Abstract
Free full text

Perforin-Independent Extracellular Granzyme B Activity Contributes to Abdominal Aortic Aneurysm
Abstract
Granzyme B (GZMB) is a serine protease that is abundantly expressed in advanced human atherosclerotic lesions and may contribute to plaque instability. Perforin is a pore-forming protein that facilitates GZMB internalization and the induction of apoptosis. Recently a perforin-independent, extracellular role for GZMB has been proposed. In the current study, the role of GZMB in abdominal aortic aneurysm (AAA) was assessed. Apolipoprotein E (APOE)−/− × GZMB−/− and APOE−/− × perforin−/− double knockout (GDKO, PDKO) mice were generated to test whether GZMB exerted a causative role in aneurysm formation. To induce aneurysm, mice were given angiotensin II (1000 ng/kg/min) for 28 days. GZMB was found to be abundant in both murine and human AAA specimens. GZMB deficiency was associated with a decrease in AAA and increased survival compared with APOE-KO and PDKO mice. Although AAA rupture was observed frequently in APOE-KO (46.7%; n = 15) and PDKO (43.3%; n = 16) mice, rupture was rarely observed in GDKO (7.1%; n = 14) mice. APOE-KO mice exhibited reduced fibrillin-1 staining compared with GDKO mice, whereas in vitro protease assays demonstrated that fibrillin-1 is a substrate of GZMB. As perforin deficiency did not affect the outcome, our results suggest that GZMB contributes to AAA pathogenesis via a perforin-independent mechanism involving extracellular matrix degradation and subsequent loss of vessel wall integrity.
An aneurysm is a permanent focal dilatation of an artery that is associated with progressive weakening of the vessel wall. Approximately 80% of all aortic aneurysms occur in the abdominal region.1 Abdominal aortic aneurysm (AAA) is an increasingly common and frequently fatal clinical condition, responsible for more than 15,000 deaths per year in the United States.1 The most significant risk factors for AAA are male gender, cigarette smoking, age, family history, and atherosclerotic disease.2,3 The incidence of AAA measuring 2.9 to 4.9 cm in diameter ranges from 1.3% in men 45 to 54 years of age to 12.5% in men 75 to 84 years of age. In women, prevalence ranges from 0% to 5.2% in the same age groups.2 Rupture occurs in approximately 20% of AAA exceeding 5 cm in diameter but is expected in over 50% of AAA greater than 7 cm.2 Currently, the only effective intervention is open surgical or endovascular repair, however operative mortality for elective open surgical repair is 5.6%.4 Endovascular repair is associated with lower operative mortality, but carries an increased risk of rupture after four years postsurgery and a greater probability of reintervention.5 Total mortality for rupture has been reported to be as high as 90%2; many such patients do not reach emergency treatment in time, and those who do have high surgical mortality.6 Because of the risk of rupture in combination with the asymptomatic nature of AAA, in 2006 the U.S. Congress passed the Screening for Abdominal Aortic Aneurysm (SAAAVE) Amendment for men over the age of 65 and men and women with a family history of AAA. Recently (September 2008), the Canadian Society for Vascular Surgery recommended a similar screening program. As such, as patient and provider awareness and education increases, it is anticipated that early-stage pharmaceutical options geared toward slowing or preventing aneurysm progression and rupture will be in great demand.1
The adventitia is thought to bear the brunt of the aortic circumferential wall stress and acts to restrict aneurysm expansion and rupture,7 whereas inflammation in this area may contribute to collagen degeneration, adventitial weakening, and AAA progression.8 Elastin degeneration, collagen fragmentation, stress distribution, and flow changes can all contribute to mechanical weakness in the aortic wall.9,10,11 In human aortic aneurysmal tissue at the end stage of disease, medial thinning, elastic fiber degeneration, adventitial hypertrophy with an accumulation of T and B lymphocytes, atherosclerosis, and thrombi are observed.12,13,14,15 Inflammation in the intraluminal thrombus adjacent to the AAA wall can also influence rupture as it is associated with increased vascular smooth muscle cell (VSMC) apoptosis, extracellular matrix (ECM) fragmentation, and perturbation of wall structural integrity and stability.16
The proteolytic mechanisms that are associated with ECM degradation in AAA are areas of active investigation. Several types of proteases, including matrix metalloproteinases−2, −7, −9, −12, cathepsins, plasminogen activators, and elastases, have been proposed to contribute to the degradation of fibrillar ECM proteins.11 In the aorta, microfibrils associate with elastin in the tunica media to form the concentric lamellae that separate individual SMC layers and confer elasticity to the aortic wall. Microfibrils also act to stabilize the vessel wall by connecting lamellar rings to one another, to SMC, and to the subendothelial basement membrane.17 Fibrillin-1 is the major scaffolding component of microfibrils and thus plays a key role in maintaining vessel wall stability.
The present study examines the role of Granzyme B (GZMB) in AAA. GZMB is a 32-kDa serine protease that is secreted along with the membrane-disruptive protein perforin (PRF1) by cytotoxic lymphocytes to induce target cell apoptosis. PRF1 is a cytolytic protein that mediates the cytoplasmic entry of GZMB into target cells.18 Over the past two decades, the majority of research on GZMB has been focused on its role in apoptosis. However, GZMB may also exhibit extracellular proteolytic activity as in vitro assays have demonstrated that GZMB can cleave extracellular proteins such as fibronectin, vitronectin, laminin, fibrinogen, von Willebrand factor, and aggrecan.19,20,21,22 Although GZMB expression was initially thought to be restricted to cytotoxic T lymphocytes and natural killer cells, it is now clear that, under conditions of cellular stress, aging, and disease, GZMB expression can be induced in other types of immune cells (macrophages, mast cells, neutrophils) in addition to nonimmune cells (keratinocytes, chondrocytes, VSMCs).23,24,25,26,27,28 As such, many cell types could potentially act as a source of GZMB in age-related degenerative diseases such as AAA.
Elevated GZMB levels have been reported in advanced human atherosclerotic and allograft vasculopathy lesions but not in healthy coronary arteries.23 In the latter study, GZMB was present in lymphocytes, macrophage foam cells, and medial and intimal SMCs, and it was found that extracellular staining increased with disease severity.23 High GZMB levels in the plasma correspond to increased carotid artery plaque instability and increased cerebrovascular events in humans.29 Furthermore, elevated GZMB production is observed in peripheral blood mononuclear cells isolated from patients with unstable angina pectoris compared with cells from patients with stable angina pectoris.30
The current study used a well-established mouse model of angiotensin II (Ang II)–induced AAA.31 In this model, macrophage accumulation in the media of the suprarenal aorta and dissection precede the formation of aneurysm and atherosclerosis in Ang II–infused apolipoprotein E–knockout (APOE-KO) mice. Although thrombi are usually constrained by adventitial tissue, rupture of the abdominal aorta and subsequent death attributable to abdominal bleeding is often observed.13 We hypothesize that GZMB is elevated in AAA and contributes to vessel wall instability and aneurysm formation. To test this, GZMB/APOE-DKO (GDKO) and PRF1/APOE-DKO (PDKO) mice were generated and infused with Ang II to induce AAA formation. In addition, human aneurysm tissues were assessed for the presence and localization of GZMB in clinical AAA and thoracic aortic aneurysm (TAA).
Materials and Methods
Mice
All procedures were done in accordance with the guidelines for animal experimentation approved by the Animal Experimentation Committee of the University of British Columbia.
