Abstract
Purpose
In 2006, we published the results of the European Organisation for Research and Treatment of Cancer phase III trial EORTC 20981 on the role of rituximab in remission induction and maintenance treatment of relapsed/resistant follicular lymphoma (FL). At that time, the median follow-up for the maintenance phase was 33 months. Now, we report the long-term outcome of maintenance treatment, with a median follow-up of 6 years.Patients and methods
Overall, 465 patients were randomly assigned to induction with either six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or rituximab plus CHOP (R-CHOP). Those in complete remission or partial remission after induction (n = 334) were randomly assigned to maintenance treatment with rituximab (375 mg/m(2) intravenously once every 3 months) or observation.Results
Rituximab maintenance significantly improved progression-free survival (PFS) compared with observation (median, 3.7 years v 1.3 years; P < .001; hazard ratio [HR], 0.55), both after CHOP induction (P < .001; HR, 0.37) and R-CHOP (P = .003; HR, 0.69). The 5-year overall survival (OS) was 74% in the rituximab maintenance arm, and it was 64% in the observation arm (P = .07). After progression, a rituximab-containing salvage therapy was given to 59% of patients treated with CHOP followed by observation, compared with 26% after R-CHOP followed by rituximab maintenance. Rituximab maintenance was associated with a significant increase in grades 3 to 4 infections: 9.7% v 2.4% (P = .01).Conclusion
With long-term follow-up, we confirm the superior PFS with rituximab maintenance in relapsed/resistant FL. The improvement of OS did not reach statistical significance, possibly because of the unbalanced use of rituximab in post-protocol salvage treatment.Free full text

Rituximab Maintenance Treatment of Relapsed/Resistant Follicular Non-Hodgkin's Lymphoma: Long-Term Outcome of the EORTC 20981 Phase III Randomized Intergroup Study
Abstract
Purpose
In 2006, we published the results of the European Organisation for Research and Treatment of Cancer phase III trial EORTC 20981 on the role of rituximab in remission induction and maintenance treatment of relapsed/resistant follicular lymphoma (FL). At that time, the median follow-up for the maintenance phase was 33 months. Now, we report the long-term outcome of maintenance treatment, with a median follow-up of 6 years.
Patients and Methods
Overall, 465 patients were randomly assigned to induction with either six cycles of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or rituximab plus CHOP (R-CHOP). Those in complete remission or partial remission after induction (n = 334) were randomly assigned to maintenance treatment with rituximab (375 mg/m2 intravenously once every 3 months) or observation.
Results
Rituximab maintenance significantly improved progression-free survival (PFS) compared with observation (median, 3.7 years v 1.3 years; P < .001; hazard ratio [HR], 0.55), both after CHOP induction (P < .001; HR, 0.37) and R-CHOP (P = .003; HR, 0.69). The 5-year overall survival (OS) was 74% in the rituximab maintenance arm, and it was 64% in the observation arm (P = .07). After progression, a rituximab-containing salvage therapy was given to 59% of patients treated with CHOP followed by observation, compared with 26% after R-CHOP followed by rituximab maintenance. Rituximab maintenance was associated with a significant increase in grades 3 to 4 infections: 9.7% v 2.4% (P = .01).
Conclusion
With long-term follow-up, we confirm the superior PFS with rituximab maintenance in relapsed/resistant FL. The improvement of OS did not reach statistical significance, possibly because of the unbalanced use of rituximab in post-protocol salvage treatment.
INTRODUCTION
In follicular lymphoma (FL), the chimeric anti-CD20 monoclonal antibody rituximab has improved response rates, progression-free survival (PFS), and overall survival (OS) to such an extent that the combination of rituximab and chemotherapy (R-chemotherapy) is the standard induction treatment in first-line as well as relapsed FL.1–4 Moreover, during the last few years, it has been shown, both in previously untreated and relapsed/refractory FL, that rituximab maintenance treatment has a clear clinical benefit after induction with R-chemotherapy, chemotherapy alone, or rituximab monotherapy.5 However, at present, there is still no proven curative treatment for FL.
