Abstract
Background and objective
Severe acute respiratory syndrome (SARS) emerged in 2003 and its long-term sequelae remain largely unclear. This study examined the long-term outcome of pulmonary function, exercise capacity, health and work status among SARS survivors.Methods
A prospective cohort study of SARS patients at the Prince of Wales Hospital, Hong Kong was conducted, with serial assessments of lung function, 6MWD and 36 item Short Form General Health Survey at 3, 6, 12, 18 and 24 months after disease onset. The work status was also recorded.Results
Serial assessments were completed by 55 of the 123 (39.9%) subjects, of whom 27 were health-care workers (HCW). The mean age of the group was 44.4 (SD 13.2) years and 19 (34.5%) were males. At 24 months, 10 (18.2%), 9 (16.4%), 6 (10.9%) and 29 (52.7%) subjects had FEV(1), FVC, TLC and DL(CO) < 80% of predicted values, respectively. The mean (SD) 6MWD increased significantly from 439.0 (89.1) m at 3 months to 460.1 (102.8) m at 6 months (P 0.016) and became steady after 6 months. However, 6MWD and 36 item Short Form General Health Survey scores were lower than the normal population throughout the study. Moreover, 29.6% of HCW and 7.1% of non-HCW had not returned to work 2 years after illness onset.Conclusions
This 2-year study of a selected population of SARS survivors, showed significant impairment of DL(CO), exercise capacity and health status persisted, with a more marked adverse impact among HCW.Free full text

The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status
ABSTRACT
Background and objective: Severe acute respiratory syndrome (SARS) emerged in 2003 and its long‐term sequelae remain largely unclear. This study examined the long‐term outcome of pulmonary function, exercise capacity, health and work status among SARS survivors.
Severe acute respiratory syndrome (SARS) emerged in 2003 and its long‐term sequelae remain largely unclear. This study examined the long‐term outcome of pulmonary function, exercise capacity, health and work status among SARS survivors.
Methods: A prospective cohort study of SARS patients at the Prince of Wales Hospital, Hong Kong was conducted, with serial assessments of lung function, 6MWD and 36 item Short Form General Health Survey at 3, 6, 12, 18 and 24
A prospective cohort study of SARS patients at the Prince of Wales Hospital, Hong Kong was conducted, with serial assessments of lung function, 6MWD and 36 item Short Form General Health Survey at 3, 6, 12, 18 and 24 months after disease onset. The work status was also recorded.
months after disease onset. The work status was also recorded.
Results: Serial assessments were completed by 55 of the 123 (39.9%) subjects, of whom 27 were health‐care workers (HCW). The mean age of the group was 44.4 (SD 13.2) years and 19 (34.5%) were males. At 24
Serial assessments were completed by 55 of the 123 (39.9%) subjects, of whom 27 were health‐care workers (HCW). The mean age of the group was 44.4 (SD 13.2) years and 19 (34.5%) were males. At 24 months, 10 (18.2%), 9 (16.4%), 6 (10.9%) and 29 (52.7%) subjects had FEV1, FVC, TLC and DLCO
months, 10 (18.2%), 9 (16.4%), 6 (10.9%) and 29 (52.7%) subjects had FEV1, FVC, TLC and DLCO <
< 80% of predicted values, respectively. The mean (SD) 6MWD increased significantly from 439.0 (89.1) m at 3
80% of predicted values, respectively. The mean (SD) 6MWD increased significantly from 439.0 (89.1) m at 3 months to 460.1 (102.8) m at 6
months to 460.1 (102.8) m at 6 months (P 0.016) and became steady after 6
months (P 0.016) and became steady after 6 months. However, 6MWD and 36 item Short Form General Health Survey scores were lower than the normal population throughout the study. Moreover, 29.6% of HCW and 7.1% of non‐HCW had not returned to work 2
months. However, 6MWD and 36 item Short Form General Health Survey scores were lower than the normal population throughout the study. Moreover, 29.6% of HCW and 7.1% of non‐HCW had not returned to work 2 years after illness onset.
years after illness onset.
Conclusions: This 2‐year study of a selected population of SARS survivors, showed significant impairment of DLCO, exercise capacity and health status persisted, with a more marked adverse impact among HCW.
This 2‐year study of a selected population of SARS survivors, showed significant impairment of DLCO, exercise capacity and health status persisted, with a more marked adverse impact among HCW.
Short abstract
This study consists of serial assessments of lung function, 6MWD, 36 item Short Form General Health Survey and work status of survivors of severe acute respiratory syndrome (SARS) over 24 months. There was persistent and significant impairment of DLCO, exercise capacity and health status, with more marked adverse impact among the health‐care workers.
months. There was persistent and significant impairment of DLCO, exercise capacity and health status, with more marked adverse impact among the health‐care workers.
INTRODUCTION
Severe acute respiratory syndrome (SARS) is an acute severe lower respiratory illness due to infection with SARS‐coronavirus.
1
,
2
Between November 2002 and August 2003 there were 8096 SARS cases globally with 900 deaths.
3
Previous studies of survivors of acute lung injury and ARDS unrelated to SARS have shown variable degrees of residual abnormalities in pulmonary function, exercise capacity and impairment in health‐related quality of life.
4
,
5
,
6
,
7
,
8
As SARS is a disease that has recently emerged, the long‐term sequelae are largely unclear. Studies on the lung function outcome of SARS survivors have been reported;
9
,
10
,
11
,
12
,
13
,
14
,
15
,
16
,
17
,
18
,
19
however, the longest duration of follow up was around 3 years and small numbers of subjects were studied.
18
,
19
We have previously reported the 6
years and small numbers of subjects were studied.
18
,
19
We have previously reported the 6 month
12
and 1
month
12
and 1 year outcomes
13
from the Prince of Wales Hospital SARS patient cohort, based on the available normative lung function data collected in the 1960s.
20
,
21
The objectives of the present study were to evaluate the 2‐year outcome of lung function, exercise capacity, health and work status of SARS survivors based on updated normative lung function data collected in Hong Kong (HK) from 2001–2003.
22
,
23
year outcomes
13
from the Prince of Wales Hospital SARS patient cohort, based on the available normative lung function data collected in the 1960s.
20
,
21
The objectives of the present study were to evaluate the 2‐year outcome of lung function, exercise capacity, health and work status of SARS survivors based on updated normative lung function data collected in Hong Kong (HK) from 2001–2003.
22
,
23
METHODS
A prospective, longitudinal study of patients with SARS who were discharged from our hospital after surviving the major outbreak in 2003 was conducted. The patients came from our previously reported cohort
12
,
13
,
24
admitted over a period of 2 weeks from March 11 to March 25, 2003. The diagnosis of SARS was based on the Centers for Disease Control and Prevention criteria at the time.