C57Bl/6 mice, APOE-KO mice (C57Bl/6 background), GZMB-KO mice (C57Bl/6 background), and PRF1-KO mice (C57Bl/6 background) were obtained from Jackson Laboratories (Bar Harbor, ME; Stock Numbers 000664, 002052, 002248, 002407). The GZMB/APOE-DKO (GDKO) mice and PRF1/APOE-DKO (PDKO) mice were generated by crossing the APOE-KO and GZMB-KO or APOE-KO and PRF1-KO mouse strains, respectively. Genotyping of the mice was performed using primers and polymerase chain reaction (PCR) protocols designed from Jackson Laboratories (GZMB primers: 5′-CTGCTACTGCTGACCTTGTCT-3′, 5′-TGAGGACAGCAATTCCATCTA-3′ and 5′-TTCCTCGTGCTTTACGGTATC-3′; APOE primers:5′-GCCTAGCCGAGGGAGAGCCG-3′, 5′-TGTGACTTGGGAGCTCTGCAGC-3′ and 5′-GCCGCCCCGACTGCATCT-3′ and PRF1 primers: 5′-GCTATCAGGACATAGCGTTGG-3′, 5′-GGAGGCTCTGAGACAGGCTA-3′ and 5′-TACCACCAAATGGGCCAAG-3′; Sigma Genosys, Oakville, Ontario). All mice were housed at the Genetic Engineered Models facility (UBC James Hogg Research Laboratories, St. Paul’s Hospital, Vancouver, BC).
Angiotensin II–Induced AAA
Mice aged three to four months received either 28 days of Ang II (Sigma Aldrich, St. Louis, MO) infusion at 1000 ng/min/kg or saline infusion from a subcutaneous 1004 model ALZET® mini osmotic pump (DURECT Corporation, Cupertino, CA) as previously described.31 Briefly, an osmotic pump was filled with the saline solution, primed at 37°C for 24 hours in saline, and surgically implanted subcutaneously posterior to the scapula of the mouse. During the implantation procedure, mice were anesthetized with gaseous anesthetic at a flow rate of 1.5 liters per minute of oxygen with 2.5% of isoflurane delivered via a Baines system using a calibrated tabletop anesthetic machine, administered from a rodent nose cone. Depth of anesthesia was monitored by toe pinch response and breathing. Eyes were protected using ocular lubricant. Postsurgical pain control consisted of a subcutaneous injection of buprenorphine. In total eight APOE-KO mice received saline and 15 received Ang II; 11 GZMB/APOE-DKO mice received saline and 14 received Ang II; five PRF1/APOE-DKO mice received saline and 16 received Ang II.
Tissue Collection and Gross Pathological Characterization
At day 28, tissues from the surviving mice were collected. Blood was collected by cardiac puncture after CO2 euthanasia in EDTA (Sarstedt Monovette, Germany) and red blood cells removed by centrifugation. The mouse was placed on ice, the chest was opened, the right atrium was cut, and a needle was placed in the left ventricle. Sterile saline, and then 4% formalin (Fisher Scientific, Fairlawn, NJ), were perfused at a constant pressure of 100 mm Hg using a pressurized tubing system until no blood was observed exiting the incision in the right atria. The heart, aorta to the iliac bifurcation, and kidneys were dissected from the mouse and photographed. At this point, a gross description of the aorta was made and grouped under the following categories: No pathology; Small localized saccular AAA below diaphragm with or without visible hematoma (28 day survival); Large dissecting AAA beginning in the suprarenal aorta and extending beyond the diaphragm, into the mid-thoracic aorta (28 day survival); Large ruptured AAA with death by exsanguination into abdominal cavity (<28 day survival). Necropsy was performed for all mice that died before 28 days to determine cause of death. Tissues were stored in fresh 10% formalin overnight before being embedded and sectioned.
Murine Tissue Embedding and Sectioning
Aortic segments were isolated from the descending aorta immediately above the diaphragm, and the thoracoabdominal aorta immediately above the renal arteries. APOE-KO, GDKO, and PDKO tissue sections were embedded in paraffin and serial sectioned into 5-μm sections.
Murine Blood and Tissue Histological Analysis
Murine abdominal aortic sections were stained with H&E, Movat pentachrome, or immunohistochemistry performed using rabbit anti-GZMB (Abcam, Cambridge, MA) and rabbit anti–fibrillin-1 (Abcam) as previously described.23 Negative control staining was performed in the same manner, with absence of primary antibody. Fibrillin-1 fragments in mouse blood serum were isolated by immunoprecipitation then analyzed by Western blotting using goat anti-human fibrillin-1 Abs (N-19 and C-19, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). To corroborate gross morphological assessments, histological evaluation for severity of disease was performed by two independent pathologists who were blinded to the experimental conditions.
RNA Extraction and RT-PCR
Human coronary artery smooth muscle cells (HCASMCs; Cambrex, Walkersville, MD) were grown in smooth muscle cell basal medium containing supplements and 5% FBS, according to the supplier’s instructions (LONZA, Walkersville, MD), and were used after reaching 80% confluence. Before treatment, cells were starved for 24 hours in serum-starved media (supplemented smooth muscle cell basal medium [LONZA] + 0.2% FBS). After starvation, cells were treated with 10 nmol/L or 25 nmol/L of GZMB (Alexis Biochemicals, Farmingdale, NY) for 24 hours. Controls were treated with PBS. At 24 hours, cells were lysed with TRIzol (Invitrogen, Carlsbad, CA), RNA was extracted according to manufacturer’s instructions, and total RNA quantified by absorbance at 260 nm. To eliminate genomic DNA contamination, total RNA (1.5 μg) was DNase I-treated (Invitrogen) according to manufacturer’s instructions. cDNA was prepared from 1 μg of total RNA using 50 μmol/L of oligo (dT)20 primer and Superscript III reverse transcriptase (Invitrogen), according to manufacturer’s instructions. All PCR reactions were performed in 25 μl volumes using Platinum TaqDNA polymerase (Invitrogen) and previously published primers for human fibrillin-1 (Forward, 5′-GTGAGATCAACATCAATGGAGC-3′; Reverse, 5′-TTACACACTCCTGGGAACACTTC-3′) and the universal GAPDH primers (Forward, 5′-CATGTTCGTCATGGGTGTGA-3′; Reverse, 5′-GACTGTGGTCATGAGTCCTT-3′).32 Equal aliquots of the PCR products were electrophoresed on a 2% agarose gel, stained with SafeView and photographed.
In Vitro GZMB ECM Cleavage
To determine whether GZMB can cleave SMC ECM, we performed an in vitro experiment as previously described.19 HCASMCs were used, as they are known to secrete fibrillin-1. Cells were cultured to confluence and serum starved for 48 hours to allow for adequate ECM synthesis and secretion, at which time cells were lysed with NH4OH so that the intact ECM remained on the plate.33 GZMB (80 nmol/L; Axxora, LLC, Farmingdale, NY) was incubated on the ECM for 24 hours at room temperature. Supernatants (containing cleaved ECM) were taken off the plate and ECM still attached to the plate was scraped off, collected, and assessed for fibrillin-1 cleavage by Western blot (Monoclonal antibody from Lab Vision/Neomarkers, Fremont, CA).
Human AAA Tissue Collection and Histological Analysis
Human AAA samples were obtained in accordance with the ethical protocols at the Karolinska Institute (Sweden). Immunohistochemistry for GZMB was performed on formalin-fixed paraffin-embedded sections as described previously.23 Briefly, sections were deparaffinized and rehydrated in xylene and ethanol. Antigen retrieval was performed by boiling slides in citrate buffer (pH 6.0) to expose antigens masked in the fixation process as per recommended protocol from antibody supplier. Background staining was blocked by incubation of sections in 10% horse serum. Sections were incubated in a 1:100 dilution of mouse anti-GZMB (a generous gift from Dr. Joseph Trapani, Melbourne, Australia, previously shown to be monospecific to GZMB34) overnight, followed by incubation with biotinylated horse anti-mouse secondary Ab (Vector laboratories, Burlingame, CA) and ABC reagent (Vector Laboratories). Staining was visualized with the DAB peroxidase substrate (Vector Laboratories), and nuclei were counterstained with hematoxylin. Negative control staining was performed in the same manner, with absence of primary antibody.