In 2006, we published the results of a large, prospective, randomized, phase III, Intergroup trial evaluating the role of rituximab in remission induction and maintenance treatment of patients with relapsed/resistant FL.6 This study showed that addition of rituximab to cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) induction resulted in increased complete and overall response rates and that rituximab maintenance strongly improved median PFS—both after induction with CHOP and rituximab plus CHOP (R-CHOP) — and OS when compared with observation.6 At that time, the median follow-up for the maintenance phase was 33 months. Now, we report the long-term outcome of maintenance treatment, with a median follow-up of 6 years from the start of maintenance.
PATIENTS AND METHODS
Patients
This randomized, phase III, Intergroup study (EORTC 20981) was conducted at 130 centers in Canada, Australia/New Zealand, Europe, and South Africa. Major eligibility criteria were as follows: age older than 18 years; CD20-positive, grades 1 to 3 FL; Ann Arbor stage III or IV at initial diagnosis; and relapse after or resistance to a maximum of two non–anthracycline-containing chemotherapy regimens.6 Written informed consent was obtained according to the local rules. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Study Design and Treatment
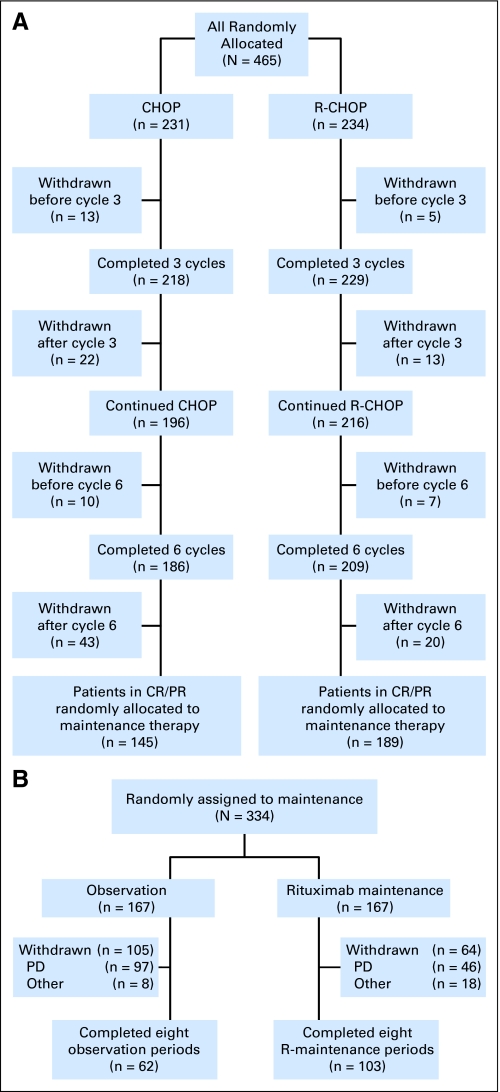
Both study design and treatment have been described in detail.6 In brief, 465 eligible patients were randomly assigned to remission induction with either six cycles of standard CHOP once every 3 weeks or R-CHOP (375 mg/m2 intravenously [IV] at day 1 of each cycle of CHOP). Those with stable disease or progression after three cycles of CHOP or R-CHOP went off study. Overall, 334 patients with a complete or partial remission after six cycles of therapy underwent a second random assignment to either observation or maintenance treatment with rituximab (375 mg/m2 IV once every 3 months until relapse or for a maximum period of 2 years). Maintenance treatment was started a median of 7 weeks (range, 3 to 16 weeks) after the end of the last induction cycle. During the 2 years of rituximab maintenance/observation, patients were seen at least every 3 months and thereafter once every 4 months. In this large, multicenter, international study, it was decided to adhere as much as possible to the daily practice in the participating countries. Thus, during maintenance/observation, computed tomography (CT) and bone marrow (BM) examinations were performed only on indication. Tumor assessments were required at each of the every-3-month visits. However, in occurrences of progression or relapse, detailed information on the date and site of progression/relapse had to be provided by the investigator, which usually required a repetition of the original radiologic assessments performed at study entry (CONSORT diagram, Fig 1).
Statistical Analysis
All primary analyses were conducted according to the intention-to-treat (ITT) principle. The primary end point for the maintenance phase was PFS, defined as interval between the date of second random assignment and date of first relapse, progression, or death. The principal analysis of PFS and OS was done with the log-rank test, and sensitivity analyses were done with Cox regression analysis, with adjustment for type of induction treatment and response. Kaplan-Meier curves were calculated to graphically show the differences between the treatment arms. P values given are all two sided.
