Abstract
Free full text

Natural immunity to pluripotency antigen OCT4 in humans
Abstract
OCT4 is a transcription factor critical for the pluripotency of human embryonal stem (ES) and induced pluipotency stem (IPS) cells. OCT4 is commonly expressed in germ-cell tumors as well as putative cancer stem cells in several tumors, and is a key determinant of oncogenic fate in germ-cell tumors. The capacity of the human immune system to recognize this critical stem-cell gene is not known, but has implications for preventing tumors with ES/IPS-based therapies and targeting stem-cell pathways in cancer. Here we show that OCT4-specific T cells can be readily detected in freshly isolated T cells from most (>80%) healthy donors. The reactivity to OCT4-derived peptides resides primarily in the CD45RO+ memory T-cell compartment and consists predominantly of CD4+ T cells. T cells reactive against OCT4-derived peptides can be readily expanded in culture using peptide-loaded dendritic cells. In contrast to healthy donors, immunity to OCT4 was detected in only 35% of patients with newly diagnosed germ-cell tumors. However, chemotherapy of germ-cell tumors led to the induction of anti-OCT4 immunity in vivo in patients lacking such responses at baseline. These data demonstrate the surprising lack of immune tolerance to this critical pluripotency antigen in humans. Harnessing natural immunity to this antigen may allow immune-based targeting of pluripotency-related pathways for prevention of cancers, including those in the setting of ES/IPS-based therapies.
Recent studies have shown that the expression of limited set of genes is sufficient to induce pluripotency in adult cells(1, 2). Pathways that regulate stemness in embryonal stem (ES) cells or induced pluripotent stem (IPS) cells also bear striking resemblance to those in cancer (3–5). Subsets of tumor cells expressing genes associated with pluripotency in ES cells have been implicated in the clonogenicity of human tumors and activation of ES-associated genes correlates with adverse outcome in several tumors (6–8). Both ES and IPS cells have considerable promise toward regenerative medicine (2). However, immunogenicity and tumorigenicity of these cells represent major potential challenges for effective translation of these approaches in the clinic (2, 9, 10).
OCT4 forms part of the core transcriptional network of human ES cells and regulates the induction of pluripotency in adult cells (11). The capacity of the human immune system to recognize this critical stem-cell gene is not known, but has potential implications for the emerging clinical applications involving therapeutics with ES/IPS-derived cells, or immune-based targeting of stem-cell pathways in cancers, and in particular germ-cell tumors (GCTs). Here, we show that most healthy humans harbor OCT4-specific memory T cells that are readily detectable in freshly isolated peripheral blood mononuclear cells (PBMCs), and demonstrate the induction of these responses in vivo in patients undergoing curative therapy for GCTs.
Results
To analyze the capacity of human T cells to recognize OCT4, mononuclear cells from healthy blood donors (n = 30) were cultured with an overlapping peptide library spanning the entire OCT4 protein (see Table S1 for the sequences of peptides in this library), and culture supernatants assayed for the presence of IFN-γ inducible protein-10 (IP10). Positive reactivity in this assay was defined as ≥ 2-fold increase in IFN-γ IP10 production in the reactive mix versus control, with a minimal absolute measurement of 100 pg/mL predetermined to be positive for the presence of antigen-specific T cells. Using this cutoff, reactivity against peptide pools derived from this library was detectable in 25 of 30 (83%) donors tested (Fig. 1 A and B). Peptide reactive IP10 production was abrogated upon depletion of CD3+ T cells before stimulation (Fig. 1C), as well as blockade of IFN-γ (Fig. 1D), demonstrating that the reactivity in this assay was dependent on peptide reactive production of IFNγ by T cells. Analysis of peptide reactive proliferation indicated that the proliferating T cells were predominantly CD4+ T cells, and antigen-dependent proliferation correlated with reactivity in the IP10 assay (Fig. 2A). The rapidity of cytokine production in response to antigen stimulation suggested that the observed immune response was a memory T-cell response. To further evaluate this finding, bulk PBMCs were depleted of CD45RO+ T cells before stimulation with OCT4 peptide library [or phytoagglutinin (PHA) as a control]. Depletion of CD45RO+ T cells led to abrogation of OCT4 reactivity, suggesting that the OCT4-specific response as measured in this assay resided predominantly in the CD45RO+ subpopulation (Fig. 2B). Together, these data indicate the presence of OCT4-specific memory T-cell response, which can be readily detected in freshly isolated PBMCs from healthy donors.