Human TAA Tissue Collection and GZMB Colocalization
Formalin-fixed paraffin-embedded human TAA samples were obtained in accordance with the ethical protocols at the Biobank Cardiovascular Registry, St. Paul’s Hospital, Vancouver, BC. Sections were de-paraffinized and rehydrated in xylene and ethanol. Antigen retrieval was performed by boiling slides in citrate buffer (pH 6.0) to expose antigens masked in the fixation process as per recommended protocol from antibody supplier. Background staining was blocked by incubation of sections in 10% goat serum. Sections were incubated in a 1:100 dilution of rabbit anti-GZMB (Abcam) overnight, followed by incubation in biotinylated goat anti-rabbit secondary Ab (Vector laboratories) and ABC reagent (Vector laboratories). GZMB staining was visualized with TSA™ Plus Fluorescence Systems cyanine 3 tyramide (PerkinElmer Life Sciences, Inc., Boston, MA). To assess for macrophages, slides were incubated with avidin and biotin to prevent interaction of labeling reagents and blocked with 10% horse serum. Sections were incubated in a 1:100 mouse anti-MAC387 (AbD Serotec, UK) overnight followed by incubation in biotinylated horse anti-mouse secondary antibody (Vector laboratories) and ABC reagent (Vector Laboratories). MAC387 staining was visualized with TSA™ Plus Fluorescence Systems fluorescein tyramide (PerkinElmer Life Sciences, Inc.). Confocal images of fluorescently labeled tissue sections were acquired with a Leica AOBS SP2 laser scanning inverted confocal microscope (Leica, Heidelberg, Germany). Excitation beams were produced by Ar (488 nm for fluorescein) and HeNe (543 nm for cyanine 3) lasers (Leica AOBS SP2 module), respectively. Images from these dual stained samples were acquired sequentially to eliminate cross talk between the emission signals and acquired images were overlaid using Volocity software (Improvisions, UK). Similarly, for colocalization of GZMB in lymphocytes, slides were first stained for GZMB with mouse anti-GZMB (Dr. Joseph Trapani) then incubated with avidin and biotin to prevent interaction of labeling reagents and blocked with 10% goat serum. Sections were incubated in a 1:100 mouse anti-CD3 (Abcam) overnight followed by incubation in biotinylated goat anti-rabbit secondary antibody (Vector laboratories) and ABC reagent (Vector laboratories). CD3 staining was visualized with TSA™ Plus Fluorescence Systems fluorescein tyramide (PerkinElmer Life Sciences, Inc.). Confocal images were acquired in a similar manner as described above. To assess GZMB colocalization in mast cells, slides were first stained for GZMB as described above, but visualized with Vector Red substrate (Vector Laboratories). Slides were then counterstained with Alcian blue to differentiate mast cells.
Statistical Analysis
Statistical differences in survival among the six groups of mice were assessed using the Log-rank (Mantel-Cox) Test. A P value (α error) of 0.05 or less was considered significant.
Results
GZMB Deficiency Protects Against Ang II–Induced AAA
Ang II–treated GDKO mice but not PDKO mice had a significant increase in 28-day survival (92.86%; n = 14, 56.25%, n = 16) when compared with APOE-KO mice (53.33%; n = 15). Survival curves (Figure 1) were analyzed by log-rank test and a statistically significant difference between survival curves was found (P = 0.002). On necropsy, all mice that died prematurely (<28 days) were found to have large aortic dissections and extensive blood clots in the abdominal cavity, suggesting that the cause of death was rupture and exsanguination. When surviving mice from Ang II–treated groups were assessed, a further 40.0% of APOE-KO mice and 43.75% of PDKO mice were found to have AAA compared with only 28.57% of GDKO mice, reducing total incidence of aneurysm-related pathology from 86.67% in APOE-KO mice and 87.50% in PDKO mice to 35.71% in GDKO mice (Figure 2I). Rupture was observed in only 1/14 GDKO mice, and aneurysms that were observed in this group were all small and localized, suggesting that GZMB contributes to the onset and progression of aneurysm. No dissection, AAA, or mortality was observed in any of the saline-infused groups. Representative images of aorta gross morphology and cross sections are shown in Figure 2, A–H.
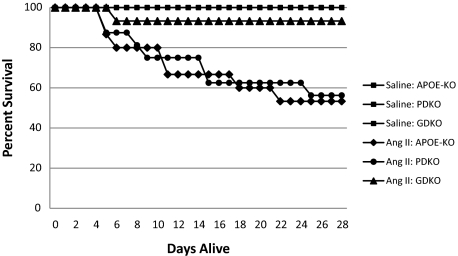
Kaplan–Meier survival curve. Mice were implanted with miniosmotic pumps containing saline or Ang II as described to induce aneurysm formation. Lines represent percentage of mice alive on each day post-implantation. No death was observed in saline control groups (n = 8 for APOE-KO, n = 11 for GDKO, n = 5 for PDKO). In contrast, 93.3% of GDKO (n = 14), 53.3% of APOE-KO (n = 15), and 58.3% of PDKO (n = 12) infused with Ang II survived to 28 days.
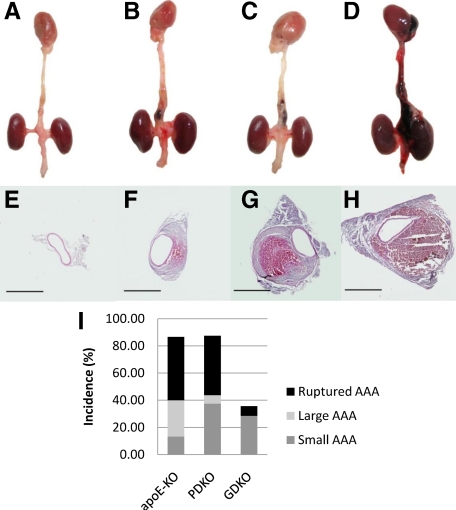
Representative gross pathology, morphology, and summary of outcomes. On euthanasia or necropsy, hearts and aortas were harvested and graded for severity of phenotype. Aortas were cross sectioned and stained with H&E to assess morphology. A and E: Heart and aorta with no visible pathology (all saline groups, APOE-KO 13.33%, GDKO 64.29%, PDKO 12.50%). B and F: Small localized AAA with or without visible hematoma (APOE 13.3%, GDKO 28.57%, PDKO 37.50%). C and G: Large dissecting AAA with visible hematoma in false lumen surrounding the abdominal aorta and extending above the diaphragm (APOE-KO 26.67%, GDKO 0%, PDKO 6.25%). D and H: Fatal ruptured dissecting AAA with abdominal bleeding (APOE-KO 46.7%, GDKO 7.14%, PDKO 43.75%) was also observed. Outcomes are summarized in (I). Scale bar: ×4 = 1 mm.
GZMB Deficiency Reduces Medial Disruption
As previously observed,13 medial disruption characterized by the loss of elastin is a predominant pathological feature of AAA; both in human aneurysms and in Ang II–induced murine AAA. In APOE-KO and PDKO mice, severe elastin breakage was frequently observed in conjunction with aneurysm development (Figure 3, C–H). Incidence of AAA and dissection was much reduced in GZMB-deficient mice. Severe medial disruption and subsequent remodelling, as seen in APOE-KO and PDKO mice, was not observed in GDKO mice (Figure 3, A and B).