25
All patients in this study had subsequent laboratory confirmation of SARS.
26
The study was approved by the Ethics Committee of the Chinese University of Hong Kong.
weeks from March 11 to March 25, 2003. The diagnosis of SARS was based on the Centers for Disease Control and Prevention criteria at the time.
25
All patients in this study had subsequent laboratory confirmation of SARS.
26
The study was approved by the Ethics Committee of the Chinese University of Hong Kong.
Assessment
All patients were followed up at the lung function laboratory at the Prince of Wales Hospital at the end of 3, 6, 12, 18 and 24 months after illness onset. Physical examination, pulmonary function testing, respiratory muscle strength measurement, 6‐minute walk test (6MWT) and the Medical Outcomes Study 36 item Short Form General Health Survey (SF‐36)
27
were performed during each visit. The health status was compared with the HK normative data collected from a random telephone survey.
28
The work status of each individual was also recorded.
months after illness onset. Physical examination, pulmonary function testing, respiratory muscle strength measurement, 6‐minute walk test (6MWT) and the Medical Outcomes Study 36 item Short Form General Health Survey (SF‐36)
27
were performed during each visit. The health status was compared with the HK normative data collected from a random telephone survey.
28
The work status of each individual was also recorded.
Lung volumes (using the nitrogen washout method), spirometry and surface area for gas exchange were performed (Vmax System; SensorMedics; Yorba Linda, CA). DLCO was determined by the single breath technique using an infrared analyser. We performed spirometry (FEV1 and FVC) according to the standards of the American Thoracic Society. 29 The results of spirometry, lung volume and DLCO were compared with the normative lung function data 20 , 21 (which were widely adopted as the reference data in HK before 2006) and also with the updated spirometric 22 and DLCO reference values. 23 Measurement of the maximum static inspiratory pressure (PImax) and the maximum static expiratory pressure (PEmax) were performed by a mouth pressure meter via a flanged mouthpiece. 30 , 31 The 6MWD was compared against normative reference data collected from a population survey of 538 normal healthy subjects in 2004 conducted by the Coordinating Committee in Physiotherapy, Hong Kong Hospital Authority. 12 , 13
Statistical analysis
Continuous variables were compared using independent sample t‐test. The lung function, 6MWD and SF‐36 of patients admitted to the Intensive Care Unit (requiring intubation or not) were compared using the non‐parametric Mann–Whitney U‐test. Repeated measures analysis of variance (anova) was used to assess serial changes in lung function test, 6MWD and SF‐36 domains. Paired sample t‐test was used to test the point of plateau in 6MWD. Univariate and multivariate analysis were performed to evaluate the potential determinants of 6MWD. Pearson correlation was used to test the correlations among SF‐36, lung function and 6MWD. Statistical analysis was performed using the Statistical Package for Social Science (SPSS) Statistical software for Window, Version 13.0 (SPSS Inc, IL, USA).
RESULTS
Of the first 138 patients hospitalized with SARS infection in March 2003, 15 (10.9%) died.
22
Among the 123 survivors, 13 (10.6%) did not attend follow up at 3 months and 6
months and 6 months,
12
and a further 13 (10.6%) did not attend the 12
months,
12
and a further 13 (10.6%) did not attend the 12 month assessment. Therefore, 97 patients attended all assessments including the 12
month assessment. Therefore, 97 patients attended all assessments including the 12 month assessment.
13
A further 14 patients (11.4%) did not attend the 18
month assessment.
13
A further 14 patients (11.4%) did not attend the 18 month assessment and a further 28 (22.8%) did not attend the 24
month assessment and a further 28 (22.8%) did not attend the 24 month assessment. Overall, 55 patients (39.9%) completed all five assessments. When comparing the SARS survivors who had completed the 24
month assessment. Overall, 55 patients (39.9%) completed all five assessments. When comparing the SARS survivors who had completed the 24 month assessment against those who had not, the former group was older with more severe disease (Table
month assessment against those who had not, the former group was older with more severe disease (Table 1).
1).
Table 1
Comparison of the characteristics and lung function of non‐defaulters and defaulters at 3 months after disease onset
months after disease onset
Non‐defaulters (n = = 55) 55) | Defaulters (n = = 55) 55) | P value | |
|---|---|---|---|
| Age, year | 44.4 (13.2) | 33.4 (8.6) | <0.001* |
| Males, % | 34.5 | 41.8 | 0.100 |
| Length of hospital stay, day | 28.2 (25.2) | 18.9 (6.8) | 0.005* |
| ICU admission, % | 21.8 | 23.8 | 0.392 |
| Mechanical ventilation, % | 7.3 | 3.6 | 0.820 |
| Peak LDH level, U/L | 460.4 (238.5) | 357.2 (145.4) | 0.011* |
| Total steroid dose (in terms of hydrocortisone, mg) | 10 805.6 (11 805.6 (11 449.4) 449.4) | 9488.6 (7247.3) | 0.461 |
| FEV1 % † | 94.2 (12.5) | 100.3 (13.7) | 0.016* |
| FVC % † | 93.3 (13.7) | 98.8 (13.1) | 0.031* |
| VC % † | 97.2 (15.9) | 106.5 (11.6) | 0.003* |
| TLC % † | 99.9 (20.0) | 108.0 (15.3) | 0.018* |
| DLCO † | 81.2 (18.8) | 88.1 (12.9) | 0.008* |
| KCO % † | 88.0 (15.6) | 90.9 (19.3) | 0.921 |
Lung function tests and respiratory muscle strength
The results of serial lung function and respiratory muscle strength testing over 24 months among SARS survivors are shown in Table
months among SARS survivors are shown in Table 2. The percentage of SARS survivors with lung function parameters
2. The percentage of SARS survivors with lung function parameters <
< 80% of predicted compared with the outdated
20
,
21
and the updated local reference values
22
,
23
and the percentages of survivors with PImax and PEmax
80% of predicted compared with the outdated
20
,
21
and the updated local reference values
22
,
23
and the percentages of survivors with PImax and PEmax <
< 80
80 cm H2O at 24
cm H2O at 24 months are shown in Table
months are shown in Table 3.
3.