Immunity to OCT4 in freshly isolated PBMCs from healthy blood donors. (A and B) Reactivity of freshly isolated human PBMCs from healthy donors to OCT4 peptide library: 2 × 105 PBMCs were cultured alone or in the presence of 3 μg/mL of OCT4 peptide mixes, or PHA, as a control. After 48 h, the culture supernatant was harvested and analyzed for the presence of IP10 by Luminex assay. (A) Data for representative healthy donors with or without detectable reactivity to OCT4 peptide library. (B) Data for reactivity to the positive OCT4 mix (represented as fold-change in reactive mix compared with control) in all 30 donors tested. Based on interassay and intra-assay variance, ≥2-fold increase in IP10 production (in reactive mix versus control), with a minimal absolute measurement of 100 pg/mL, was predetermined to be positive for the presence of antigen-specific T cells, as discussed under Materials and Methods. (C) Bulk PBMCs or those depleted of CD3+ T cells were cultured alone (control) or with OCT4 peptide library. After 48 h, the culture supernatant was harvested and analyzed for the presence of IP10 by Luminex assay. Data are representative of similar experiments on three donors. (D) PBMCs were cultured alone or OCT4 peptide mix in the presence of either anti-IFN-γ blocking antibody or isotype control antibody. After 48 h, the culture supernatant was harvested and analyzed for the presence of IP10 by Luminex assay. Data are representative of similar experiments on three donors.
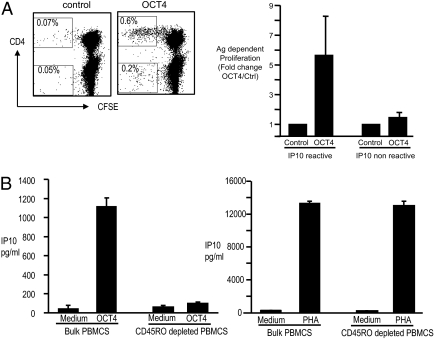
Antigen dependent proliferation of OCT4-specific memory T cells. (A) Proliferative response to OCT4 peptide library: 2 × 105 PBMCs were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) and cultured with 1 μg/mL of anti-CD28 and anti-CD49d antibody alone (control) or in the presence of OCT4-derived peptides (3 μg/mL). (Left) Proliferation of CD3+-gated cells in a representative donor. Note the predominant proliferation of CD4+ T cells. (Right) Antigen-dependent proliferation in six donors with or without reactivity in the IP10 assay, as in Fig 1A. (B) PBMCs were depleted of CD45RO+ T cells using immunomagnetic beads (or left undepleted) before stimulation with OCT4 peptides (Left), or PHA as a control (Right).
To analyze the fine specificity of the anti-OCT4 response and confirm reactivity to the peptide mix, PBMCs were cultured against individual peptides from the reactive peptide mix in 10 donors. Although individual donors had reactivity to different peptides within the pool, some hot-spot areas of promiscuous reactivity were also identified (Fig. 3A). Analysis of areas of hot spots of reactivity by BLAST (National Center for Biotechnology Information) demonstrated that these sequences represented highly conserved regions within OCT4 protein, without homology to known pathogen-derived sequences (Table S2). To further analyze the capacity of functional OCT4-specific T cells to expand in culture, T cells were stimulated with peptide-pulsed autologous dendritic cells (DCs). The presence of peptide-specific IFNγ-secreting CD4+ T cells was then analyzed by intracellular cytokine flow cytometry following peptide-specific restimulation (Fig. 3B). Together, these data demonstrate that T cells responding to peptide epitopes derived from OCT4 can be readily detected and expanded in culture using stimulation with peptide-loaded autologous DCs. Together, these data demonstrate the lack of immune tolerance to this critical ES antigen in humans, and show that these T cells can be readily harnessed for potential therapeutic utility after ex vivo stimulation.