RESULTS
Patients
A total of 474 patients with relapsed/resistant FL were randomly assigned to receive induction treatment with CHOP or R-CHOP. Nine patients had to be excluded because of missing informed consent forms. Therefore, all analyses were restricted to 465 patients (231 CHOP and 234 R-CHOP). Recruitment was stopped after the preplanned second interim analysis for efficacy, because the criteria for early discontinuation were met both for induction and maintenance.6 The characteristics of the patients and the results of the induction phase of the study have been described in detail.6 In brief, the addition of rituximab significantly increased complete response (CR) rates: there was a 15.6% CR rate in patients receiving CHOP and a 29.5% CR rate in patients receiving R-CHOP (P < .001). Overall response rates were 72.3% and 85.1% after CHOP and R-CHOP induction treatment, respectively (P < .001).6
The 334 patients randomly assigned to the maintenance phase were well balanced for baseline characteristics at study entry, Follicular Lymphoma International Prognostic Index (FLIPI) score (≥ 2 in 70% and 66% in the observation and maintenance arms, respectively), type of induction treatment received, and response to induction (in both arms: 29% CR and 71% PR). In both arms, more patients had received R-CHOP during induction (59% in the observation arm and 55% in the maintenance arm), reflecting the higher efficacy of R-CHOP compared with CHOP in terms of response induction.
Efficacy: Maintenance Phase
Overall, 334 eligible patients were randomly assigned to rituximab maintenance treatment for 2 years (n = 167) or observation (n = 167). In each study arm, one patient did not start allocated treatment because of progression immediately after random assignment. Median follow-up from second random assignment was 6 years, and 75% of the patients had a follow-up of at least 5 years. Only 3% of all patients were lost to follow-up (ie, no follow-up data available for at least 1 year).
Median PFS from second random assignment was 3.7 years in the maintenance arm and was 1.3 years in the observation arm (P < .0001, log-rank test; hazard ratio [HR], 0.55; Fig 2). As shown in Figure 3, the advantage of rituximab maintenance was observed both after CHOP induction and R-CHOP induction. After CHOP induction, rituximab maintenance resulted in a median PFS from second random assignment of 3.1 years versus 1 year in the observation arm (HR, 0.37; P < .001). After R-CHOP induction, these figures were 4.4 years and 1.9 years, respectively (HR, 0.69; P = .043). Similarly, rituximab maintenance resulted in a highly significant increase in PFS, both in patients who had a partial remission (PR) after induction treatment and in those who had obtained a complete remission. In patients with a PR after remission induction, rituximab maintenance resulted in a median PFS from second random assignment of 3.4 years versus 1.3 years in the observation arm (HR, 0.58; P < .001). For patients in complete remission, median PFS was 4.4 years in the rituximab maintenance arm versus 1.2 years in the observation arm (HR, 0.48; P = .003; Fig 4). We have performed an unplanned exploratory subgroup analysis according to baseline disease characteristics. Only in the subgroup with bulky disease, rituximab maintenance did not result in improved PFS. The improvement in PFS was comparable in the FLIPI low, intermediate, and high subgroups.
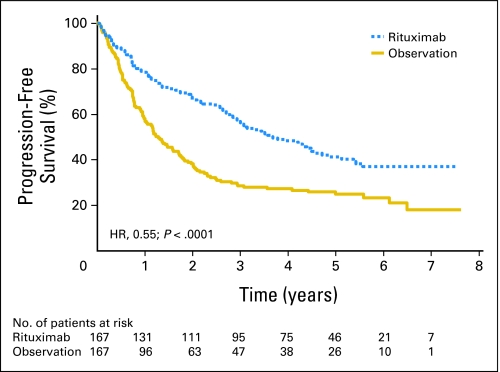
Effect of rituximab maintenance treatment on progression-free survival (PFS). Kaplan-Meier plot of PFS from second random assignment after rituximab maintenance therapy (n = 167) and observation (n = 167). Rituximab maintenance, median 3.7 years; observation, median 1.3 years. HR, hazard ratio.