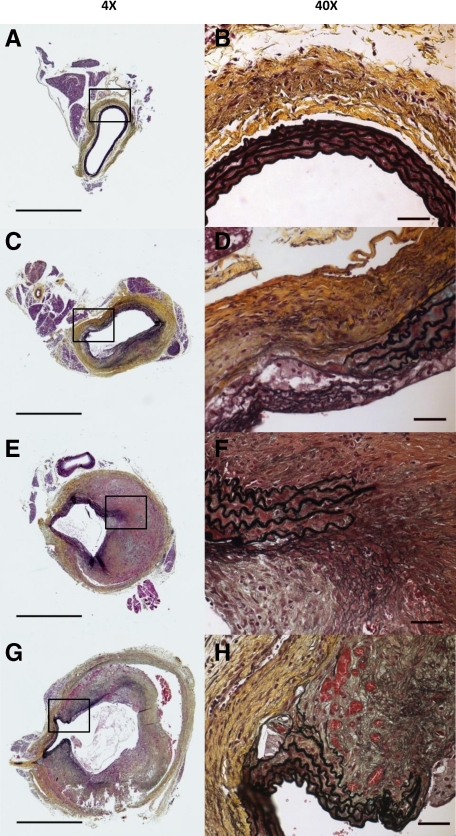
GZMB deficiency reduces medial disruption. Formalin-fixed tissues collected from mice that survived to 28 days were assessed using Movat Pentachrome as described. A and B: Representative aorta from GDKO mouse with no medial disruption and minimal adventitial thickening. C and D: Aorta from APOE-KO with elastin breakage, pronounced adventitial thickening, but no visible hematoma. E and F: Aorta from PDKO mouse with small AAA, elastin breakage, remodeled medial hematoma. G and H: Aorta from PDKO mouse with dissection, elastin breakage, and pronounced dilation. Scale bars: ×4 = 1 mm, ×40 = 50 μm.
GZMB Is Elevated in Ang II–Induced AAA
GZMB was elevated in the aortas of APOE-KO and PDKO mice that developed AAA after Ang II infusion (Figure 4A–F). No GZMB immunopositivity was observed in healthy age-matched controls that received saline infusion. GZMB was particularly abundant in the thrombus, both in trapped lymphocytes and in the extracellular space, as well as in the adventitia of large dissecting AAA.
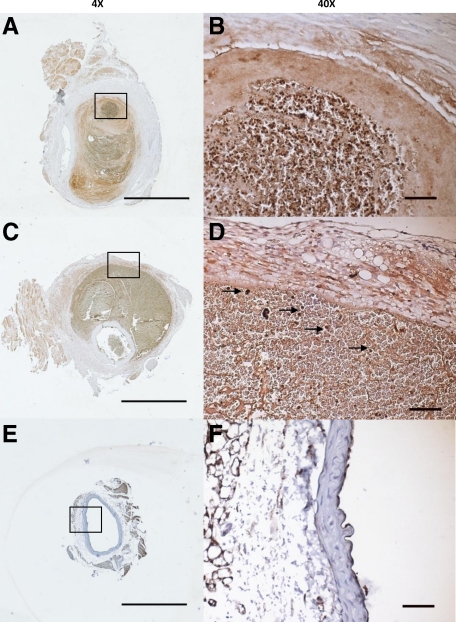
GZMB immunostaining in APOE-KO and PDKO AAA. GZMB-immunopositivity is observed in the medial thrombus of an APOE-KO mouse that suffered a small saccular AAA (A and B) and the adventitia of a PDKO mouse that suffered a large dissecting AAA (C and D). Trapped lymphocytes in the thrombus are indicated by arrows. GZMB staining is not observed in APOE-KO mice that received saline infusion (E and F). Scale bars: ×4 = 1 mm, ×40 = 50 μm.
Fibrillin-1 Staining Is Reduced in APOE-KO Mice as Compared with GDKO Mice
Immunostaining with anti–fibrillin-1 antibody revealed greater levels of fibrillin-1 in the tunica media of GDKO mice versus APOE-KO in Ang II treatment groups. Interestingly, in areas around the thrombus of AAA, minimal fibrillin-1 staining was observed suggesting that these areas could be weakened because of a lack of fibrillin-1 (Figure 5, A and B).
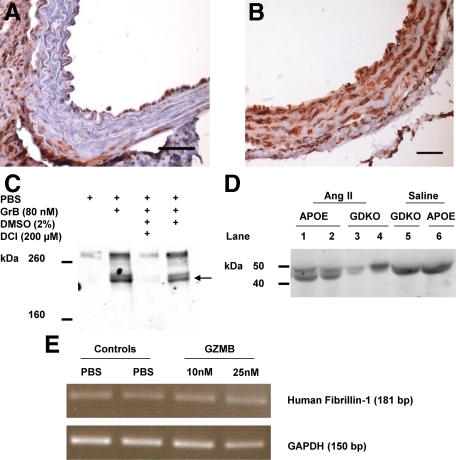
A: Fibrillin-1 immunostaining in APOE-KO AAA is reduced compared with GDKO. A and B: Decreased fibrillin-1 staining, as indicated by red color, was observed in Ang II–infusion APOE-KO mice versus Ang II–infusion GDKO mice. Scale bar = 50 μm. C: GZMB cleaves fibrillin-1 from HCASMC-generated ECM in vitro. HCASMCs were cultured to confluency, lysed, and ECM was biotinylated as described. Human GZMB, isolated from IL-2 stimulated lymphocytes was then added to biotinylated ECM, and supernatants were collected to assess ECM fragments that were released from the plate. GZMB-mediated fibrillin-1 cleavage was attenuated by the GZMB inhibitor DCI but not by the inhibitor solvent control DMSO. Fibrillin-1 fragments from the supernatant are indicated by an arrow. Gel is representative of six independent experiments. D: Fibrillin-1 fragmentation is prevented in GDKO mouse serum compared with APOE-KO after Ang II infusion. Fibrillin-1 in mouse serum was isolated by immunoprecipitation and analyzed by Western blotting. APOE-KO mice with aneurysm show a distinct band at approximately 45 kDa that is not observed in GDKO on Ang II and APOE-KO on saline. E: In vitro fibrillin-1 expression is not directly affected by GZMB treatment. HCASMCs were treated with GZMB and assessed for fibrillin-1 transcription by RT-PCR. No difference was observed in cells that received treatment compared with controls.
Granzyme B Cleaves Fibrillin-1 in Vitro
HCASMCs were cultured to confluency and serum starved for 48 hours at which time cells were lysed and GZMB activity was determined as described. When treated with GZMB, a 210-kDa fibrillin-1 fragment was detected in the supernatant indicating that fibrillin-1 is susceptible to GZMB-mediated proteolytic cleavage (Figure 5C). As the antibody is monoclonal, the other fragment was not recognizable as it did not contain the epitope. We also observed an increase in full-length fibrillin-1 in the supernatant suggesting that GZMB was also weakening the adhesion of this protein to other ECM proteins or cleaving other proteins. The full-length protein was observed to dissociate from the plate in all six repetitions of the assay (data not shown). Fibrillin-1 cleavage was not observed in the plates that received PBS alone, or in the presence of the serine protease inhibitor, 3,4-Dichloroisocoumarin (DCI), confirming fibrillin-1 is indeed a substrate for GZMB. The latter two treatments also were associated with reduced full-length fibrillin-1 in the supernatant.
Fibrillin-1 Fragmentation Is Prevented in GDKO Mice Blood Serum
Mouse blood serum levels of fibrillin-1 were assessed by immunoprecipitation and Western blotting. All samples tested have a band at approximately 50 kD representing immunoglobulin heavy chain; however, it was observed that samples from APOE-KO mice with aneurysms after infusion with Ang II have a distinct second band at approximately 45 kDa that is not seen in samples from GDKO mice that received similar treatment or in APOE-KO mice that received the saline control, suggesting that significant fibrillin-1 fragmentation is prevented in GDKO compared with APOE-KO after Ang II infusion (Figure 5D).