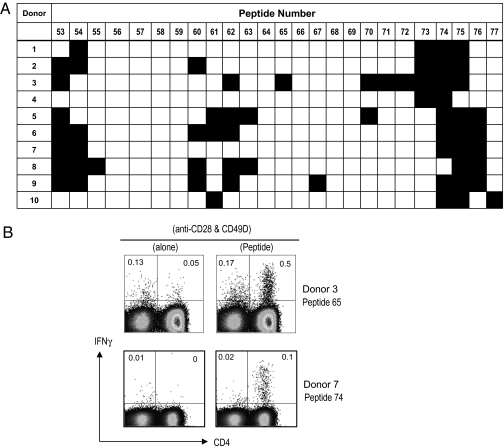
Fine specificity of OCT4-specific T-cell immunity and activation of peptide-specific T cells. (A) Reactivity to individual peptides: PBMCs were cultured with individual peptides from submix 3 to determine the specific reactive peptide. After 48 h, culture supernatant was harvested and analyzed for the presence of IP10 by Luminex assay. The figure shows the reactivity against individual peptides for 10 healthy donors. (B) Expansion of peptide-reactive T cells with peptide-pulsed DCs. Monocyte-derived mature DCs loaded with active peptide (as identified in experiments in Fig 2A) were used to stimulate autologous T cells. The presence of peptide-specific IFNγ-producing T cells was analyzed by intracellular cytokine flow cytometry following restimulation with anti-CD28 and anti-CD49d, with or without stimulating peptide. Data shown are representative of four different donors.
OCT4 has emerged as an important biomarker and critical determinant of oncogenic fate in GCTs (12, 13). Therefore, we prospectively analyzed the presence of OCT4-specific T cells in patients with newly diagnosed GCT, at baseline and after the completion of therapy. Of 21 patients analyzed, 20 had pathologic evidence for predominant seminoma or embryonal carcinoma, subtypes typically associated with highest OCT4 expression (Table S3) (12). Reactivity to viral antigens (CMV, EBV, and influenza; CEF) and PHA was monitored as a control. In contrast to the finding with healthy donors, only 8 of 21 patients with GCT (38%) had measurable OCT4-specific T cell immunity at baseline (P < 0.05) (Fig. 4A). An intriguing aspect of GCTs is the high curability of these tumors, even in the setting of advanced cancers (14). Recent studies have suggested that the ability of some chemotherapies to induce immunogenic death of tumor cells may contribute to their antitumor effects in vivo (15). However, whether specific immunity to tumor-associated antigens is induced after curative therapy for GCT is not known. In contrast to analysis of baseline samples, the presence of OCT4-specific T cells was detected at the completion of chemotherapy in 10 of 12 patients (83%) tested, including 5 patients who lacked such responses at baseline (Fig. 4B). The induction of antigen-specific T cells after therapy was specific for OCT4, as there were no significant changes in virus-specific T-cell responses (Fig. 4B). There was also no correlation between reactivity to OCT4 and candida, as an additional control for human antigen-specific CD4+ T-cell responses (r2 = 0.002) (Fig. S1). As testicular tumors commonly lack MHC II (16), we hypothesized that the induction of OCT4-specific T cells might involve the uptake of dying tumor cells by DCs. Stimulation of autologous T cells with DCs loaded with dying embryonal carcinoma cells led to the induction of OCT4-specific immune responses in culture (Fig. S2). Therefore, DCs can process and present OCT4 from dying GCT cells. Together, these data suggest that chemotherapy-induced cell death may lead to the induction of tumor immunity in vivo.