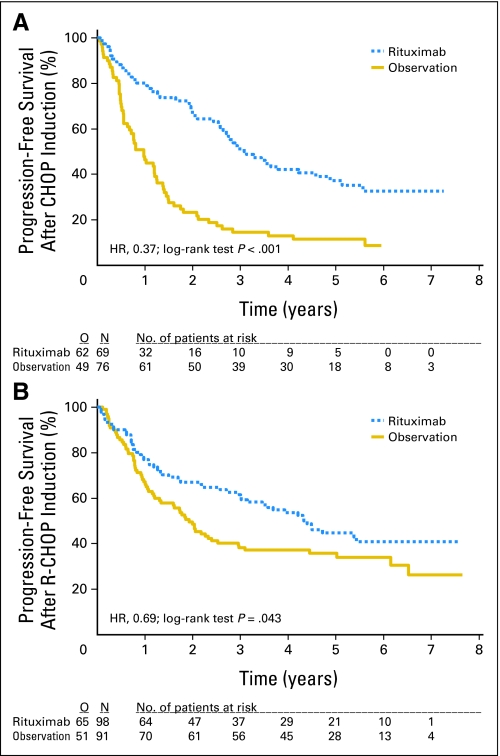
Effect of rituximab maintenance treatment on progression-free survival (PFS) after remission induction with either cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or rituximab plus CHOP (R-CHOP). Kaplan-Meier plots of PFS and overall survival from second random assignment. (A) PFS after CHOP remission induction (n = 145). Rituximab maintenance, median 3.1 years; observation, median 1.0 year. (B) PFS after R-CHOP remission induction (n = 189). Rituximab maintenance, median 4.4 years; observation, median 1.9 years. HR, hazard ratio; O, observed; N, number.
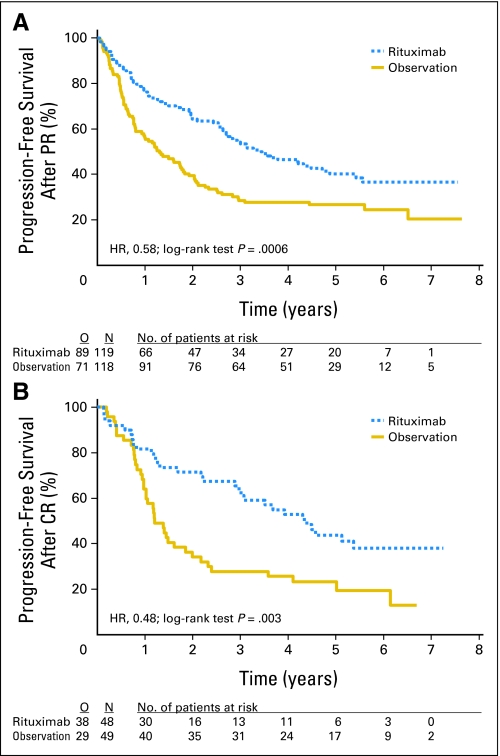
Effect of rituximab maintenance treatment on progression-free survival (PFS) in patients in partial remission (PR) or complete remission (CR) after remission induction treatment. Kaplan-Meier plots of PFS and overall survival from second random assignment. (A) PFS in patients with PR (n = 237). Rituximab maintenance, median 3.4 years; observation, median 1.3 years. (B) PFS in patients with CR (n = 97). Rituximab maintenance, median 4.4 years; observation, median 1.2 years. HR, hazard ratio; O, observed; N, number.
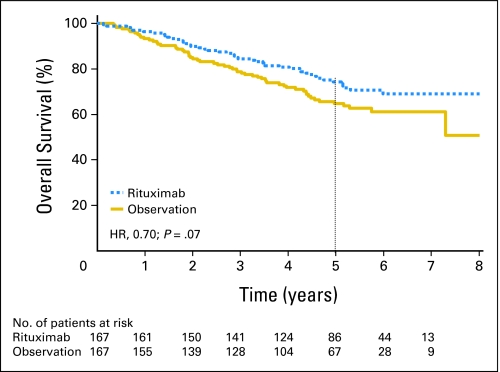
Rituximab maintenance treatment increased 5-year OS rates (from second random assignment) from 64.7% in the observation group to 74.3% in the rituximab maintenance group (P = .07, log-rank test; Fig 5). The hazard ratio for rituximab maintenance compared with observation was 0.70 (95% CI, 0.48 to 1.03) Subgroup analysis according to induction regimen showed a strong trend for improved OS in both patient groups—in patients responding to CHOP (HR, 0.59; P = .05), and, although much less, in those responding to R-CHOP (HR, 0.80; P = .42) induction therapy. However, these differences were not statistically significant. Importantly, Figures 2 and and33 show that 35% to 40% of patients had experienced progression or relapse during or within 6 months after rituximab maintenance.