Table 2
Results of serial pulmonary function tests and respiratory muscle strength (% of predicted) among SARS survivors (n =
= 55)
55)
| Tests conducted % predicted † | 3 months months | 6 months months | 12 months months | 18 months months | 24 months months | P‐value for trend |
|---|---|---|---|---|---|---|
| FVC | 93.3 (13.7) | 95.1 (13.7) | 95.2 (12.9) | 93.6 (12.6) | 92.7 (13.1) | 0.13 |
| FEV1 | 94.2 (12.5) | 94.3 (12.2) | 94.2 (12.1) | 93.7 (12.3) | 91.0 (14.2) | 0.09 |
| FEF25–75 | 105.4 (30.6) | 99.1 (30.6) | 99.1 (32.3) | 99.5 (30.1) | 94.0 (35.4) | 0.03* |
| TLC | 99.9 (20.0) | 104.6 (18.4) | 101.6 (18.4) | 100.9 (14.9) | 97.6 (15.4) | 0.10 |
| VC | 97.2 (15.9) | 97.7 (15.8) | 97.6 (14.3) | 95.5 (14.1) | 95.0 (14.7) | 0.05 |
| RV | 103.5 (50.6) | 117.0 (39.1) | 108.2 (47.1) | 110.1 (29.1) | 100.5 (34.2) | 0.27 |
| DLCO | 81.2 (18.8) | 78.5 (19.1) | 76.8 (15.4) | 77.8 (13.5) | 77.7 (13.3) | 0.43 |
| KCO | 88.0 (15.6) | 89.4 (12.3) | 94.8 (10.5) | 93.5 (11.6) | 95.1 (10.4) | 0.003* |
| PImax | 118.4 (30.1) | 116.3 (27.4) | 116.0 (29.6) | 114.4 (21.3) | 102.4 (33.3) | 0.02* |
| PEmax | 76.3 (16.0) | 80.0 (15.5) | 81.0 (18.2) | 74.2 (17.5) | 75.1 (23.2) | 0.11 |
Table 3
Percentage of SARS survivors with lung function parameters <
< 80% of predicted of the old and the updated local reference values, and the percentage of survivors with PImax and PEmax less than 80
80% of predicted of the old and the updated local reference values, and the percentage of survivors with PImax and PEmax less than 80 cm H2O at 24
cm H2O at 24 months
months
| Percentage of patients with impairment using the old reference value † , % (n) | Percentage of patients with impairment using the new reference value ‡ , % (n) | |
|---|---|---|
| FEV1 | 12.7 (7) | 18.2 (10) |
| FVC | 14.5 (8) | 16.4 (9) |
| VC | 14.5 (8) | |
| TLC | 10.9 (6) | |
| DLCO | 43.6 (24) | 52.7 (29) |
| KCO | 5.5 (3) | 21.8 (12) |
PI max < < 80, cm H2O 80, cm H2O | 34.5 (19) | |
PE max < < 80, cm H2O 80, cm H2O | 10.9 (6) |
According to the American Thoracic Society criteria of severity of DLCO impairment, 20 (36.4%), 7 (12.7%) and 2 (3.6%) patients had mild, moderate and severe impairment, respectively, based on the updated reference values. 23 Two patients (3.6%) had obstructive spirometric abnormality; one of these had a history of COPD and pneumoconiosis and the other had a significant smoking history (25 pack‐years). Three patients (5.5%) had restrictive abnormality without any comorbidity that would account for the abnormal lung function result.
6‐minute walk test
The mean (SD) 6MWD increased significantly from 439.0 (89.1) m at 3 months to 460.1 (102.8) m at 6
months to 460.1 (102.8) m at 6 months (P‐value
months (P‐value =
= 0.016) and then became steady. The mean (SD) 6MWD at 12
0.016) and then became steady. The mean (SD) 6MWD at 12 months, 18
months, 18 months and 24
months and 24 months were 464.7 (101.9) m, 466.3 (91) m and 462.6 (120) m, respectively. The P‐value for trend from 3
months were 464.7 (101.9) m, 466.3 (91) m and 462.6 (120) m, respectively. The P‐value for trend from 3 months to 24
months to 24 months was 0.45.
months was 0.45.
The SARS survivors’ exercise capacity was generally lower than that of normal subjects (Table 4). None had significant hypoxia after the 6MWT. There were no significant associations between 6MWD and any of the following: BMI, total steroid dose, length of hospital stay, admission to ICU, baseline LDH, peak LDH, baseline CRP, peak CRP, FEV1%, FVC%, DLCO%, gas transfer corrected for alveolar volume (KCO%) at 24
4). None had significant hypoxia after the 6MWT. There were no significant associations between 6MWD and any of the following: BMI, total steroid dose, length of hospital stay, admission to ICU, baseline LDH, peak LDH, baseline CRP, peak CRP, FEV1%, FVC%, DLCO%, gas transfer corrected for alveolar volume (KCO%) at 24 months post SARS.
months post SARS.
Table 4
6MWD among SARS survivors (n =
= 48)
†
at 24
48)
†
at 24 months after illness onset in comparisons with Hong Kong normative data
‡
months after illness onset in comparisons with Hong Kong normative data
‡
| Normal Mean (SD), meters | 24‐month mean (SD), meters | Mean difference, meters | 95% CI | P value | |
|---|---|---|---|---|---|
Age group 21 to 30 years (n years (n = = 9) 9) | |||||
 Males Males | 651 (105); [n = = 80] 80] | 542 (76); [n = = 3] 3] | −109 | −231.1 to 13.1 | 0.080 |
 Females Females | 600 (84); [n = = 85] 85] | 496 (105); [n = = 6] 6] | −104 | −175.6 to −32.4 | 0.005* |
Age group 31 to 40 years (n years (n = = 13) 13) | |||||
 Males Males | 645 (93); [n = = 78] 78] | 510 (109); [n = = 6] 6] | −135 | −214.3 to −55.7 | 0.001* |
 Females Females | 606 (86); [n = = 108] 108] | 516 (70); [n = = 7] 7] | −90 | −155.9 to −24.1 | 0.008* |
Age group 41 to 50 years (n years (n = = 16) 16) | |||||
 Males Males | 623 (80); [n = = 38] 38] | 550 (139); [n = = 3] 3] | −73 | −174.9 to 28.9 | 0.155 |
 Females Females | 541 (67); [n = = 79] 79] | 459 (104); [n = = 13] 13] | −82 | −125.4 to −38.6 | <0.001* |
Age group 51 to 60 years (n years (n = = 10) 10) | |||||
 Males Males | 588 (68); [n = = 23] 23] | 375 (19); [n = = 3] 3] | −213 | −4.303 to −290 | 0.007* |
 Females Females | 534 (89); [n = = 33] 33] | 373 (174); [n = = 7] 7] | −161 | 2.447 to −326 | 0.055 |
 Of statistical significance.
Of statistical significance. Four males and three females aged 61
Four males and three females aged 61 years or above were not included as no normative data were available.
years or above were not included as no normative data were available. The normative reference data were collected from a population survey of 538 normal healthy subjects in 2004 by the Coordinating Committee in Physiotherapy, HK Hospital Authority, on two separate days.
