was performed using SYBR Green I technology on a Bio-Rad iQ5 system (Bio-Rad Laboratories). Primer sequences were forward 5′-GGGCTCTAAAGTTCTCTTAATGGGC-3′ and reverse 5′-CTCTAACACACCCTTAGCATTTCC-3′ for V. phoeniceum, forward 5′-TACAGTGACAATTGCTCCTCCCGT-3′ and reverse 5′-GGCATGTCCAAGATCAGCAGCAAA-3′ for tobacco, and forward 5′-CGCGGAAGTTTGAGGCAATAA-3′ and reverse 5′-TCGGCCAAGGCTATAGACTCGT-3′ for 18S rRNA. The 50-μL quantitative RT-PCRs contained 1× iQ SYBR Green PCR supermix (Bio-Rad Laboratories), 300 nM of each primer, and 1 μL template cDNA. The amplification protocol consisted of an initial cycle of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 55 °C for 1 min. Primer specificity was evaluated by melting curve analysis. All samples were amplified in triplicate and averaged. Data analysis was performed using the relative standard curve method.
Sucrose Uptake Assay.
Leaf discs (1.6-mm diameter) from the first fully expanded mature leaf were collected with a belt punch (C. S. Osborne) at 14:00 to 15:00 and incubated in Mes [2-(N-Morpholino) ethanesulfonic acid] buffer pH 5.5, containing 9.9 Bq [14C]sucrose and 1 mM unlabeled sucrose, with or without PCMBS (5 mM) for 1 h, and washed three times with ice-cold buffer for a total of 1 h. Sugar extraction and scintillation counting procedures were as described (18). The PCMBS-insensitive portion of uptake was subtracted from total uptake to obtain PCMBS-sensitive values. Thirty leaf discs were used per replicate and three replicates were conducted per experiment. For autoradiography, leaf discs were flash frozen, lyophilized, pressed thin, and applied to BioMax MR film (Kodak) (32).
Photosynthesis and Carbohydrate Content.
A LI-COR 6400 (LI-COR Biotechnology) was used to analyze CO2 uptake in the first three fully expanded leaves of each plant at 600 μmol m−2 s−1 with a CO2 concentration of 360 μmol m−2 s−1. Sugars were analyzed by HPLC as described (18, 46), using xylitol as internal standard.
Acknowledgments.
We thank L. Cheng, P. Li, W. Miller, and R. Harmon for the use of equipment; Bertrand Lasseur, Edwin Reidel, and Jingying Liu for assistance; and Andre Jagendorf for critical reading of the manuscript. This study was supported by National Science Foundation Grant IOB 0444119 (to R.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. FJ797307).
This article contains supporting information online at www.pnas.org/cgi/content/full/0904189106/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0904189106
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2774004?pdf=render
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/reprint/106/44/18849.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/abstract/106/44/18849
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/full/106/44/18849
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Protocol for analyzing the movement and uptake of isotopically labeled signaling molecule azelaic acid in Arabidopsis.
STAR Protoc, 5(2):102944, 11 Mar 2024
Cited by: 0 articles | PMID: 38470913 | PMCID: PMC10945267
The transcription factor CmERFI-2 represses CmMYB44 expression to increase sucrose levels in oriental melon fruit.
Plant Physiol, 192(2):1378-1395, 01 May 2023
Cited by: 2 articles | PMID: 36938625
Leaf photosynthesis is positively correlated with xylem and phloem areas in leaf veins in rice (Oryza sativa) plants.
Ann Bot, 129(5):619-631, 01 Apr 2022
Cited by: 7 articles | PMID: 35143609 | PMCID: PMC9007091
Sucrose Utilization for Improved Crop Yields: A Review Article.
Int J Mol Sci, 22(9):4704, 29 Apr 2021
Cited by: 33 articles | PMID: 33946791 | PMCID: PMC8124652
Review Free full text in Europe PMC
Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants.
Int J Mol Sci, 22(1):E318, 30 Dec 2020
Cited by: 39 articles | PMID: 33396811 | PMCID: PMC7795015
Review Free full text in Europe PMC
Go to all (38) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Nucleotide Sequences (4)
- (3 citations) ENA - CAA57727
- (2 citations) ENA - FJ797307
- (1 citation) ENA - FM164638
- (1 citation) ENA - CAQ58422
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides.
Proc Natl Acad Sci U S A, 104(49):19619-19624, 28 Nov 2007
Cited by: 43 articles | PMID: 18048337 | PMCID: PMC2148338
Phloem loading in cucumber: combined symplastic and apoplastic strategies.
Plant J, 98(3):391-404, 12 Feb 2019
Cited by: 27 articles | PMID: 30604489
Sucrose transporter1 functions in phloem loading in maize leaves.
J Exp Bot, 60(3):881-892, 30 Jan 2009
Cited by: 129 articles | PMID: 19181865 | PMCID: PMC2652052
Photosynthetic acclimation in the context of structural constraints to carbon export from leaves.
Photosynth Res, 94(2-3):455-466, 09 Jan 2007
Cited by: 30 articles | PMID: 17211580
Review