Post-Protocol Treatment
To investigate why the highly improved PFS after rituximab maintenance did not translate into a significant OS advantage, we recorded the salvage regimens used in patients relapsing during or after rituximab maintenance or observation. In total, 41% of patients who experienced progression received rituximab-containing regimens as (part of) salvage therapy. This varied according to treatment arm, from 59% after CHOP followed by observation to 26% after R-CHOP followed by rituximab maintenance (Table 1). Rituximab was given as single-agent salvage treatment in 53% of the patients experiencing relapse in the observation group, whereas this was the case in 38% of the patients experiencing relapse in the maintenance group. In all four subgroups, a comparable low percentage (< 10%) of the patients had either autologous stem-cell transplantation or allogeneic stem-cell transplantation as part of their salvage treatment. Although secondary/second primary tumors were reported in 8% and 5% of the patients in the observation arm and rituximab maintenance arm, respectively, no histologic transformation has been reported. However, because detailed post-protocol information is lacking, we cannot exclude that (as expected), in a small number of patients, histologic transformation has occurred.
Table 1.
Post-Protocol Treatment
| Post-Protocol Lymphoma Treatment | Induction and Maintenance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHOP + OBS (n = 69) | R-CHOP + OBS (n = 98) | CHOP + RITUX (n = 76) | R-CHOP + RITUX (n = 91) | Total (n = 334) | ||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Chemotherapy | 34 | 49.3 | 33 | 33.7 | 26 | 34.2 | 35 | 38.5 | 128 | 38.3 |
| Radiotherapy | 16 | 23.2 | 18 | 18.4 | 13 | 17.1 | 16 | 17.6 | 63 | 18.9 |
| Autologous stem-cell transplantation | 3 | 4.3 | 8 | 8.2 | 6 | 7.9 | 5 | 5.5 | 22 | 6.6 |
| Allogeneic stem-cell transplantation | 5 | 7.2 | 7 | 7.1 | 8 | 10.5 | 4 | 4.4 | 24 | 7.2 |
| Rituximab, single agent | 26 | 37.7 | 13 | 13.3 | 8 | 10.5 | 5 | 5.5 | 52 | 15.6 |
| Rituximab, combination | 20 | 29.0 | 14 | 14.3 | 13 | 17.1 | 8 | 8.8 | 55 | 16.5 |
| Other | 8 | 11.6 | 12 | 12.2 | 6 | 7.9 | 17 | 18.7 | 43 | 12.9 |
| Progression status and rituximab post-protocol treatment | ||||||||||
    No progression No progression | 8 | 11.6 | 34 | 34.7 | 32 | 42.1 | 41 | 45.1 | 115 | 34.4 |
    Progression, no rituximab Progression, no rituximab | 25 | 36.2 | 42 | 42.9 | 26 | 34.2 | 37 | 40.7 | 130 | 38.9 |
    Progression, rituximab Progression, rituximab | 36 | 52.2 | 22 | 22.4 | 18 | 23.7 | 13 | 14.3 | 89 | 26.6 |
    % rituximab among patients with progression % rituximab among patients with progression | 59.0 | 34.4 | 40.9 | 26.0 | 40.6 | |||||
NOTE. The upper part of the Table is not cumulative, as one patient may have received different successive post-protocol treatments. The lower part of the table shows the proportion of patients who have received rituximab as part of salvage therapy.
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone; OBS, observation; RITUX, rituximab.