GZMB does not Alter in Vitro Fibrillin-1 Expression
Fibrillin-1 transcription levels in HCASMCs after treatment with GZMB were assessed by RT-PCR (Figure 5E). It was found that there was no difference in fibrillin-1 expression in HCASMCs treated with GZMB compared with controls, suggesting that GZMB does not have any direct effect on fibrillin-1 transcription levels.
GZMB Is Elevated in Human AAA
Human AAA tissue and healthy nonatherosclerotic aorta tissue were assessed for the presence of GZMB. No GZMB immunopositivity was observed in control healthy aorta (Figure 6A), whereas GZMB was abundantly present in AAA tissue (Figure 6, B–G). GZMB positivity was noted in phagocytic cells trapped in the intima and fibrin platelet-red cell thrombus, and also in thrombic material attached to the deep atheroma. GZMB was also noted in cells in proximity to the medial neovasculature, in adventitial collagen layers, in remote adventitia containing nerves, and particularly in large collections of lymphocytes in the adventitia. Moderate staining was observed extracellularly and in the occasional SMC. GZMB positivity was also found in thrombic material with red cells, suggestive of GZMB in the plasma. Intense granular cytoplasmic GZMB was also identified in neuroganglion cells of the remote adventitia.
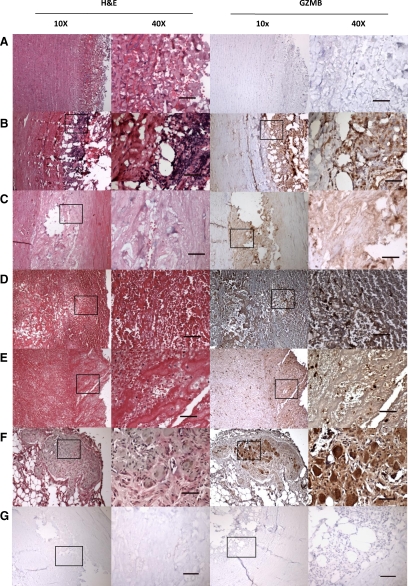
GZMB expression in nonatherosclerotic human aorta versus human AAA. No GZMB immunopositivity was observed in control healthy aorta (A), whereas GZMB was abundantly present in AAA tissue (B–F). GZMB positivity was observed in the media and adventitia but particularly in the adventitial lymphocytes and extracellular connective tissue layers of AAA tissue (B). Staining in the medial thrombus (C) was largely extracellular, however, within the intraluminal thrombus (D–E), GZMB positivity was noted in cellular elements trapped and scattered throughout the fibrin platelet red cell thrombus. GZMB was also noted in remote adventitia containing nerves, and in large collections of lymphocytes in the adventitia. Intense granular cytoplasmic GZMB was identified in nerve ganglion cells of the remote adventitia (F). Negative control, no GZMB primary antibody, shows lack of non-specific staining (G). Scale bar ×40 = 50 μm.
GZMB Colocalizes to Immune Cells in Human TAA
Human TAA tissue was assessed for the presence of GZMB in various immune cell types (Figure 7, A–H). GZMB was found to colocalize to mast cells in the adventitia when visualized by immunohistochemistry. Macrophages in the intima, adventitia, and intraluminal thrombus, and CD3+ lymphocytes in the intima, media, adventitia, and intraluminal thrombus were found to contain GZMB when visualized by confocal microscopy.
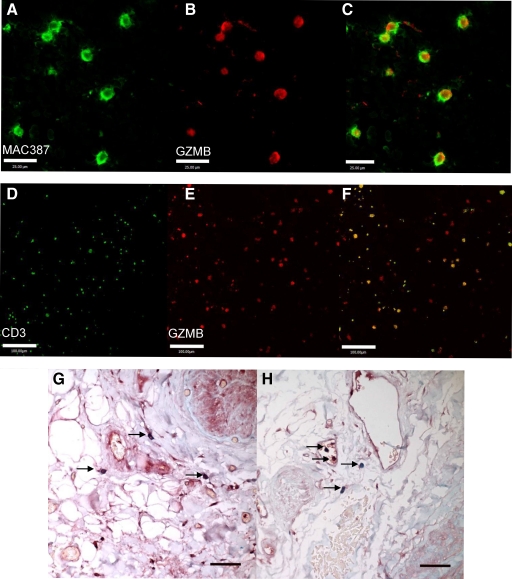
GZMB colocalization with immune cells in human TAA. GZMB colocalization is observed in macrophages of the intraluminal thrombus when visualized by confocal microscopy. GZMB positivity is seen in red (B), macrophage marker MAC387 in green (A), and combined image in C. Scale bar = 25 μm. GZMB also colocalizes to lymphocytes in the intraluminal thrombus. GZMB positivity is seen in red (E), CD3 in green (D), and combined image in F. Scale bar = 100 μm. GZMB expression was observed in mast cells (arrows in G and H) of the adventitia in sections of human TAA stained for GZMB (red) and counterstained with Alcian blue. Scale bar: ×40 = 50 μm.
Discussion
In the current study we demonstrate that GZMB is abundant in AAA and plays a pathogenic role in aortic dissection and aneurysm in Ang II–treated APOE-KO mice. Although GZMB was absent in healthy nonatherosclerotic human aorta, intense immunopositivity was observed in human AAA tissue. This complements previous studies where GZMB expression has been shown to increase in both the lesion and plasma as atherosclerotic disease severity increases.23,29 As it is difficult to demonstrate a causative relationship between GZMB and AAA in humans, we used a well-established mouse model of AAA. In this model, macrophage accumulation in the media, medial disruption, and dissection precede AAA and atherosclerosis.13 We showed that GZMB deficiency resulted in a significant decrease in the total incidence of AAA from 86.67% in APOE-KO and 87.5% in PDKO mice to 35.71% in GDKO. Incidence of rupture was also reduced from 46.67% in APOE-KO and 43.75% in PDKO to 7.14% in GDKO resulting in a much improved survival rate for GZMB-deficient mice (Figures 1 and and2).2). It is important to note that the GZMB-KO mouse35 is a cluster knockdown in which some of the less abundant ‘orphan’ granzymes, unique to mice located close to GZMB on chromosome 14 (C, F, D, and G) have been reported to exhibit reduced expression.36 However, it is unlikely that this would affect the outcome of this study as these granzymes are not present in humans and GZMB is highly expressed in the area of injury in both mice and humans, whereas these other granzymes are not detectable in the vasculature (unpublished observations) and their physiological role in general has not been determined.
The results from the PDKO group is of particular note because PRF1 is essential for GZMB-mediated internalization and induction of apoptosis in target cells.18 As GZMB is only one of multiple granzymes released toward target cells, many studies previously used PRF1-KO mice to evaluate the role of the granule pathway in disease with the assumption that PRF1 is necessary for granzyme internalization and apoptosis. In this scenario, if PRF1 deficiency did not affect outcome, it was often indirectly concluded that granzymes were not involved or did not contribute to disease outcome. One shortcoming of this approach, as demonstrated in the present study, was that it ignored the possibility that granzymes could exhibit PRF1-independent extracellular activity that might contribute to disease.
As PRF1 deficiency in this model does not provide any protective effect, it is unlikely that the classical GZMB/PRF1-mediated apoptosis pathway is involved in AAA, and this would suggest that the pathological effects exerted by GZMB are largely extracellular. In support of this concept, GZMB is capable of cleaving numerous extracellular proteins (reviewed in Boivin et al37). This does not rule out a role for GZMB in cell death as extracellular GZMB can induce PRF1-independent detachment-mediated apoptosis, or anoikis, of VSMCs in vitro through the cleavage of ECM.19 Although we did not observe a significant difference in SMC apoptosis at 28 days postimplant (data not shown), it is often difficult to capture apoptotic cells in vivo as apoptotic cell debris is rapidly removed by neighboring cells and it is possible that measurable levels of apoptosis may be observed at an earlier time-point. Nonetheless, the protective effect of GZMB deficiency on AAA is clearly evident.