Immunity to OCT4 in patients with GCTs. (A) Reactivity of freshly isolated human PBMCs from newly diagnosed GCT patients (n = 21) or healthy controls (n = 30) to OCT4 peptide library (as in Fig 1B) or PHA as a control. Positive reactivity was predetermined to be ≥2-fold increase in IP10 production (in reactive mix versus control), with a minimal absolute measurement of 100 pg/mL, as discussed under Materials and Methods. (B) Effect of antitumor therapy on OCT4 immunity: PBMCs from GCT patients (n = 12) were analyzed at baseline and at the completion of therapy for reactivity to OCT4 and to viral antigens (CEF) as a control. Data shown are reactivity at baseline and at the completion of therapy. Bold lines represent mean reactivity.
Discussion
In this article, we have demonstrated that most healthy humans have naturally occurring memory T-cell responses to specific epitopes derived from OCT4, indicating that most individuals lack immune tolerance to this critical pluripotency antigen. Several studies have examined the presence of self-antigen reactive T cells in healthy individuals and patients with cancer or autoimmunity. Detection of T cells specific for MelanA/MART1 (CD8+, naive), carcinoembryonic antigen (CD4, naive), wild-type p53 (CD8/CD4, naive), cyclin B1 (CD4/CD8, naive/memory), glutamic acid decarboxylase, and insulin (CD4/CD8, naive) was feasible in T cells from healthy individuals (17–21). However, none of these nor similar studies evaluated the immunogenicity of ES-restricted antigens in humans or mice, which may provide a potential mechanism for immune-mediated surveillance (22) against unrestrained growth of pluripotent cells, as well as GCT. The presence of rare OCT4-expressing cells with pluripotent potential in adult tissues has recently been suggested to play a role in tissue regeneration in mice (12, 23, 24). The possibility of immune surveillance against GCT is supported by the observation that the immunity to OCT4 is reduced in GCT patients compared with healthy donors and increased risk of these tumors in patients with immunodeficiency (25). In prior studies, we have shown that T-cell responses against another ES antigen, SOX2, are commonly detected in patients with preneoplastic gammopathy and correlate with reduced risk of progression to overt myeloma (26). However, in contrast to OCT4, anti-SOX2 responses were not detected in healthy donors using similar assays (26). The reason underlying the differences in immune reactivity between these two core ES factors in healthy individuals is not known, but may relate to differences in their immunogenicity, or patterns of aberrant expression (12, 23, 24). Further studies are needed to explore the full spectrum of immunogenic ES-specific antigens and understand the mechanism of induction and maintenance of these immune responses.
Our finding that anti-OCT4 T cells are specifically induced in vivo in response to therapy of GCTs demonstrates that these T cells are functional in vivo and can respond to stimulation. Most of the OCT4-specific T cells consist of CD4+ T cells. As most testicular tumors are typically MHC II-negative (16), the induction of OCT4 T cells may involve the uptake of dying tumor cells and presentation of tumor-derived antigens by DCs. Tumor-specific CD4+ Th1 cells can mediate antitumor effects against MHC II-negative cells by multiple mechanisms, including the activation of macrophage-mediated innate resistance and antiangiogenesis (27–29). These data also provide evidence that curative therapy of GCTs can lead to the induction of tumor antigen-specific T-cell responses in vivo. Further studies are needed to understand whether the observed induction of tumor immunity associated with chemotherapy is critical for the long-term cure of GCTs.