Safety
During rituximab maintenance treatment, neutropenia was the only significant toxicity; 11.5% experienced grades 3 to 4 neutropenia in the rituximab maintenance arm versus 6.0% in the observation arm. This probably contributed to the increased grades 3 to 4 infection rates of 9.7% in the maintenance group and 2.4% during observation (P = .01). The majority of these infections were in the ear-nose-throat area. No opportunistic or viral infections were observed. In the observation arm, serum immunoglobulin (Ig) G levels increased from 6.6 g/L at second random assignment to 7.3 g/L at the end of the 2-year observation period. In the maintenance arm, IgG levels at second random assignment and at the end of the maintenance period were 6.5 g/L and 6.3 g/L, respectively. No patient had to be withdrawn because of persisting IgG levels less than 3g/L.
Only seven of the 167 patients withdrew from maintenance treatment because of toxicity: four had recurrent infections, one had severe neutropenia, one had ventricular arrhythmia, and one had persistent general complaints. There were no deaths related to rituximab maintenance treatment.
DISCUSSION
The most important conclusions from this long-term follow-up of the large, randomized EORTC 20981 Intergroup study in patients with relapsed/resistant FL study are as follows: We confirm the strong improvement of PFS by maintenance treatment with rituximab. Rituximab maintenance treatment results in superior PFS both after remission induction treatment with CHOP and R-CHOP and in patients in complete remission and PR after remission induction. Rituximab maintenance treatment is well tolerated, with an increase in the incidence of grades 3 to 4 infections as the only significant adverse effect, which required only 4% of patients to discontinue maintenance. Rituximab maintenance treatment results in a 10% improvement of 5-year OS.
The improvement in OS was not statistically significant (P = .07). That the highly improved PFS after rituximab maintenance did not translate into a significant OS advantage might partially be due to the effect of the unbalanced use of rituximab in post-protocol salvage treatment regimens. Not surprisingly, rituximab was used most abundantly in patients who had neither received rituximab during induction treatment nor as maintenance. Because our trial was initiated already in 1998 in all four treatment subgroups, an unknown percentage of the patients did not receive rituximab-containing salvage treatment because of reimbursement problems.
In a number of randomized studies, the clinical benefit of rituximab maintenance treatment has been shown after various remission induction regimens: after rituximab monotherapy in previously untreated patients as well as patients who experienced relapse,7,8 after first-line chemotherapy9 and after R-chemotherapy induction in patients who experienced relapse.6,10 In a recent meta-analysis, rituximab maintenance was found to improve not only PFS but also OS in patients with FL.11 Moreover, this meta-analysis showed a significant increase in (bacterial) infections. However, this rarely necessitated discontinuation of the rituximab maintenance, and it did not result in death.11
Of course, an important caveat about conclusions of this study is that, because the trial was initiated already in 1998, none of the patients had received rituximab as part of their first or second remission induction treatment. Whether the results of R-chemotherapy induction and rituximab maintenance will be similar in patients who have already received prior treatment with rituximab is still an open question. The efficacy of maintenance treatment after first-line induction treatment with R-chemotherapy was addressed in the international PRIMA (Primary Rituximab and Maintenance) study, which has enrolled 1,217 patients. The first results are eagerly awaited.
Hainsworth et al7 compared rituximab maintenance with rituximab re-treatment at disease progression in a randomized, phase II study in patients with relapsed or refractory indolent NHL. They found an approximately four-fold increase in PFS by rituximab maintenance (31.1 months v 7.4 months). However, the rituximab benefit (defined as date of study entry to date of next lymphoma treatment with cytotoxic drugs) was similar in both groups (31.3 months v 27.4 months, respectively). Unfortunately, assessment of quality of life was not part of this rather small study in a heterogeneous group of patients. The important issue of maintenance treatment versus re-treatment on relapse is the topic of an ongoing large, randomized, phase III study (RESORT [Rituximab Extended Schedule or Retreatment] trial).