The normative reference data were collected from a population survey of 538 normal healthy subjects in 2004 by the Coordinating Committee in Physiotherapy, HK Hospital Authority, on two separate days.SARS, severe acute respiratory syndrome.
Health‐related quality of life among severe acute respiratory syndrome survivors
There was impairment in all SF‐36 domains (P <
< 0.01) at 24
0.01) at 24 months, except for role limitation due to emotional problems and mental heath for the age group 18–40
months, except for role limitation due to emotional problems and mental heath for the age group 18–40 years (Fig.
years (Fig. 1). Significant worsening of bodily pain was observed: 65.8 (SD, 4.01), 59.4 (SD, 3.8), 60.5 (SD, 3.7), 54.4 (SD, 3.6) and 53.8 (SD, 3.5) at 3, 6, 12, 18 and 24
1). Significant worsening of bodily pain was observed: 65.8 (SD, 4.01), 59.4 (SD, 3.8), 60.5 (SD, 3.7), 54.4 (SD, 3.6) and 53.8 (SD, 3.5) at 3, 6, 12, 18 and 24 months, respectively (P 0.025). The only two significant positive correlations between lung function indices and SF‐36 domains were between FVC (r
months, respectively (P 0.025). The only two significant positive correlations between lung function indices and SF‐36 domains were between FVC (r =
= 0.363, P
0.363, P <
< 0.01) and FEV1 (r
0.01) and FEV1 (r =
= 0.3, P
0.3, P <
< 0.05) with physical functioning. However, 6MWD had significant positive correlations with all SF 36 domains except for emotional problems and mental heath.
0.05) with physical functioning. However, 6MWD had significant positive correlations with all SF 36 domains except for emotional problems and mental heath.
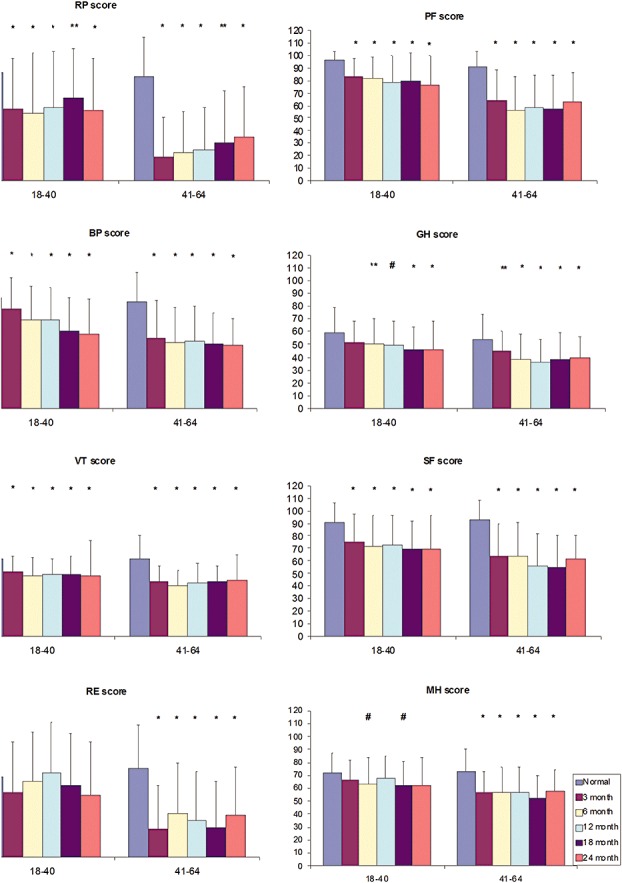
Health status (Short Form General Health Survey—SF‐36) among survivors of severe acute respiratory syndrome at 3, 6, 12, 18 and 24 months after illness onset in comparisons with Hong Kong normative data stratified into different age groups. The vertical axis represents the SF‐36 domain score in mean (SD) ranging from 0 (minimum) to 100 (maximum), whereas the horizontal axis defines age groups in years. BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, emotional problem; RP, physical problems; SF, social functioning; VT, vitality. *Significant at P
months after illness onset in comparisons with Hong Kong normative data stratified into different age groups. The vertical axis represents the SF‐36 domain score in mean (SD) ranging from 0 (minimum) to 100 (maximum), whereas the horizontal axis defines age groups in years. BP, bodily pain; GH, general health; MH, mental health; PF, physical functioning; RE, emotional problem; RP, physical problems; SF, social functioning; VT, vitality. *Significant at P <
< 0.01; **Significant at P
0.01; **Significant at P <
< 0.03; #Significant at P
0.03; #Significant at P <
< 0.01.
0.01.
Comparisons of patients who required ICU support versus those who were treated on the medical wards
The lung function results at 24 months showed significantly lower mean (SD) TLC% (88.3(14.7) vs. 100.7 (12.8)%; 95% CI 3.7–21.1, P
months showed significantly lower mean (SD) TLC% (88.3(14.7) vs. 100.7 (12.8)%; 95% CI 3.7–21.1, P =
= 0.006) and VC% (87.8 (15.5) vs. 97.3(12.6); 95% CI 0.75–18.2%, P
0.006) and VC% (87.8 (15.5) vs. 97.3(12.6); 95% CI 0.75–18.2%, P =
= 0.034) in survivors who had required ICU support compared with those treated on the medical wards. However, there was no significant difference in 6MWD and SF‐36 assessment between two groups. The ICU patients were further stratified into intubated (n
0.034) in survivors who had required ICU support compared with those treated on the medical wards. However, there was no significant difference in 6MWD and SF‐36 assessment between two groups. The ICU patients were further stratified into intubated (n =
= 4) and non‐intubated groups (n
4) and non‐intubated groups (n =
= 8). The intubated group had a significantly lower median (interquartile range) DLCO% (56.5 (3.3) vs. 82 (25.8); P
8). The intubated group had a significantly lower median (interquartile range) DLCO% (56.5 (3.3) vs. 82 (25.8); P =
= 0.042) and a lower KCO% (93.5(16.3) vs. 126.5 (22.3); P
0.042) and a lower KCO% (93.5(16.3) vs. 126.5 (22.3); P =
= 0.007). There was no significant difference in 6MWD and SF‐36 domain scores between two groups at 24
0.007). There was no significant difference in 6MWD and SF‐36 domain scores between two groups at 24 months.
months.
Work status
The percentages of patients who had returned to work over 24 months are shown in Table
months are shown in Table 5. At 2
5. At 2 years, up to 29.6% of HCW but only 7.1% of non‐HCW had not returned to work.
years, up to 29.6% of HCW but only 7.1% of non‐HCW had not returned to work.