The ability for GZMB to cleave ECM components such as fibronectin, vitronectin, laminin, and aggrecan19,20,22 is documented. Here, we present evidence for fibrillin-1 as another extracellular substrate for GZMB. Fibrillin-1 is the major scaffolding component of microfibrils and plays a key role in maintaining vessel wall stability. In the aorta, fibrillin-1 associates with elastin to form the concentric elastic lamellae of the tunica media that confer elasticity to the vessel. In addition, microfibrils not associated with elastin act to stabilize the vessel wall by connecting lamellar rings to one another, to SMCs, and to the subendothelial basement membrane.17,38 Together with collagen, fibrillin-1 microfibrils in the adventitia provide load-bearing support for the entire vessel.39
Reduced fibrillin-1 staining was observed in the aneurismal region of the aortas of APOE-KO mice when compared with GDKO mice, suggesting that GZMB could contribute to the loss of fibrillin-1 (Figure 5, A and B). Subsequently, when GZMB was added to SMC-generated ECM in vitro, distinct fibrillin-1 cleavage fragments were observed indicating that GZMB does indeed possess the ability to cleave fibrillin-1. Furthermore, Western blot analysis of mouse blood serum showed the presence of an extra fibrillin-1 fragment in APOE-KO samples that was not observed in GDKO mice after infusion of Ang II (Figure 5D) suggesting that GZMB deficiency prevents the degradation of fibrillin-1. In addition to this, in vitro GZMB treatment of HCASMCs did not have any direct effect on fibrillin-1 transcription levels (Figure 5E), supporting the assertion that any decrease in fibrillin-1 observed in the aortas of APOE-KO mice was most likely attributed to proteolytic cleavage by GZMB. It should be noted that the fibrillin-1 fragment size detected in mouse serum differs from the fragments observed in the human SMC-generated ECM supernatant. There could be a number of reasons for this discrepancy. Firstly, it is possible that human GZMB cleaves human fibrillin-1 at a different site on the protein compared with their mouse counterparts. Indeed, previous studies have shown that mouse and human granzymes can differ in substrate specificities.40 Secondly, different antibodies were required to detect mouse versus human fibrillin-1, as such, while we were unable to detect the larger fragment seen in the in vitro study, it is possible that fibrillin-1 might have multiple cleavage sites or that the murine antibody does not detect this fragment. These results may be of relevance to the fibrillin-1 deficiencies associated with Marfan syndrome, but whether structural changes in fibrillin-1 attributable to mutations associated with Marfan syndrome predispose it to GZMB cleavage is unknown.
Fibrillin-1–null mice die perinatally from ruptured aortic aneurysm and impaired lung function.41 They have abnormally smooth elastic lamellae and exhibit a loss in VSMC attachments normally mediated by fibrillin-1.42 Hence, the degradation of fibrillin-1 by GZMB could contribute to medial disruption and subsequent fragmentation of elastic lamellae that is commonly observed in aneurysms as shown in Figure 3, but also affect VSMC attachment and phenotype, ultimately resulting in a decrease in VSMCs, loss of structural integrity and a predisposition toward dilation, dissection, and the subsequent formation of aneurysms or rupture.43 We are currently performing a high-throughput screen to identify additional GZMB substrates in the ECM.
The greatest concern on diagnosis of an aneurysm is the significantly increased risk of fatal aortic rupture. GZMB-deficiency appears not only to reduce incidence of AAA formation but also considerably reduces the incidence of aortic rupture in our model. Although premature fatal rupture occurred in more than half of APOE-KO and PDKO mice, only 1 in 14 GDKO mice was found to have died from rupture and exsanguination.
In this model, the preliminary dissection that disrupts the media and results in a visible hematoma is usually constrained initially by the tunica adventitia, allowing for remodeling of the thrombus and reforming of the endothelium.13 Combining the observation of greatly reduced rupture in GDKO mice with the extensive GZMB immunopositivity observed in the adventitia of both human and murine AAA (Figures 4 and and6),6), it is possible that increased GZMB activity contributes to adventitial degeneration and weakening thereby facilitating expansion and susceptibility to rupture. This could account for the fact that AAA observed in APOE-KO and PDKO were more likely to have expanded with loss of adventitia and progressed to premature rupture of the vessel wall. When the APOE-KO and PDKO mice that died prematurely were examined, large blood clots were always found in the abdominal cavity and thrombus material often spanned the entire length of the aorta from the kidneys to the heart. It is exciting to speculate that GZMB inhibition could limit aneurysm expansion by maintaining adventitial structural integrity; however, this requires further elucidation.
In both human and murine AAA samples, we observed substantial GZMB positivity in lymphocytes trapped in the intraluminal thrombus. In addition, GZMB colocalized to macrophages (intima, adventitia, and thrombus) and lymphocytes (intima, media, adventitia, and thrombus) and mast cells (adventitia) in human thoracic aortic aneurysms (Figure 7), readily demonstrating a source of GZMB in situ. A role for GZMB in AAA rupture would be consistent with previous studies demonstrating that the aneurysm wall covered with thrombus exhibits increased inflammation, mast cell activation,44 and association with neovessels in the media and adventitia,45 macrophage infiltration,13,46,47,48 SMC apoptosis, and ECM degradation, thus subjecting this region to greater risk of rupture.16 Based on the histology in human and murine AAA, GZMB could contribute to AAA rupture through adventitial weakening in addition to its effects initiated at the intraluminal thrombus.
One of the major risk factors for aneurysm formation is advanced age. During aging, the elastin to collagen ratio is reduced thereby leading to arterial stiffness and reduced compliance during contraction.49,50,51 Chronic inflammation during atherosclerosis is associated with vessel wall remodeling and a loss of integrity. GZMB levels increase in the intima, media, and adventitia with the severity of atherosclerotic disease,23 a condition that is associated with increased risk for developing AAA. Elevated plasma levels of GZMB are found in patients with unstable versus stable carotid plaques and are associated with an increased occurrence of cerebral vascular events suggesting that GZMB contributes to plaque instability.29 Furthermore, a recent study has suggested a link between GZMB and unstable angina pectoris in which mononuclear cells from unstable angina pectoris patients exhibited greater GZMB production compared with cells from stable angina pectoris or healthy controls.30 As GZMB has been previously found to retain its activity in plasma,52 it is not unreasonable to propose a mechanism whereby chronic inflammation with macrophage infiltration and mast cell activation could lead to increased GZMB levels in and around the vessel wall. GZMB then cleaves ECM components such as fibrillin-1, contributing to the loss of elastic lamellae, medial degeneration, vessel wall instability, and subsequent aneurysm formation, after which further assault by GZMB on the adventitial layer that maintains the structural integrity of the vessel could lead to rupture of the aorta.
The role of GZMB in Ang II–induced AAA is multifactorial. Based on this study, we can conclude that GZMB is elevated in mouse and human AAA tissues and that GZMB-deficiency has a protective role against dissection and AAA formation and rupture in mice. Our results support an extracellular role for GZMB as PRF1 deficiency exhibited no protective effect. As such, GZMB is most likely acting through the degradation of ECM proteins, in particular fibrillin-1, thereby leading to mechanical weakness. As such, GZMB could be an attractive therapeutic target worthy of consideration for the prevention of vascular pathologies that are associated with ECM degeneration.
Acknowledgments
We thank Amrit Samra and Crystal Leung for technical assistance with histology, Thomas Abraham for assistance with confocal imaging, and Dr. Joseph Trapani for the anti-human GZMB Ab. We also thank Dr. Alan Daugherty for technical assistance and for providing us with the murine AAA protocol.