Questions still to be answered relate to the optimal schedule and duration of rituximab maintenance. Thus far, a number of different rituximab maintenance schedules have been used, ranging from a single rituximab infusion every 3 months for up to 2 years to once-weekly rituximab infusions for 4 weeks repeated every 6 months for up to 2 years. In a recent pharmacokinetic study, patients without progressive disease after a standard course of rituximab subsequently received single infusions of rituximab when the serum level of the drug decreased to less than 25 μg/mL, (ie, the level considered to be the minimum required for clinical activity of rituximab). In the 12-month follow-up period, 95% of the patients required three or fewer doses of rituximab to continuously maintain their serum rituximab levels greater than 25 μg/mL.12 However, the level of 25 μg/mL has been chosen rather arbitrarily. To date, there are no data demonstrating the optimal rituximab serum level for maintenance therapy. The large, randomized trials required to establish the best rituximab dosing schedule are not likely ever to be performed. However, because all maintenance schedules have been shown to be effective, more important than establishing the best dosing schedule is the issue of optimal duration of rituximab maintenance treatment. Should the optimal duration be the maximum period that has been shown to be both effective and safe (ie, 2 years), or should rituximab maintenance therapy be continued until relapse occurs? Currently available evidence appears to indicate that extended rituximab dosing is safe in the vast majority of patients studied. However, follow-up times are still relatively short for most studies, and isolated reports of patients developing late-onset neutropenia,13 Pneumocystis carinii pneumonia,14 cutaneous squamous cell carcinoma,15 and progressive multifocal leukoencephalopathy16 after rituximab therapy have been published. Therefore, patients must be carefully monitored to confirm the safety of rituximab maintenance therapy. The first analysis of the SAKK35/03 study –comparing a brief maintenance treatment of every 2 months for a total of four doses with maintenance treatment every 2 months for 5 years—did not reveal unexpected toxicity.17 Other important issues related to prolonged rituximab maintenance are the incidence of histologic transformation, possible selection for CD20-negative relapses, and the optimal treatment for rituximab refractoriness (ie, progression or relapse occurring during or within 6 months after maintenance treatment, as observed in 35% to 40% of our patients). All these questions need to be addressed in ongoing and future prospective trials.
Appendix
Table A1.
Overall Survival and Comparison of Rituximab Maintenance After CHOP or R-CHOP Induction and Rituximab Treatment or Retreatment at Progression After CHOP or R-CHOP Followed by Observation
| Variable | Observation (n = 34) | Rituximab (n = 167) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Patients with event | 9 | 26.5 | 18 | 10.8 |
| Patients without events* | 25 | 73.5 | 149 | 89.2 |
| Time to event, days | ||||
    Median† Median† | NR | NR | ||
    95% CI 95% CI | 1290 to NR | NR to NR | ||
    25th and 75th percentiles 25th and 75th percentiles | 1290 | — | — | — |
    Range‡ Range‡ | 127-2,014 | 50-1,989 | ||
| P by log-rank test | .0436 | |||
    Hazard ratio Hazard ratio | 0.45 | |||
    95% CI 95% CI | 0.20 to 1.00 | |||
| P by Wald Test | .0493 | |||
| Patients remaining at risk at month 12 | 32 | 146 | ||
    Event-free rate Event-free rate | 0.94 | 0.96 | ||
    95% CI 95% CI | 0.86 to 1.00 | 0.93 to 0.99 | ||
NOTE. Unstratified analysis.
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone; NR, not reached.
