Abstract
Free full text

New Mechanisms of Pulmonary Fibrosis
Abstract
The understanding of the pathogenesis of pulmonary fibrosis continues to evolve. The initial hypothetical model suggested chronic inflammation as the cause of pulmonary fibrosis, whereas a subsequent hypothesis posited epithelial injury and impaired wound repair as the etiology of fibrosis without preceding inflammation. Over the past decade, several concepts have led to refinement of these hypotheses. These include the following: (1) the importance of the integrity of the alveolar-capillary barrier basement membrane (BM) to conserving the architecture of the injured lung; (2) conversely, that the failure of reepithelialization and reendothelialization of this BM results in pathologic fibrosis; (3) transforming growth factor-β is necessary but not sufficient to the pathologic fibrosis of the lungs; (4) the role of persistent antigens in the pathogenesis of usual interstitial pneumonia; and (5) the contribution of epithelial-to-mesenchymal transformation and bone marrow-derived progenitor cells in the pathogenesis of lung fibrosis. In this review, we will discuss these evolving conceptual mechanisms for the pathogenesis of pulmonary fibrosis relevant to idiopathic pulmonary fibrosis.
Our understanding of the pathogenesis of idiopathic pulmonary fibrosis (IPF) continues to evolve. The initial hypothetical model of the development of this illness, proposed > 3 decades ago, suggested that chronic inflammation was the underlying cause of pulmonary fibrosis.1,2 This hypothesis was subsequently called into question based on the following two clinical observations: (1) that measures of tissue inflammation correlate poorly to the severity or outcome of pulmonary fibrosis; and (2) that the use of immunosuppressive drugs does not influence the natural history of the disease.3 Instead, a competing hypothesis was proposed that the pathogenesis of IPF is the consequence of epithelial injury of unknown cause followed by abnormal wound healing, but is independent of preceding inflammation.
Over the past decade, the following several concepts have lead to refinement of these hypotheses: (1) the loss of alveolar-capillary barrier basement membrane (BM) integrity represents the “point of no return” that favors pathologic fibrosis instead of reestablishing normal lung architecture; (2) the failure of reepithelialization and reendothelialization in the context of the loss of the BM integrity in usual interstitial pneumonia (UIP) associated with IPF leads to destroyed lung architecture and pathologic fibrosis; (3) transforming growth factor (TGF)-β is necessary but not entirely sufficient to promote permanent fibrosis; (4) a persistent injury/antigen/irritant is necessary for the propagation of fibrosis; and (5) epithelial to mesenchymal transition (EMT) and a bone marrow-derived progenitor cell (the fibrocyte) are critical cellular mechanisms in the regulation of fibrosis. In this review, we will discuss these evolving conceptual mechanisms for the pathogenesis of pulmonary fibrosis relevant to IPF.
Loss of Alveolar-Capillary Barrier BM Integrity as the Point of No Return
The alveolar-capillary barrier represents the gas exchange surface of the lungs and is, by far, the largest surface area within the lungs that separates the interior of the body from the environment. Ultrastructurally, this barrier is composed of the type I alveolar epithelial cell, the capillary endothelial cell, and their respective BMs. Over about one-half of the barrier, the two BMs are fused, and in the remainder they are separated by a thin sheet of interstitium. Since both the epithelial and the endothelial cells are flat cells with large surface areas but little cytoplasm, most of the barrier is composed of four phospholipid bilayers, minimal cytoplasm and the BMs, and is as thin as 0.3 μm.4
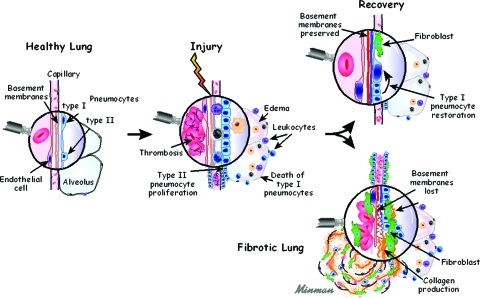
Acute lung injury and normal repair of the alveolar-capillary barrier results in a rapid restoration of tissue integrity and function following a variety of insults. Following injury to this barrier, the process immediately begins with hemorrhage and extravasation of plasma into lung tissue.5–7 Platelet activation and degranulation also occurs during coagulation, leading to the release of a number of lipid mediators and cytokines into the provisional matrix.5–7 These lipid mediators and cytokines are either important growth factors or chemotaxins that incite leukocyte, endothelial cell, fibroblast/myofibroblast, and epithelial cell activation.5–7 The transition of tissue repair of the alveolar-capillary barrier from acute inflammation to the deposition of extracellular matrix (ECM) is an essential event. If the BM is intact and the stimulus for the original injury has been removed, the deposition of ECM is remodeled/reabsorbed and occurs concurrently with the reepithelialization and reendothelialization of the alveolar-capillary barrier.5–7 The normal repair process is complete when the epithelium and endothelium have reestablished their normal spatial orientation on the BM7,8 (Fig 1).