These findings also have several therapeutic implications. Cells derived from IPS cells are likely to reach the clinic in the near future, but carry a real risk of tumor formation, particularly if the ES genes were to be reactivated in vivo (9, 30). Boosting immunity to OCT4 may therefore be important to minimize the tumorigenicity of these cells in the clinic. A growing body of data points to the importance of shared pathways of “stemness” in the biology of human cancer and ES cells (5, 31). OCT4 is essential for pluripotency of ES and IPS cells. OCT4 has been shown to act as a dose-dependent oncogenic fate determinant in mice and thought to play a critical role in the pathogenesis of GCT (32). However, interactions between transcriptional networks regulating pluripotency/stemness may be cell type/context-dependent, particularly in the setting of transformed cells. For example, pluripotency in some variant ES lines was found to be independent of OCT4 (33). Therefore, further study is needed to elucidate the functional significance of OCT4 expression in putative cancer stem cells in different cancers. An important consideration when targeting putative cancer stem-cell genes is the potential impact on normal stem cells. It is therefore of interest that OCT4 is dispensable for the function of adult stem cells in mice, making it an attractive target on cancer stem cells (34). There is a long history of targeting embryonal tissues toward vaccines against cancer, as reviewed recently (35). Notably in these studies, the protective effect was most evident only with early but not late embryonic tissues and lacked precise definition of antigenic targets (35). Interestingly, a recent study demonstrated that vaccination of mice with ES/IPS cells mediated protection in a colon-cancer model (36). The immunogenic epitopes of OCT4 as identified here may therefore serve as the basis of a vaccine for prevention or therapy of several cancers, or adoptive T cell-based therapies, including in the setting of allogeneic stem cell transplantation. Immunity to stemness genes may be critical for harnessing the immune system against cancer (37).
Materials and Methods
Healthy Donors and Patients with GCTs.
Peripheral blood was collected from healthy donors, as well as patients with GCTs, after obtaining informed consent approved by the Institutional Review Boards at Yale University, The Rockefeller University, or the Memorial Sloan Kettering Cancer Center. Buffy coats derived from healthy blood donors were purchased from the New York Blood Center.
Synthesis of OCT4 Peptide Library.
Peptides were synthesized in the Proteomics Resource Center at The Rockefeller University, as described (26). Overlapping sequences from the OCT4 protein were determined and optimized for synthesis by using an epitope library fragment-generation program PeptGen, developed by Los Alamos National Laboratories, as part of the HIV Immunology Database at http://www.hiv.lanl.gov. All peptides were created in microtiter plate (96-well) format using an Intavis MultiPep parallel peptide synthesizer/spotter (Intavis) on precoupled Wang (p-Alkoxy-benzyl alcohol) resins (Bachem) loaded at 10 μMol per well, using Fmoc-protected amino acids (Anaspec). Deprotection of the amine was accomplished with 20% piperidine (Aldrich) in NMP (N-methylpyrrolidinone). Repetitive coupling reactions were conducted using 0.6 M HATU/ HOBT and 0.4 M NMM using NMP (EMD) as the primary solvent. Simultaneous resin cleavage and side-chain deprotection were achieved by treatment with 0.8 mL per well concentrated, sequencing grade, trifluoroacetic acid (Fisher) with triisopropylsilane (Fluka), water, and 3,6 dioxa-1,8-octane-dithiol (Aldrich) in a ratio of 95:2:2:1 over a 4-h time frame. Following vacuum filtration to a collection plate, a standard ether precipitation was performed on all individual peptides in 10-mL cold tert-butyl methyl ether (TBME, Aldrich). After pellet formation by centrifugation, excess ether was removed by vacuum aspiration. Peptides were then treated with 0.5 mL 8 M acetic acid and washed with 10 mL of cold TBME again. The precipitates were again allowed to form and then spun. The excess ether was then removed and the pellets allowed to dry overnight. Peptide pellets were redissolved in 20% acetonitrile and HPLC-grade water and lyophilized. All crude products were subsequently analyzed by reversed-phase HPLC (Waters Chromatography) using a Merck Chromolith Performance C18 column. Individual peptide integrity was verified by MALDI mass spectrometry using a PerSeptive/Applied Biosystems Voyager (PE/ABI) delayed-extraction spectrometer system. The library consisted of 95 peptides (15-mer overlapping by 11 amino acids), and was divided into four submixes of pooled peptides as follows: Mix 1, peptides 1 to 25; Mix 2, peptides 26 to 50; Mix 3, peptides 51 to 75; and Mix 4, peptides 76 to 95. The sequences of individual peptides in this library are noted in Table S1.