Footnotes
Supported by Grants No. 2U10 CA11488-28 through 5U10 CA11488-35 from the National Cancer Institute, Bethesda, MD; by the Koningin Wilhelmina Fund, the Netherlands; and by F. Hoffmann-La Roche, Pharmaceuticals Division.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: ISRCTN65655917.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Robert E. Marcus, Roche (C), Celgene (C); Eva Kimby, Roche (C); Anton Hagenbeek, Roche (C) Stock Ownership: None Honoraria: Marinus H.J. van Oers, Roche; Richard Klasa, Roche; Robert E. Marcus, Roche; Eva Kimby, Roche; Andrej Vranovsky, Roche Research Funding: Martine Van Glabbeke, Roche; Eva Kimby, Roche; Andrej Vranovsky, Roche Expert Testimony: None Other Remuneration: Robert E. Marcus, Roche
AUTHOR CONTRIBUTIONS
Conception and design: Marinus H.J. van Oers, Martine Van Glabbeke, Richard Klasa, Robert E. Marcus, Max Wolf, Eva Kimby, Anton Hagenbeek
Administrative support: Marinus H.J. van Oers, Livia Giurgea
Provision of study materials or patients: Marinus H.J. van Oers, Richard Klasa, Robert E. Marcus, Max Wolf, Eva Kimby, Mars van t Veer, Andrej Vranovsky, Harald Holte, Anton Hagenbeek
Collection and assembly of data: Marinus H.J. van Oers, Martine Van Glabbeke, Livia Giurgea, Max Wolf, Andrej Vranovsky, Harald Holte
Data analysis and interpretation: Marinus H.J. van Oers, Martine Van Glabbeke, Livia Giurgea, Anton Hagenbeek
Manuscript writing: Marinus H.J. van Oers, Anton Hagenbeek
Final approval of manuscript: Marinus H.J. van Oers, Martine Van Glabbeke, Livia Giurgea, Richard Klasa, Robert E. Marcus, Max Wolf, Eva Kimby, Mars van t Veer, Andrej Vranovsky, Harald Holte, Anton Hagenbeek
REFERENCES
Articles from Journal of Clinical Oncology are provided here courtesy of American Society of Clinical Oncology
Full text links
Read article at publisher's site: https://doi.org/10.1200/jco.2009.26.5827
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2903319?pdf=render
Free to read at intl.jco.org
http://intl.jco.org/cgi/content/abstract/28/17/2853
Subscription required at intl.jco.org
http://intl.jco.org/cgi/reprint/28/17/2853.pdf
Subscription required at intl.jco.org
http://intl.jco.org/cgi/content/full/28/17/2853
Citations & impact
Impact metrics
Article citations
Outcomes of the transformation of follicular lymphoma to diffuse large B-cell lymphoma in the rituximab era: A population-based study.
Cancer Med, 13(8):e7120, 01 Apr 2024
Cited by: 2 articles | PMID: 38629251 | PMCID: PMC11022146
Investigator choice of standard therapy versus sequential novel therapy arms in the treatment of relapsed follicular lymphoma (REFRACT): study protocol for a multi-centre, open-label, randomised, phase II platform trial.
BMC Cancer, 24(1):370, 25 Mar 2024
Cited by: 0 articles | PMID: 38528445 | PMCID: PMC10962099
Budget Impact of Introducing Fixed-Duration Mosunetuzumab for the Treatment of Relapsed or Refractory Follicular Lymphoma After Two or More Lines of Systemic Therapy in the USA.
Pharmacoeconomics, 42(5):569-582, 01 Feb 2024
Cited by: 0 articles | PMID: 38300452 | PMCID: PMC11039538
Risk of Severe Infections Secondary to the Use of Targeted Therapies in Hematological Malignancies.
Cureus, 16(1):e52050, 10 Jan 2024
Cited by: 0 articles | PMID: 38344573 | PMCID: PMC10857843
Review Free full text in Europe PMC
Cost-effectiveness of obinutuzumab plus bendamustine in Chinese patients with relapse and refractory follicular lymphoma.
J Comp Eff Res, 12(12):e230073, 02 Nov 2023
Cited by: 0 articles | PMID: 37916709 | PMCID: PMC10734320
Go to all (187) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: results of a prospective randomized phase 3 intergroup trial.
Blood, 108(10):3295-3301, 27 Jul 2006
Cited by: 364 articles | PMID: 16873669
Phase III randomized intergroup trial of CHOP plus rituximab compared with CHOP chemotherapy plus (131)iodine-tositumomab for previously untreated follicular non-Hodgkin lymphoma: SWOG S0016.
J Clin Oncol, 31(3):314-320, 10 Dec 2012
Cited by: 89 articles | PMID: 23233710 | PMCID: PMC3732010
First-line R-CVP versus R-CHOP induction immunochemotherapy for indolent lymphoma with rituximab maintenance. A multicentre, phase III randomized study by the Polish Lymphoma Research Group PLRG4.
Br J Haematol, 188(6):898-906, 02 Dec 2019
Cited by: 10 articles | PMID: 31792945 | PMCID: PMC7154735
Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma.
Drugs, 70(11):1445-1476, 01 Jul 2010
Cited by: 113 articles | PMID: 20614951
Review
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: U10 CA011488
Grant ID: 5U10 CA11488-35
Grant ID: CA11488-28