Table 5
The number and percentage of SARS survivors (n =
= 55) who had returned to work within 24
55) who had returned to work within 24 months
months
3 months months | 6 months months | 12 months months | 18 months months | 24 months months | |
|---|---|---|---|---|---|
Full‐time work (n = = 41) 41) | 23 (56.1%) | 30 (73.1%) | 33 (80.4%) | 33 (80.4%) | 32 (78.0%) |
Health‐care workers (n = = 27) 27) | 10 (37.0%) | 18 (66.7%) | 21 (77.8%) | 21 (77.8%) | 19 (70.4%) |
Non‐health‐care workers (n = = 14) 14) | 13 (92.9%) | 12 (85.7%) | 12 (85.7%) | 12 (85.7%) | 13 (92.9%) |
SARS, severe acute respiratory syndrome.
DISCUSSION
This prospective cohort study has shown that 52% of SARS survivors had persistent impairment in DLCO and that exercise capacity and health status were significantly lower than the normal controls of the same age groups at 24 months post‐illness. In addition, only 78% of SARS survivors had returned to work and 29% of the HCW had not resumed duty.
months post‐illness. In addition, only 78% of SARS survivors had returned to work and 29% of the HCW had not resumed duty.
With more than one half of the patients having abnormal DLCO at 24 months, the data suggest impairment in the intra‐alveolar diffusion pathway. Su et
months, the data suggest impairment in the intra‐alveolar diffusion pathway. Su et al.
18
reported 8/13 (61.5%) patients in Taiwan and Liu et
al.
18
reported 8/13 (61.5%) patients in Taiwan and Liu et al.
19
reported 5.4% of 39 patients in Beijing who had impairment in DLCO at 14
al.
19
reported 5.4% of 39 patients in Beijing who had impairment in DLCO at 14 months and 36
months and 36 months, respectively. However, these two studies were limited by very small sample sizes of highly selected patients.
18
,
19
The static trend of DLCO impairment over 24
months, respectively. However, these two studies were limited by very small sample sizes of highly selected patients.
18
,
19
The static trend of DLCO impairment over 24 months in the current study was different from other long‐term follow‐up studies that showed improvement in DLCO over 1
months in the current study was different from other long‐term follow‐up studies that showed improvement in DLCO over 1 year in Singapore,
16
and in Beijing.
17
,
19
This might be explained by different methodologies in measuring DLCO among the studies. Studies of ARDS survivors unrelated to SARS have shown significant restrictive and obstructive impairment in pulmonary function tests.
32
,
33
,
34
Up to 76% of ARDS survivors at 5
year in Singapore,
16
and in Beijing.
17
,
19
This might be explained by different methodologies in measuring DLCO among the studies. Studies of ARDS survivors unrelated to SARS have shown significant restrictive and obstructive impairment in pulmonary function tests.
32
,
33
,
34
Up to 76% of ARDS survivors at 5 years after recovery had impairment in DLCO.
34
It is difficult to interpret the clinical significance of the trend of KCO found, as the alveolar volume effect has not been validated in lung diseases in which lung pathology has reduced carbon monoxide (CO) uptake properties as well as alveolar volume.
35
,
36
years after recovery had impairment in DLCO.
34
It is difficult to interpret the clinical significance of the trend of KCO found, as the alveolar volume effect has not been validated in lung diseases in which lung pathology has reduced carbon monoxide (CO) uptake properties as well as alveolar volume.
35
,
36
All the spirometric and lung volume parameters remained static during the study period except for a decreasing trend in the mean of the forced expiratory flow (FEF)%25–75. The percentages of patients with spirometry and lung volume impairment and restrictive abnormality at 24 months were similar to the previous 3–12
months were similar to the previous 3–12 month follow‐up studies on SARS survivors.
9
,
11
,
15
The overall pattern of lung function impairment in our study suggests small airway disease and impairment in the diffusion pathway in the SARS survivors. There were 33% and 27.8% of SARS survivors who had abnormal total CXR scores at 6
month follow‐up studies on SARS survivors.
9
,
11
,
15
The overall pattern of lung function impairment in our study suggests small airway disease and impairment in the diffusion pathway in the SARS survivors. There were 33% and 27.8% of SARS survivors who had abnormal total CXR scores at 6 months and 12
months and 12 months, respectively, in our cohort.
12
,
13
The lung function impairment findings are consistent with the CT images of SARS survivors showing persistent ground glass opacity, reticular opacities and traction bronchiectasis suggesting fibrosis.
37
,
38
,
39
Despite the absence of bronchiolitis in the imaging of SARS patients during the acute phase, air trapping was often present and persisted in the CT scanning of SARS survivors 6
months, respectively, in our cohort.
12
,
13
The lung function impairment findings are consistent with the CT images of SARS survivors showing persistent ground glass opacity, reticular opacities and traction bronchiectasis suggesting fibrosis.
37
,
38
,
39
Despite the absence of bronchiolitis in the imaging of SARS patients during the acute phase, air trapping was often present and persisted in the CT scanning of SARS survivors 6 months post‐infection. These findings probably reflected damage to the ciliated respiratory epithelia during the acute disease but were radiologically occult in most patients.
39
Our findings are also consistent with the histological features of SARS cases. The predominant pathological finding in SARS was diffuse alveolar damage in the early phase of the disease
24
but in the later course of disease, dense septal and alveolar fibrosis were seen.
40
A direct correlation was found between the extent of fibrosis and the duration of the illness.
41
,
42
The restrictive abnormality of lung function might have also been partially due to respiratory muscle weakness, as demonstrated by persistent abnormal PEmax and decreasing trend of PImax over 24
months post‐infection. These findings probably reflected damage to the ciliated respiratory epithelia during the acute disease but were radiologically occult in most patients.
39
Our findings are also consistent with the histological features of SARS cases. The predominant pathological finding in SARS was diffuse alveolar damage in the early phase of the disease
24
but in the later course of disease, dense septal and alveolar fibrosis were seen.
40
A direct correlation was found between the extent of fibrosis and the duration of the illness.
41
,
42
The restrictive abnormality of lung function might have also been partially due to respiratory muscle weakness, as demonstrated by persistent abnormal PEmax and decreasing trend of PImax over 24 months.
months.
The discrepancies of the percentages of SARS survivors with abnormal lung function based on different reference values may have significant implications on compensation decisions, with more SARS patients having abnormal lung function parameters using the updated reference data 22 , 23 than the outdated values, 20 , 21 which were used by the local health authority for judging the extent of impairment.
Previous studies on SARS survivors have reported persistent impairment in exercise capacity up to 14 months post‐illness.
12
,
13
,
15
,
18
This observation was similar to that of survivors of ARDS unrelated to SARS.