Detection of OCT4-Reactive T Cells in Fresh PBMCs.
PBMCs were separated by density-gradient centrifugation using Ficoll-Hypaque (GE HealthCare): 2 × 105 PBMCs in 200 μL of media were cultured in the presence of 3 μg/mL of peptide pools derived from OCT4 peptide library. A mixture of MHC class I-restricted peptides derived from influenza virus, EBV and CMV (CEF mix), candida, or PHA were used as positive controls (26). The composition of the OCT4-peptide library pools is noted in the Table S1. After 48 h, supernatants were collected and assayed for the production of IFN-γ IP10 by Luminex, using the manufacturer's directions (Luminex Corp and Upstate), and analyzed by Beadview software (Upstate). In some experiments, CD3+ T cells were depleted by negative selection, or stimulation by specific antigens was carried out in the presence of IFNγ-blocking mAbs (Biolegend) to confirm the specificity of IP10 production. In the assay validation experiments, the variation between replicates, including the nonreactive submixes (intraassay), as well as reanalysis of the same sample (interassay), was analyzed. The coefficient of variation (% CV) in these experiments was mean (range) 8.5% (4–23%) for intraassay and 6% (1.5–30%) for interassay measurements. Based on these considerations, ≥ 2-fold increase in IP10 production, with a minimal absolute measurement of 100 pg/mL, was predetermined to be positive for the presence of antigen-specific T cells, as previously described (26). In several donors, the reactivity to a submix was further confirmed by individually testing each individual peptide from the reactive submix, to identify the specific peptide responsible for the reactivity.
CFSE Proliferation Assay.
PBMCs obtained as described above were labeled with CFSE cell tracker dye (0.5 μM; Molecular Probes) to monitor proliferation. CFSE-labeled PBMCs were cultured with 1 μg/mL of anti-CD28 and anti-CD49d (BD Biosciences) alone or with OCT4 peptide mixes (3 μg/mL), CEF mix (3 μg/mL), or PHA (2 μg/mL). After 7 d of culture, PBMCs were stained with anti-CD3; CD4 and T-cell proliferation was analyzed using the FACSCalibur (Becton Dickinson). The data were analyzed using the FlowJo software.
DC Generation and Expansion of OCT4-Specific T Cells.
DCs were generated from monocytes isolated by CD14 magnetic beads (Miltenyi Biotech) and cultured for 5 d in the presence of GM-CSF (Immunex) and IL-4 (R&D Systems), as described (26). Day-5 DC were matured overnight with inflammatory cytokines [10 ng/mL IL-1β, 1,000 U/mL IL-6, 10 ng/mL TNF-α (all from R&D Systems), and prostaglandin E2 (1 μg/mL, Sigma-Aldrich)] and pulsed for 2 h with OCT4-derived peptide; 2 μg/mL CD14- responder T cell-enriched fraction was added to U-bottom 96-well plates at 2 × 105 cells per well in 200 μL of medium and stimulated with mature peptide pulsed DC at DC: responder ratio of 1:30. IL2 (20 U/mL) was added to the DC:T-cell cocultures on days 4, 7, and 14. IL7 and IL15 (both 5 ng/mL) were added on day 14. After two restimulations with peptide-loaded DCs, the T cells were analyzed for the presence of peptide-reactive T cells using intracellular cytokine assay. For some experiments, immature DCs were fed with irradiated embryonal carcinoma tumor cells (N-tera), before maturation and use as antigen-presenting cells to stimulate autologous T cells (38).