Footnotes
Address reprint requests to David Granville, Ph.D., James Hogg Research Laboratories, Providence Heart and Lung Institute, St. Paul’s Hospital, University of British Columbia, 166-1081 Burrard Street, Vancouver, British Columbia, Canada, V6Z 1Y6. E-mail: [email protected].
Supported by grants from the Canadian Institutes of Health Research (CIHR; to M.F.A., B.M.M., D.J.G.), the Heart and Stroke Foundation of Canada (to D.J.G.), and the Canadian Foundation for Innovation (to M.F.A., B.M.M., D.J.G.). C.M.C. and L.S.A. are both recipients of MSFHR trainee awards and CIHR Canada Graduate Scholarships. W.A.B. is a recipient of a MSFHR trainee award and an NSERC Canada Graduate Scholarship. A.H. is a recipient of a CIHR Canada Graduate Scholarship. S.J.W. is a recipient of a CIHR Fellowship award and a CIHR-IMPACT Strategic Training Post-Doctoral Fellowship. D.M.C. is a recipient of a Heart and Stroke Foundation of Canada Research Fellowship and a CIHR-IMPACT Strategic Training Post-Doctoral Fellowship. D.J.G. is a Founder/Consultant to viDA Therapeutics, Inc.
C.M.C. and L.S.A. contributed equally to this work.
References
- Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. [Europe PMC free article] [Abstract] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. [Abstract] [Google Scholar]
- Annambhotla S, Bourgeois S, Wang X, Lin PH, Yao Q, Chen C. Recent advances in molecular mechanisms of abdominal aortic aneurysm formation. World J Surg. 2008;32:976–986. [Europe PMC free article] [Abstract] [Google Scholar]
- Heller JA, Weinberg A, Arons R, Krishnasastry KV, Lyon RT, Deitch JS, Schulick AH, Bush HL, Jr, Kent KC. Two decades of abdominal aortic aneurysm repair: have we made any progress? J Vasc Surg. 2000;32:1091–1100. [Abstract] [Google Scholar]
- Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. [Abstract] [Google Scholar]
- Johansson G, Swedenborg J. Little impact of elective surgery on the incidence and mortality of ruptured aortic aneurysms. Eur J Vasc Surg. 1994:489–493. [Abstract] [Google Scholar]
- Sonesson B, Lanne T, Vernersson E, Hansen F. Sex difference in the mechanical properties of the abdominal aorta in human beings. J Vasc Surg. 1994;20:959–969. [Abstract] [Google Scholar]
- Beckman EN. Plasma cell infiltrates in atherosclerotic abdominal aortic aneurysms. Am J Clin Pathol. 1986;85:21–24. [Abstract] [Google Scholar]
- Wilmink AB, Quick CR. Epidemiology and potential for prevention of abdominal aortic aneurysm. Br J Surg. 1998;85:155–162. [Abstract] [Google Scholar]
- Upchurch GR, Jr, Schaub TA. Abdominal aortic aneurysm. Am Fam Physician. 2006;73:1198–1204. [Abstract] [Google Scholar]
- Wassef M, Baxter BT, Chisholm RL, Dalman RL, Fillinger MF, Heinecke J, Humphrey JD, Kuivaniemi H, Parks WC, Pearce WH, Platsoucas CD, Sukhova GK, Thompson RW, Tilson MD, Zarins CK. Pathogenesis of abdominal aortic aneurysms: a multidisciplinary research program supported by the National Heart. Lung, and Blood Institute. J Vasc Surg. 2001;34:730–738. [Abstract] [Google Scholar]
- Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [Europe PMC free article] [Abstract] [Google Scholar]
- Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. [Abstract] [Google Scholar]
- Koch AE, Haines GK, Rizzo RJ, Radosevich JA, Pope RM, Robinson PG, Pearce WH. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response, Am J Pathol. 1990;137:1199–1213. [Europe PMC free article] [Abstract] [Google Scholar]
- Anidjar S, Kieffer E. Pathogenesis of acquired aneurysms of the abdominal aorta. Ann Vasc Surg. 1992;6:298–305. [Abstract] [Google Scholar]
- Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, Swedenborg J. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. [Abstract] [Google Scholar]
- Reinhardt DP, Keene DR, Corson GM, Poschl E, Bachinger HP, Gambee JE, Sakai LY. Fibrillin-1: organization in microfibrils and structural properties. J Mol Biol. 1996;258:104–116. [Abstract] [Google Scholar]
- Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nature Rev Immunol. 2006;6:940–952. [Abstract] [Google Scholar]
- Choy JC, Hung VH, Hunter AL, Cheung PK, Motyka B, Goping IS, Sawchuk T, Bleackley RC, Podor TJ, McManus BM, Granville DJ. Granzyme B induces smooth muscle cell apoptosis in the absence of perforin: involvement of extracellular matrix degradation. Arterioscler Thromb Vasc Biol. 2004;24:2245–2250. [Abstract] [Google Scholar]
- Buzza MS, Zamurs L, Sun J, Bird CH, Smith AI, Trapani JA, Froelich CJ, Nice EC, Bird PI. Extracellular matrix remodeling by human granzyme B via cleavage of vitronectin, fibronectin, and laminin. J Biol Chem. 2005;280:23549–23558. [Abstract] [Google Scholar]
- Buzza MS, Dyson JM, Choi H, Gardiner EE, Andrews RK, Kaiserman D, Mitchell CA, Berndt MC, Dong JF, Bird PI. Antihemostatic activity of human granzyme B mediated by cleavage of von Willebrand factor. J Biol Chem. 2008;283:22498–22504. [Abstract] [Google Scholar]
- Froelich CJ, Zhang X, Turbov J, Hudig D, Winkler U, Hanna WL. Human granzyme B degrades aggrecan proteoglycan in matrix synthesized by chondrocytes. J Immunol. 1993;151:7161–7171. [Abstract] [Google Scholar]
- Choy JC, McDonald PC, Suarez AC, Hung VH, Wilson JE, McManus BM, Granville DJ. Granzyme B in atherosclerosis and transplant vascular disease: association with cell death and atherosclerotic disease severity. Mod Pathol. 2003;16:460–470. [Abstract] [Google Scholar]
- Pardo J, Wallich R, Ebnet K, Iden S, Zentgraf H, Martin P, Ekiciler A, Prins A, Mullbacher A, Huber M, Simon MM. Granzyme B is expressed in mouse mast cells in vivo and in vitro and causes delayed cell death independent of perforin. Cell Death Differ. 2007;14:1768–1779. [Abstract] [Google Scholar]
- Wagner C, Stegmaier S, Hansch GM. Expression of granzyme B in peripheral blood polymorphonuclear neutrophils (PMN), myeloid cell lines and in PMN derived from haemotopoietic stem cells in vitro. Mol Immunol. 2008;45:1761–1766. [Abstract] [Google Scholar]
- Berthou C, Michel L, Soulie A, Jean-Louis F, Flageul B, Dubertret L, Sigaux F, Zhang Y, Sasportes M. Acquisition of granzyme B and Fas ligand proteins by human keratinocytes contributes to epidermal cell defense. J Immunol. 1997;159:5293–5300. [Abstract] [Google Scholar]
- Horiuchi K, Saito S, Sasaki R, Tomatsu T, Toyama Y. Expression of granzyme B in human articular chondrocytes. J Rheumatol. 2003;30:1799–1810. [Abstract] [Google Scholar]
- Hernandez-Pigeon H, Jean C, Charruyer A, Haure MJ, Baudouin C, Charveron M, Quillet-Mary A, Laurent G. UVA induces granzyme B in human keratinocytes through MIF: implication in extracellular matrix remodeling. J Biol Chem. 2007;282:8157–8164. [Abstract] [Google Scholar]
- Skjelland M, Michelsen AE, Krohg-Sorensen K, Tennoe B, Dahl A, Bakke S, Brosstad F, Damas JK, Russell D, Halvorsen B, Aukrust P. Plasma levels of granzyme B are increased in patients with lipid-rich carotid plaques as determined by echogenicity. Atherosclerosis. 2007;195:142–146. [Abstract] [Google Scholar]