In contrast, the pathogenesis of fibrosis in patients with diffuse parenchymal lung diseases is associated with the following events: (1) the loss of type I epithelial cells and endothelial cells7–13; (2) the loss of the integrity of the BM of the alveolar-capillary barrier with collapse of the alveolar structures and fusion of their BMs8–14; (3) the proliferation of type II alveolar epithelial cells and endothelial cells on an inappropriate ECM without reestablishment of normal alveolar structures8–14; and (4) the recruitment and proliferation of fibroblasts and myofibroblasts, with the deposition of mature ECM leading to end-stage alveolar fibrosis.8–14 This process results in the loss of alveolar structures that can include the entire anatomical lobule.8–14 The evolution of ECM deposition is associated with a hypocellular state and a loss of cells that produce proteinases, which could normally be involved in remodeling/reabsorbing the ECM.8–14 The process leads to the end-stage histopathology of UIP (Fig 1).
The integrity of the alveolar-capillary barrier BM has been underappreciated as the pivotal determinant of whether the injured lung returns to normal or is replaced by a scar, with complete loss of the alveolar structures. The loss of this structure and function is dependent on the magnitude of persistent inflammation and exposure to specific proteinases related to the significant loss of the epithelium and endothelium of the alveolar-capillary barrier.8,14 Therefore, the integrity of the alveolar-capillary barrier BM dictates whether normal reepithelialization and endothelialization will occur with preservation of the alveolar structures; or whether the loss of BM integrity of the alveolar-capillary barrier will contribute to alveolar collapse and fusion of adjacent BMs, contributing to the loss of alveolar structures, including the entire anatomical lobule.7,8,14
Failure of Reepithelialization and Reendothelialization Due to the Loss of Alveolar-Capillary Barrier BM Leads to Destroyed Lung Architecture and Fibrosis
An ultrastructural analysis of tissue from the lungs of patients with IPF demonstrated the following: (1) both type I pneumocyte and endothelial cell injury with loss of integrity of the alveolar-capillary barrier BM8–14; (2) intraalveolar deposition of ECM with accumulation of fibroblasts and myofibroblasts; and (3) obliteration and fibrosis of the alveoli with fusion of adjacent alveolar-capillary barrier BM residual structures, leading to the development of fibroblastic foci.8–14 A three-dimensional reconstruction of UIP has demonstrated thickening of the visceral pleura, extensive vascular remodeling, and fibroblastic foci that appear to be interconnected by fascial planes of connective tissue.15 Moreover, the nature of these interconnected fibroblastic foci highlights that these structures are not anatomically separate entities.15 This supports the notion that fibroblastic foci may represent the loss of alveolar architecture of an entire anatomical respiratory lobule of the lung. The anatomical respiratory lobule structure represents the respiratory unit of the lung, consisting of a terminal bronchiole, respiratory bronchioles, alveolar ducts, alveoli with associated pulmonary arteries, alveolar capillaries, and a pulmonary venule supported by alveolar-capillary barrier BM and interstitial ECM.16 Physiologically, these lobular structures are interconnected, as shown by the ability of the lung tissue to demonstrate collateral ventilation. Therefore, the respiratory lobule (airspace or vascular side) may represent the anatomical target of persistent or recurrent injury that leads to the loss of alveolar-capillary barrier BM integrity with organizing fibrosis, which ultimately destroys the integrity of the entire respiratory lobule, leading to the formation of the fibroblastic foci of UIP. In other words, the above findings support the notion that UIP is a process that may result in the targeted loss of respiratory lobules leading to the formation of fibroblastic foci.