4
,
33
The decrease in exercise capacity has been attributed to several factors. Diffusion impairment and respiratory muscle weakness might result in exertional dyspnoea and limit performance of 6MWT. However, the reduced exercise capacity after hospital discharge in SARS survivors could not be accounted for by impairment of pulmonary function alone,
12
,
13
,
18
,
19
whereas extra‐pulmonary causes such as physical deconditioning, muscle weakness and poor motivation were contributing factors. Several reasons for muscle weakness were suggested, including viral‐induced myositis at initial presentation, muscle wasting and deconditioning due to prolonged bed rest, steroid myopathy and critical illness‐associated poly‐neuropathy or myopathy.
12
,
13
,
18
,
19
,
43
months post‐illness.
12
,
13
,
15
,
18
This observation was similar to that of survivors of ARDS unrelated to SARS.
4
,
33
The decrease in exercise capacity has been attributed to several factors. Diffusion impairment and respiratory muscle weakness might result in exertional dyspnoea and limit performance of 6MWT. However, the reduced exercise capacity after hospital discharge in SARS survivors could not be accounted for by impairment of pulmonary function alone,
12
,
13
,
18
,
19
whereas extra‐pulmonary causes such as physical deconditioning, muscle weakness and poor motivation were contributing factors. Several reasons for muscle weakness were suggested, including viral‐induced myositis at initial presentation, muscle wasting and deconditioning due to prolonged bed rest, steroid myopathy and critical illness‐associated poly‐neuropathy or myopathy.
12
,
13
,
18
,
19
,
43
The persistent impairment of 6MWD was an important contributor to the reduced quality of life over 24 months. In addition to the physical impairment, mental impairment is expected as the major SARS outbreak in 2003 was a traumatic experience for the SARS survivors.
44
A striking finding was that 10 patients in our cohort had suffered from depression or post‐traumatic stress disorder requiring psychiatric support. This finding was echoed by the observation that 10–18% of SARS survivors in HK had symptoms related to post‐traumatic stress disorder, anxiety and depression.
44
Among SARS survivors in Canada, psychiatrist consultations accounted for the greatest numbers of health‐care visits.
15
Similarly impaired health status was reported in ARDS survivors unrelated to SARS.
4
,
8
,
33
,
34
months. In addition to the physical impairment, mental impairment is expected as the major SARS outbreak in 2003 was a traumatic experience for the SARS survivors.
44
A striking finding was that 10 patients in our cohort had suffered from depression or post‐traumatic stress disorder requiring psychiatric support. This finding was echoed by the observation that 10–18% of SARS survivors in HK had symptoms related to post‐traumatic stress disorder, anxiety and depression.
44
Among SARS survivors in Canada, psychiatrist consultations accounted for the greatest numbers of health‐care visits.
15
Similarly impaired health status was reported in ARDS survivors unrelated to SARS.
4
,
8
,
33
,
34
It was reported that 66% of SARS patients in Canada had returned to full‐time work 1 year after SARS.
15
Our study had higher percentages of patients returning to work at 1 and 2
year after SARS.
15
Our study had higher percentages of patients returning to work at 1 and 2 years. Studies on ARDS unrelated to SARS have reported that 49% and 65% of ARDS survivors returned to full‐time work at 1
years. Studies on ARDS unrelated to SARS have reported that 49% and 65% of ARDS survivors returned to full‐time work at 1 year and 2
year and 2 years, respectively.
4
,
33
In our study, the lower percentage of HCW returning to work compared with non‐HCW may be explained by the profound psychological trauma of the SARS outbreak at our hospital that involved a high percentage of HCW.
24
HCW with SARS had significantly higher stress levels, higher depression, anxiety and post‐traumatic symptoms 1
years, respectively.
4
,
33
In our study, the lower percentage of HCW returning to work compared with non‐HCW may be explained by the profound psychological trauma of the SARS outbreak at our hospital that involved a high percentage of HCW.
24
HCW with SARS had significantly higher stress levels, higher depression, anxiety and post‐traumatic symptoms 1 year after the outbreak than non‐health‐care SARS survivors.
45
year after the outbreak than non‐health‐care SARS survivors.
45
There are several limitations to this study. First, although this study had the largest sample size for a 2‐year follow‐up study, only 55 of the 123 (39.9%) survivors in the early cohort
24
completed serial assessments over 24 months. The high default rate was likely to be related to patients’ efforts to reduce the SARS‐associated stigma.
46
Those who had returned for follow up had more severe disease at onset and lower lung function parameters at 3
months. The high default rate was likely to be related to patients’ efforts to reduce the SARS‐associated stigma.
46
Those who had returned for follow up had more severe disease at onset and lower lung function parameters at 3 months. The results might therefore not be representative of the entire cohort. Second, 27% of SARS survivors in our cohort had medical comorbidities. This might have introduced additional impairment of exercise capacity and health status. Third, cardiopulmonary exercise testing was not performed in this study as most patients complained of generalized muscle weakness on initial follow up.
12
,
13
As a result, the extra‐pulmonary factors contributing to the 6MWT could not be measured. Finally, respiratory muscle strength was assessed by mouth pressure in our study. Low PEmax or PImax values might be due to poor motivation and technical difficulties such as mouth leakage.
30
months. The results might therefore not be representative of the entire cohort. Second, 27% of SARS survivors in our cohort had medical comorbidities. This might have introduced additional impairment of exercise capacity and health status. Third, cardiopulmonary exercise testing was not performed in this study as most patients complained of generalized muscle weakness on initial follow up.
12
,
13
As a result, the extra‐pulmonary factors contributing to the 6MWT could not be measured. Finally, respiratory muscle strength was assessed by mouth pressure in our study. Low PEmax or PImax values might be due to poor motivation and technical difficulties such as mouth leakage.
30
In summary, 2 years after SARS onset, more than 50% of this highly selected group of SARS survivors had impairment in DLCO. Their exercise capacity and health status were remarkably lower than that of the general population and 30% of HCW had not returned to work. SARS can lead to persistent mental and physical abnormalities in survivors, with a greater adverse impact on HCW. Health authorities should provide good support and follow up for these patients including HCW.
years after SARS onset, more than 50% of this highly selected group of SARS survivors had impairment in DLCO. Their exercise capacity and health status were remarkably lower than that of the general population and 30% of HCW had not returned to work. SARS can lead to persistent mental and physical abnormalities in survivors, with a greater adverse impact on HCW. Health authorities should provide good support and follow up for these patients including HCW.
Supporting information
Table S1 24‐month lung function test.
Table S2 24‐month 6MWT distance (in meters) stratified by age and gender, using Mann–Whitney U‐test and all values are expressed as median (IQR).