- Tsuru R, Kondo H, Hojo Y, Gama M, Mizuno O, Katsuki T, Shimada K, Kikuchi M, Yashiro T. Increased granzyme B production from peripheral blood mononuclear cells in patients with acute coronary syndrome. Heart. 2008;94:305–310. [Abstract] [Google Scholar]
- Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. J Clin Invest. 2000;105:1605–1612. [Europe PMC free article] [Abstract] [Google Scholar]
- Samuel CS, Sakai LY, Amento EP. Relaxin regulates fibrillin 2, but not fibrillin 1, mRNA and protein expression by human dermal fibroblasts and murine fetal skin. Arch Biochem Biophys. 2003;411:47–55. [Abstract] [Google Scholar]
- Munshi HG, Stack MS. Adams JC, editor. San Diego CA: Elsevier Science,; Analysis of Matrix Degradation. 2002:pp. 195–205. [Abstract] [Google Scholar]
- Sedelies KA, Sayers TJ, Edwards KM, Chen W, Pellicci DG, Godfrey DI, Trapani JA. Discordant regulation of granzyme H and granzyme B expression in human lymphocytes. J Biol Chem. 2004;279:26581–26587. [Abstract] [Google Scholar]
- Heusel JW, Wesselschmidt RL, Shresta S, Russell JH, Ley TJ. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. [Abstract] [Google Scholar]
- Pham CT, MacIvor DM, Hug BA, Heusel JW, Ley TJ. Long-range disruption of gene expression by a selectable marker cassette. Proc Natl Acad Sci USA. 1996;93:13090–13095. [Europe PMC free article] [Abstract] [Google Scholar]
- Boivin WA, Cooper DM, Hiebert PR, Granville DJ. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Lab Invest. 2009;Epub 2009 Sept 21:1–26. [Europe PMC free article] [Abstract] [Google Scholar]
- Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19:151–154. [Abstract] [Google Scholar]
- Tilson DM EJ, Brophy CM. Greenhalgh M MJ, editor. Plymouth UK: Latimer Trend,; Tensile strength and collagen in abdominal aortic aneurysm disease. 1990:pp. 97–104. [Google Scholar]
- Kaiserman D, Bird CH, Sun J, Matthews A, Ung K, Whisstock JC, Thompson PE, Trapani JA, Bird PI. The major human and mouse granzymes are structurally and functionally divergent. J Cell Biol. 2006;175:619–630. [Europe PMC free article] [Abstract] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, Merkel CA, Sukoyan M, Kerkis A, Hazeki N, Keene DR, Sakai LY, Ramirez F. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J Biol Chem. 2006;281:8016–8023. [Europe PMC free article] [Abstract] [Google Scholar]
- Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, Biery NJ, Dietz HC, Sakai LY, Ramirez F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. [Europe PMC free article] [Abstract] [Google Scholar]
- Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res. 2001;88:37–43. [Abstract] [Google Scholar]
- Tsuruda T, Kato J, Hatakeyama K, Kojima K, Yano M, Yano Y, Nakamura K, Nakamura-Uchiyama F, Matsushima Y, Imamura T, Onitsuka T, Asada Y, Nawa Y, Eto T, Kitamura K. Adventitial mast cells contribute to pathogenesis in the progression of abdominal aortic aneurysm. Circ Res. 2008;102:1368–1377. [Abstract] [Google Scholar]
- Mayranpaa MI, Trosien JA, Fontaine V, Folkesson M, Kazi M, Eriksson P, Swedenborg J, Hedin U. Mast cells associate with neovessels in the media and adventitia of abdominal aortic aneurysms. J Vasc Surg. 2009;50:388–395. discussion 395–386. [Abstract] [Google Scholar]
- Maeda I, Mizoiri N, Briones MP, Okamoto K. Induction of macrophage migration through lactose-insensitive receptor by elastin-derived nonapeptides and their analog. J Pept Sci. 2007;13:263–268. [Abstract] [Google Scholar]
- Shiraya S, Miyake T, Aoki M, Yoshikazu F, Ohgi S, Nishimura M, Ogihara T, Morishita R. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis. 2009;202:34–40. [Abstract] [Google Scholar]
- Hance KA, Tataria M, Ziporin SJ, Lee JK, Thompson RW. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261. [Abstract] [Google Scholar]
- Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. [Abstract] [Google Scholar]
- Diez J. Arterial stiffness and extracellular matrix. Adv Cardiol. 2007;44:76–95. [Abstract] [Google Scholar]
- Agrotis A. The genetic basis for altered blood vessel function in disease: large artery stiffening. Vasc Health Risk Manag. 2005;1:333–344. [Europe PMC free article] [Abstract] [Google Scholar]
- Kurschus FC, Kleinschmidt M, Fellows E, Dornmair K, Rudolph R, Lilieb H, Jenne DE. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. [Abstract] [Google Scholar]
Articles from The American Journal of Pathology are provided here courtesy of American Society for Investigative Pathology
Full text links
Read article at publisher's site: https://doi.org/10.2353/ajpath.2010.090700
Read article for free, from open access legal sources, via Unpaywall:
http://ajp.amjpathol.org/article/S0002944010604130/pdf
Free to read at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/abstract/176/2/1038
Free after 12 months at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/reprint/176/2/1038.pdf
Free after 12 months at ajp.amjpathol.org
http://ajp.amjpathol.org/cgi/content/full/176/2/1038
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Reassessing granzyme B: unveiling perforin-independent versatility in immune responses and therapeutic potentials.
Front Immunol, 15:1392535, 23 May 2024
Cited by: 0 articles | PMID: 38846935 | PMCID: PMC11153694
Review Free full text in Europe PMC
Granzyme serine proteases in inflammation and rheumatic diseases.
Nat Rev Rheumatol, 20(6):361-376, 30 Apr 2024
Cited by: 0 articles | PMID: 38689140
Review
Potential role of extracellular granzyme B in wet age-related macular degeneration and fuchs endothelial corneal dystrophy.
Front Pharmacol, 13:980742, 20 Sep 2022
Cited by: 5 articles | PMID: 36204224 | PMCID: PMC9531149
Review Free full text in Europe PMC
Effective Natural Killer Cell Degranulation Is an Essential Key in COVID-19 Evolution.
Int J Mol Sci, 23(12):6577, 13 Jun 2022
Cited by: 4 articles | PMID: 35743021 | PMCID: PMC9224310
AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis.
Biomedicines, 10(1):94, 02 Jan 2022
Cited by: 26 articles | PMID: 35052774 | PMCID: PMC8773452
Review Free full text in Europe PMC
Go to all (40) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Serpina3n attenuates granzyme B-mediated decorin cleavage and rupture in a murine model of aortic aneurysm.
Cell Death Dis, 2:e209, 08 Sep 2011
Cited by: 40 articles | PMID: 21900960 | PMCID: PMC3186906
Perforin and granzyme B have separate and distinct roles during atherosclerotic plaque development in apolipoprotein E knockout mice.
PLoS One, 8(10):e78939, 24 Oct 2013
Cited by: 14 articles | PMID: 24205352 | PMCID: PMC3811993
Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice.
Cardiovasc Res, 96(3):401-410, 07 Aug 2012
Cited by: 65 articles | PMID: 22871592 | PMCID: PMC3500043
Reassessing granzyme B: unveiling perforin-independent versatility in immune responses and therapeutic potentials.
Front Immunol, 15:1392535, 23 May 2024
Cited by: 0 articles | PMID: 38846935 | PMCID: PMC11153694
Review Free full text in Europe PMC