Cytokine Mediators of Lung Fibrosis and Loss of Tissue Architecture
TGF-β is a pleiotropic cytokine that has been found to be highly associated in promoting fibrosis. TGF-β itself has been found to promote fibrosis in the lung. Adenoviral transgenic delivery and the overexpression of active TGF-β promotes marked fibrosis in rodent models.17 However, in another study,18 conditional transgenic mice were generated that express active TGF-β in airway cells. TGF-β overexpression led to peribronchial fibrosis with extension to the adjacent lung parenchyma.18 In contrast, if they “turned off” the TGF-β transgene after peribronchial fibrosis has developed, the fibrosis was completely reabsorbed with the return of normal peribronchial and lung parenchymal architecture.18 These findings demonstrate that TGF-β was necessary to induce fibrosis, but was not entirely sufficient to maintain fibrosis. In another study,19 adenoviral delivery of human interleukin (IL)-1β resulted in marked inflammation and destruction of the lung architecture followed by persistent fibrosis, which appeared to be mediated by the sustained expression of TGF-β. In contrast to the conditional expression of TGF-β, the overexpression of IL-1β led to the loss of integrity of the alveolar-capillary barrier BM with failure of reepithelialization or reendothelialization, leading to destroyed lung architecture (ie, the point of no return with ultimate fibrosis). Thus, TGF-β appears to be necessary but not sufficient to perpetuate fibrosis and loss of lung architecture.
Other cytokine growth factors have long been implicated in the pathogenesis of pulmonary fibrosis. Platelet-derived growth factor and insulin-like growth factor are spontaneously generated in the lungs of patients with diverse interstitial lung diseases,20–26 whereas the production of hepatocyte growth factor by lung fibroblasts is reduced in IPF patients.27 Treatment with platelet-derived growth factor receptor tyrosine kinase inhibitors in murine models has been shown to result in attenuated fibrosis in radiation-induced and transgenic TGF-β-induced pulmonary fibrosis models.28,29 Similarly, pharmacologic inhibition of the epidermal growth factor receptor tyrosine kinase resulted in attenuated fibrosis in the bleomycin model.30
The T-helper type 2 cytokine IL-13 is also expressed in the lungs in many human fibrotic lung diseases, including at least some patients with IPF31 as well as in animal models of lung fibrosis. Transgenic overexpression of IL-13 by airway epithelial cells has been shown to induce lung fibrosis in rodent models,32 whereas antibody-mediated neutralization or genetic deletion of IL-13, as well as the targeting of IL-13-producing cells with an immunotoxin in mouse models resulted in protection from lung fibrosis.33–35 In contrast to profibrotic effects of IL-13 in models of liver fibrosis, IL-13-mediated fibrosis in the lungs appears to be mediated via TGF-β.36,37
The Pathogenesis of Usual Interstitial Pneumonitis Is a Process Related to a Persistent “Antigen” That Promotes Chronic Inflammation, Destruction of Lung Architecture, and Progressive Fibrosis
In addition to IPF (which is defined as idiopathic UIP), the histologic pattern of UIP is also found in asbestosis (in the context of a persistent inorganic irritant), hypersensitivity pneumonitis (recurrent exposure to exogenous antigens), and collagen vascular diseases such as rheumatoid arthritis or scleroderma (recurrent exposure to self antigens).14 Thus, in each of the illnesses when UIP is attributable to a known cause, it is the result of a persistent or repetitive insult that leads to chronic inflammation, epithelial and endothelial injury of the alveolar-capillary barrier with loss of its BM, alveolar collapse and fusion, and fibroblast/myofibroblast activation and ECM deposition.8–14 A gene microarray analysis38 of pulmonary fibrosis has found genes related to smooth muscle markers, proteins involved in ECM formation, degradation, and signaling; and genes normally associated with chronic inflammation and immune responses, such as cytokines, chemokines, antioxidants, complement, amyloid, and Igs. The latter findings are suggestive of a chronic inflammation/immune response in the lungs of patients with IPF. In support of this notion is the finding of tertiary lymphoid tissue with the accumulation of reactivated memory T cells, B cells, and locally maturing dendritic cells in the lungs of IPF patients.39 The presence of tertiary lymphoid tissue in IPF lungs represents an immunologic response that may play a central role in sustaining chronic inflammation that could be resistant to antiinflammatory agents. Further support of the concept of an immunologic response in the lungs of IPF patients has come from40,41 imaging studies that demonstrate a direct correlation of the presence of mediastinal lymphadenopathy on chest CT scans and fibrosis in patients with IPF. These studies40,41 independently demonstrated the baseline prevalence of mediastinal lymphadenopathy in IPF patients to be approximately 55%. Moreover, radiographic quantification of the magnitude of ground-glass opacities, fibrosis, and progression of fibrosis in IPF patients correlated directly with the degree of lymphadenopathy.40 Taken together, the above studies at the molecular, cellular, and imaging levels support the concept of the potential of a persistent or recurrent antigen exposure in the lungs of patients with IPF that correlates with the progression of fibrosis.