Flow Cytometry for the Detection of Intracellular Cytokines.
Antigen-specific cells were analyzed by flow cytometry-based assay for the detection of intracellular cytokines. Briefly, T cells were cultured with anti-CD28 anti-CD49d (both at 5 μg/mL; BD Biosciences), alone or with OCT4 peptides (3 μg/mL) in the presence of Brefeldin A. After 5 h in culture, T cells were fixed and permeabilized in 100 μL Cytofix/Cytoperm solution using the manufacturer's instructions and stained for intracellular IFN-γ and surface markers (CD3, CD8). The presence of peptide-specific IFNγ-producing T cells was analyzed by flow cytometry. In some experiments, mature DCs alone or loaded with OCT4 Mix 3 were used to assess the generation of OCT4-specific T cells.
Statistical Analysis.
The reactivity against OCT4 between two groups was compared using the Mann Whitney test, and significance set at P < 0.05.
Acknowledgments
This study was supported in part by funds from the Dana Foundation and the National Institutes of Health (CA109465, CA135110, CA106802, and AI054375).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0915086107/DCSupplemental.
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.0915086107
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/content/pnas/107/19/8718.full.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/reprint/107/19/8718.pdf
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/full/107/19/8718
Free after 6 months at www.pnas.org
http://www.pnas.org/cgi/content/abstract/107/19/8718
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102382807
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.0915086107
Article citations
Human iPSC-Derived Renal Cells Change Their Immunogenic Properties during Maturation: Implications for Regenerative Therapies.
Cells, 11(8):1328, 13 Apr 2022
Cited by: 2 articles | PMID: 35456007 | PMCID: PMC9032821
No Tumorigenicity of Allogeneic Induced Pluripotent Stem Cells in Major Histocompatibility Complex-matched Cynomolgus Macaques.
Cell Transplant, 30:963689721992066, 01 Jan 2021
Cited by: 3 articles | PMID: 33588604 | PMCID: PMC7894586
Strategies for Cancer Immunotherapy Using Induced Pluripotency Stem Cells-Based Vaccines.
Cancers (Basel), 12(12):E3581, 30 Nov 2020
Cited by: 6 articles | PMID: 33266109 | PMCID: PMC7760556
Review Free full text in Europe PMC
Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma.
J Immunother Cancer, 8(2):e001066, 01 Aug 2020
Cited by: 38 articles | PMID: 32788236 | PMCID: PMC7422651
Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods.
Cells, 9(3):E719, 14 Mar 2020
Cited by: 33 articles | PMID: 32183385 | PMCID: PMC7140629
Review Free full text in Europe PMC
Go to all (57) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
OCT4: biological functions and clinical applications as a marker of germ cell neoplasia.
J Pathol, 211(1):1-9, 01 Jan 2007
Cited by: 148 articles | PMID: 17117392
Review
OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma.
Am J Surg Pathol, 28(7):935-940, 01 Jul 2004
Cited by: 151 articles | PMID: 15223965
Spontaneous and therapy-induced immunity to pluripotency genes in humans: clinical implications, opportunities and challenges.
Cancer Immunol Immunother, 60(3):413-418, 23 Nov 2010
Cited by: 6 articles | PMID: 21104412 | PMCID: PMC3574640
Review Free full text in Europe PMC
Oct4-induced pluripotency in adult neural stem cells.
Cell, 136(3):411-419, 01 Feb 2009
Cited by: 595 articles | PMID: 19203577
Funding
Funders who supported this work.
NCI NIH HHS (6)
Grant ID: CA109465
Grant ID: CA135110
Grant ID: R01 CA106802
Grant ID: R01 CA135110
Grant ID: CA106802
Grant ID: R01 CA109465
NIAID NIH HHS (2)
Grant ID: AI054375
Grant ID: K08 AI054375