Table S3 Quality of life as assessed by SF‐36 at 24 months.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
We thank the Research Fund for the Control of Infectious Diseases (Food and Health Bureau, HKSAR) for funding this study. We would like to thank the following colleagues who have offered tremendous help in this study: M Tong (nurse), PY Chan (nurse), MY Leung (nurse), and Catherine Ho (research assistant). Finally, we would like to acknowledge the Co‐ordinating Committee in Physiotherapy, HK Hospital Authority, for collecting the updated normative data on 6MWD.
REFERENCES
 al.
Identification of a novel coronavirus in patients with severe acute respiratory syndrome.
N. Engl. J. Med.
2003; 348: 1967–76.
[Abstract] [Google Scholar]
al.
Identification of a novel coronavirus in patients with severe acute respiratory syndrome.
N. Engl. J. Med.
2003; 348: 1967–76.
[Abstract] [Google Scholar] al.
Aetiology: ss's postulates fulfilled for SARS virus.
Nature
2003; 423: 240.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Aetiology: ss's postulates fulfilled for SARS virus.
Nature
2003; 423: 240.
[Europe PMC free article] [Abstract] [Google Scholar] al.
One‐year outcomes in survivors of the acute respiratory distress syndrome.
N. Engl. J. Med.
2003; 348: 683–93.
[Abstract] [Google Scholar]
al.
One‐year outcomes in survivors of the acute respiratory distress syndrome.
N. Engl. J. Med.
2003; 348: 683–93.
[Abstract] [Google Scholar] al.
Long‐term follow‐up and bronchial reactivity testing in survivors of the adult respiratory distress syndrome.
Am. Rev. Respir. Dis.
1978; 117: 449–54.
[Abstract] [Google Scholar]
al.
Long‐term follow‐up and bronchial reactivity testing in survivors of the adult respiratory distress syndrome.
Am. Rev. Respir. Dis.
1978; 117: 449–54.
[Abstract] [Google Scholar] al.
Clinical determinants of abnormalities in pulmonary functions in survivors of the adult respiratory distress syndrome.
Am. Rev. Respir. Dis.
1989; 139: 1163–8.
[Abstract] [Google Scholar]
al.
Clinical determinants of abnormalities in pulmonary functions in survivors of the adult respiratory distress syndrome.
Am. Rev. Respir. Dis.
1989; 139: 1163–8.
[Abstract] [Google Scholar] al.
Pulmonary function and health‐related quality of life in survivors of acute respiratory distress syndrome.
Am. J. Respir. Crit. Care Med.
2003; 167: 690–4.
[Abstract] [Google Scholar]
al.
Pulmonary function and health‐related quality of life in survivors of acute respiratory distress syndrome.
Am. J. Respir. Crit. Care Med.
2003; 167: 690–4.
[Abstract] [Google Scholar] al.
Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome.
Eur. Respir. J.
2004; 24: 436–42.
[Abstract] [Google Scholar]
al.
Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome.
Eur. Respir. J.
2004; 24: 436–42.
[Abstract] [Google Scholar] al.
Follow‐up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge.
Chest
2005; 127: 2119–24.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Follow‐up study on pulmonary function and lung radiographic changes in rehabilitating severe acute respiratory syndrome patients after discharge.
Chest
2005; 127: 2119–24.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors.
Thorax
2004; 59: 889–91.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors.
Thorax
2004; 59: 889–91.
[Europe PMC free article] [Abstract] [Google Scholar] al.
The impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors.
Thorax
2005; 60: 401–9.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
The impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors.
Thorax
2005; 60: 401–9.
[Europe PMC free article] [Abstract] [Google Scholar] al.
The 1‐year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors.
Chest
2005; 128: 2247–61.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
The 1‐year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors.
Chest
2005; 128: 2247–61.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Dynamic changes of serum SARS‐coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge.
Respir. Res.
2005; 6: 5.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Dynamic changes of serum SARS‐coronavirus IgG, pulmonary function and radiography in patients recovering from SARS after hospital discharge.
Respir. Res.
2005; 6: 5.
[Europe PMC free article] [Abstract] [Google Scholar] al.
One year outcomes and health care utilization in survivors of severe acute respiratory syndrome.
Arch. Intern. Med.
2007; 167: 1312–20.
[Abstract] [Google Scholar]
al.
One year outcomes and health care utilization in survivors of severe acute respiratory syndrome.
Arch. Intern. Med.
2007; 167: 1312–20.
[Abstract] [Google Scholar] al.
1‐year pulmonary function and health status in survivors of severe acute respiratory syndrome.
Chest
2005; 128: 1393–400.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
1‐year pulmonary function and health status in survivors of severe acute respiratory syndrome.
Chest
2005; 128: 1393–400.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome.
Respiration
2007; 74: 511–16.
[Abstract] [Google Scholar]
al.
Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome.
Respiration
2007; 74: 511–16.
[Abstract] [Google Scholar] al.
Changes in pulmonary function in SARS patients during the three‐year convalescent period.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue
2007; 19: 536–8.
[Abstract] [Google Scholar]
al.
Changes in pulmonary function in SARS patients during the three‐year convalescent period.
Zhongguo Wei Zhong Bing Ji Jiu Yi Xue
2007; 19: 536–8.
[Abstract] [Google Scholar] al.
A major outbreak of severe acute respiratory syndrome in Hong Kong.
N. Engl. J. Med.
2003; 348: 1986–94.
[Abstract] [Google Scholar]
al.
A major outbreak of severe acute respiratory syndrome in Hong Kong.
N. Engl. J. Med.
2003; 348: 1986–94.
[Abstract] [Google Scholar] al.
Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak.
Thorax
2004; 59: 414–20.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak.
Thorax
2004; 59: 414–20.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF 36 health survey.
J. Clin. Epidemiol.
1998; 51: 1139–47.
[Abstract] [Google Scholar]
al.
Tests of scaling assumptions and construct validity of the Chinese (HK) version of the SF 36 health survey.
J. Clin. Epidemiol.
1998; 51: 1139–47.
[Abstract] [Google Scholar] al.
Population based norming of the Chinese (HK) version of the SF 36 health survey.
Hong Kong Pract.
1999; 21: 460–70.
[Google Scholar]
al.
Population based norming of the Chinese (HK) version of the SF 36 health survey.
Hong Kong Pract.
1999; 21: 460–70.
[Google Scholar] al.
Clinical usefulness of the single‐breath pulmonary diffusing capacity test.
Am. Rev. Respir. Dis.
1961; 84: 789–806.
[Abstract] [Google Scholar]
al.
Clinical usefulness of the single‐breath pulmonary diffusing capacity test.
Am. Rev. Respir. Dis.
1961; 84: 789–806.
[Abstract] [Google Scholar] al.
Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization.
Chest
2006; 129: 384–92.
[Abstract] [Google Scholar]
al.