EMT and Fibrocytes Are Critical Cellular Players in the Regulation of Fibrosis
Since lung fibroblasts and myofibroblasts play an important role in ECM deposition in pulmonary fibrosis, the origin of the expanded populations of these cells in the lungs is of substantial interest. There is one classic theory and two contemporary theories for the origin of fibroblasts/myofibroblasts.13,42 The classic concept is that tissue injury induces the activation of a resident fibroblast to proliferate and express constituents of the ECM. The contemporary theory is that tissue injury with the presence of TGF-β induces epithelial cells to transition to a mesenchymal phenotype, the fibroblast/myofibroblast that subsequently contributes to fibroproliferation.43–46 Another contemporary theory is that circulating fibrocytes are mesenchymal progenitor cells that home and extravasate into sites of tissue injury, differentiate into fibroblasts/myofibroblasts, and contribute to the generation of ECM during fibroproliferation13,42 (Fig 2).
With regard to EMT, several studies43–45 have demonstrated that alveolar type II pneumocytes can serve as progenitor cells that, in response to TGF-β, can undergo differentiation to fibroblasts/myofibroblasts with the generation of ECM. In addition, studies have suggested that EMT can be found in the lungs and can contribute to fibrosis in IPF patients.47 In contrast, a recent study48 using immunohistochemistry of appropriate epithelial and mesenchymal cell markers found little evidence for EMT in the lungs of patients with pulmonary fibrosis. However, the disparity of these findings should not reduce the enthusiasm for a burgeoning field of investigation for the importance of EMT to the progression of pulmonary fibrosis.
Fibrocytes were first identified in 1994 as circulating progenitor cells that migrated into wounds and contributed to wound repair.49 Circulating fibrocytes appear to originate from the bone marrow, and these cells constitutively express markers of hematopoietic cells (CD45, major histocompatibility complex II, and CD34) and stromal cells (collagens I and III, and fibronectin).13,49–53 In addition, fibrocytes can undergo differentiation into fibroblasts/myofibroblasts with the gain in expression of α-smooth muscle actin in response to TGF-β and endothelin.50,54–57 In mouse models of pulmonary fibrosis,50,58 inhibition of the trafficking and extravasation of fibrocytes into the lungs is associated with a marked reduction in pulmonary fibrosis. One study59 of patients with idiopathic fibrotic interstitial lung disease demonstrated that the number of circulating fibrocytes were an order of magnitude higher than that in healthy control subjects. This study was further confirmed by another study60 in which lung tissue from IPF patients demonstrated the presence of fibrocytes that coexpressed markers of collagen production and α-smooth muscle actin; the number of fibrocytes in the lung tissue of these patients directly correlated with the number of fibroblastic foci. Moreover, the measurement of elevated levels of circulating fibrocytes in IPF patients has been confirmed in patients with IPF, and has been shown61 to directly correlate with both exacerbations of their disease and as a biomarker for worse prognosis. The results of the above studies underscore the importance of fibrocyte extravasation into the lungs during the pathogenesis of pulmonary fibrosis, and indicate that circulating fibrocytes may contribute to the expansion of the fibroblast/myofibroblast population in patients with IPF and other fibroproliferative disorders of the lung.
Conclusions
In summary, the loss of the alveolar-capillary barrier BM integrity is critical in determining the point of no return, which leads to the promotion of fibrosis. The loss of epithelial cells, endothelial cells, and alveolar-capillary barrier BM integrity in patients with UIP associated with IPF leads to destroyed lung architecture and perpetual fibrosis. The anatomical target that leads to the hallmark features of UIP may be the anatomical respiratory lobule. TGF-β is necessary but not entirely sufficient to promote permanent fibrosis. Persistent injury/antigen/irritant is critical for the propagation of fibrosis in the context of the loss of BM integrity and the failure of reepithelialization and reendothelialization. IPF is an example of a process related to the persistence of antigens, chronic inflammation, and fibrosis. EMT and circulating fibrocytes are critical players in the regulation of fibrosis, and their biology has relevance for the future consideration of therapeutic targets to attenuate the progression of pulmonary fibrosis. With the appreciation of the complexity of pulmonary fibrosis, we hope over the next decade that we will see further progress in our understanding of the pathogenesis of this devastating disorder.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest with any companies/organizations whose products or services discussed in this article.
Abbreviations:
| BM | basement membrane |
| ECM | extracellular matrix |
| EMT | epithelial to mesenchymal transition |
| IL | interleukin |
| IPF | idiopathic pulmonary fibrosis |
| TGF | transforming growth factor |
| UIP | usual interstitial pneumonia |
Footnotes
Funding/Support: National Institutes of Health grants CA87879; and HL66027.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
Articles from Chest are provided here courtesy of American College of Chest Physicians
Full text links
Read article at publisher's site: https://doi.org/10.1378/chest.09-0510
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2773361?pdf=render
Free to read at www.chestjournal.org
http://intl.chestjournal.org/cgi/content/abstract/136/5/1364
Subscription required at intl.chestjournal.org
http://intl.chestjournal.org/cgi/content/full/136/5/1364
Subscription required at www.chestjournal.org
http://intl.chestjournal.org/cgi/reprint/136/5/1364.pdf
Citations & impact
Impact metrics
Article citations
Salvianolic acid B in fibrosis treatment: a comprehensive review.
Front Pharmacol, 15:1442181, 30 Jul 2024
Cited by: 0 articles | PMID: 39139645 | PMCID: PMC11319160
Review Free full text in Europe PMC
Interstitial Lung Disease in Patients with Mixed Connective Tissue Disease: A Retrospective Study.
Int J Gen Med, 17:2091-2099, 13 May 2024
Cited by: 0 articles | PMID: 38766599 | PMCID: PMC11100959
Epithelium-derived exosomes promote silica nanoparticles-induced pulmonary fibroblast activation and collagen deposition via modulating fibrotic signaling pathways and their epigenetic regulations.
J Nanobiotechnology, 22(1):331, 12 Jun 2024
Cited by: 0 articles | PMID: 38867284
Multidimensional biomarker approach integrating tumor markers, inflammatory indicators, and disease activity indicators may improve prediction of rheumatoid arthritis-associated interstitial lung disease.
Clin Rheumatol, 43(6):1855-1863, 05 May 2024
Cited by: 1 article | PMID: 38704780
Effects of Natural Products through Inhibiting Endoplasmic Reticulum Stress on Attenuation of Idiopathic Pulmonary Fibrosis.
Drug Des Devel Ther, 18:1627-1650, 17 May 2024
Cited by: 1 article | PMID: 38774483 | PMCID: PMC11108075
Review Free full text in Europe PMC
Go to all (190) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A new diagnostic system for fibrosing alveolitis (interstitial pneumonitis) based on the functional anatomy of the lung.
Chest, 69(2 suppl):253-256, 01 Feb 1976
Cited by: 0 articles | PMID: 1248294
Endothelial fenestration of the alveolar capillaries in interstitial fibrotic lung diseases.
Acta Pathol Jpn, 42(3):177-184, 01 Mar 1992
Cited by: 12 articles | PMID: 1570740
What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis.
Proc Am Thorac Soc, 5(3):305-310, 01 Apr 2008
Cited by: 87 articles | PMID: 18403324 | PMCID: PMC2645241
Epithelial origin of myofibroblasts during fibrosis in the lung.
Proc Am Thorac Soc, 3(4):377-382, 01 Jun 2006
Cited by: 323 articles | PMID: 16738204 | PMCID: PMC2658689
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: R01 CA087879
Grant ID: CA87879
NHLBI NIH HHS (3)
Grant ID: HL66027
Grant ID: R01 HL066027
Grant ID: R01 HL073848