Updated spirometric reference values for adult Chinese in Hong Kong and implications on clinical utilization.
Chest
2006; 129: 384–92.
[Abstract] [Google Scholar] al.
Reference values of diffusing capacity of non‐smoking Chinese in Hong Kong.
Respirology
2007; 12: 599–606.
[Abstract] [Google Scholar]
al.
Reference values of diffusing capacity of non‐smoking Chinese in Hong Kong.
Respirology
2007; 12: 599–606.
[Abstract] [Google Scholar] al.
Comparison of two different mouthpieces for the measurement of Pimax and Pemax in normal and weak subjects.
Eur. Respir. J.
1988; 9: 863–7.
[Abstract] [Google Scholar]
al.
Comparison of two different mouthpieces for the measurement of Pimax and Pemax in normal and weak subjects.
Eur. Respir. J.
1988; 9: 863–7.
[Abstract] [Google Scholar] al.
Long‐term assessment of lung function in survivors of severe ARDS.
Chest
2003; 123: 845–53.
[Abstract] [Google Scholar]
al.
Long‐term assessment of lung function in survivors of severe ARDS.
Chest
2003; 123: 845–53.
[Abstract] [Google Scholar] al.
Two year outcomes, health care use, and costs of survivors of Acute Respiratory Distress Syndrome.
Am. J. Respir. Crit. Care Med.
2006; 174: 538–44.
[Abstract] [Google Scholar]
al.
Two year outcomes, health care use, and costs of survivors of Acute Respiratory Distress Syndrome.
Am. J. Respir. Crit. Care Med.
2006; 174: 538–44.
[Abstract] [Google Scholar] al.
Pulmonary function and health‐related quality of life in a sample of long‐term survivors of the acute respiratory distress syndrome.
Intensive Care Med.
2000; 26: 1304–11.
[Abstract] [Google Scholar]
al.
Pulmonary function and health‐related quality of life in a sample of long‐term survivors of the acute respiratory distress syndrome.
Intensive Care Med.
2000; 26: 1304–11.
[Abstract] [Google Scholar] al.
Standardisation of the single‐breath determination of carbon monoxide uptake in the lung.
Eur. Respir. J.
2005; 26: 720–35.
[Abstract] [Google Scholar]
al.
Standardisation of the single‐breath determination of carbon monoxide uptake in the lung.
Eur. Respir. J.
2005; 26: 720–35.
[Abstract] [Google Scholar] al.
Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin‐section CT.
Radiology
2005; 236: 1067–75.
[Abstract] [Google Scholar]
al.
Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin‐section CT.
Radiology
2005; 236: 1067–75.
[Abstract] [Google Scholar] al.
Severe acute respiratory syndrome thin‐section computed tomography features, temporal changes and clinical radiological correlation during the convalescent period.
J. Comput. Assist. Tomogr.
2004; 28: 790–5.
[Abstract] [Google Scholar]
al.
Severe acute respiratory syndrome thin‐section computed tomography features, temporal changes and clinical radiological correlation during the convalescent period.
J. Comput. Assist. Tomogr.
2004; 28: 790–5.
[Abstract] [Google Scholar] al.
Evolution of pulmonary pathology in severe acute respiratory syndrome.
J. Formos. Med. Assoc.
2005; 104: 75–81.
[Abstract] [Google Scholar]
al.
Evolution of pulmonary pathology in severe acute respiratory syndrome.
J. Formos. Med. Assoc.
2005; 104: 75–81.
[Abstract] [Google Scholar] al.
Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS).
J. Clin. Pathol.
2004; 57: 260–5.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS).
J. Clin. Pathol.
2004; 57: 260–5.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Pulmonary pathology of severe acute respiratory syndrome in Toronto.
Mod. Pathol.
2005; 18: 1–10.
[Europe PMC free article] [Abstract] [Google Scholar]
al.
Pulmonary pathology of severe acute respiratory syndrome in Toronto.
Mod. Pathol.
2005; 18: 1–10.
[Europe PMC free article] [Abstract] [Google Scholar] al.
Neuromuscular disorders in severe acute respiratory syndrome.
Arch. Neurol.
2004; 61: 1669–73.
[Abstract] [Google Scholar]
al.
Neuromuscular disorders in severe acute respiratory syndrome.
Arch. Neurol.
2004; 61: 1669–73.
[Abstract] [Google Scholar] al.
Stress and psychological distress among SARS survivors 1
al.
Stress and psychological distress among SARS survivors 1 year after the outbreak.
Can. J. Psychiatry
2007; 52: 233–40.
[Abstract] [Google Scholar]
year after the outbreak.
Can. J. Psychiatry
2007; 52: 233–40.
[Abstract] [Google Scholar]Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1440-1843.2010.01720.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7192220?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1440-1843.2010.01720.x
Article citations
Serum proteomics for the identification of biomarkers to flag predilection of COVID19 patients to various organ morbidities.
Clin Proteomics, 21(1):61, 01 Nov 2024
Cited by: 0 articles | PMID: 39487396 | PMCID: PMC11531188
Quantitative susceptibility mapping at 7 T in COVID-19: brainstem effects and outcome associations.
Brain, awae215, 07 Oct 2024
Cited by: 0 articles | PMID: 39375207 | PMCID: PMC7616766
Rehabilitation and functional outcomes of COVID-19 patients in a rehabilitation hospital in Qatar.
Qatar Med J, 2024(3):45, 24 Sep 2024
Cited by: 0 articles | PMID: 39372687 | PMCID: PMC11450274
Evaluation of pulmonary arterial stiffness in post mild COVID-19 patients: a pilot prospective study.
J Cardiovasc Imaging, 32(1):25, 28 Aug 2024
Cited by: 0 articles | PMID: 39198895 | PMCID: PMC11351102
Study of Postacute Sequelae of COVID-19 Using Digital Wearables: Protocol for a Prospective Longitudinal Observational Study.
JMIR Res Protoc, 13:e57382, 16 Aug 2024
Cited by: 0 articles | PMID: 39150750 | PMCID: PMC11364950
Go to all (281) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors.
Chest, 128(4):2247-2261, 01 Oct 2005
Cited by: 219 articles | PMID: 16236881 | PMCID: PMC7094276
Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors.
Thorax, 60(5):401-409, 01 May 2005
Cited by: 294 articles | PMID: 15860716 | PMCID: PMC1758905
Medium-Term Disability and Long-Term Functional Impairment Persistence in Survivors of Severe COVID-19 ARDS: Clinical and Physiological Insights.
Arch Bronconeumol, 60(10):619-626, 28 May 2024
Cited by: 0 articles | PMID: 38853119
Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome.
Respiration, 74(5):511-516, 05 Sep 2006
Cited by: 15 articles | PMID: 16960439





